The Safety of Long-Term Proton Pump Inhibitor Use on Cardiovascular Health: A Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Protocol
2.2. Search Strategy
2.3. Study Selection Criteria
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
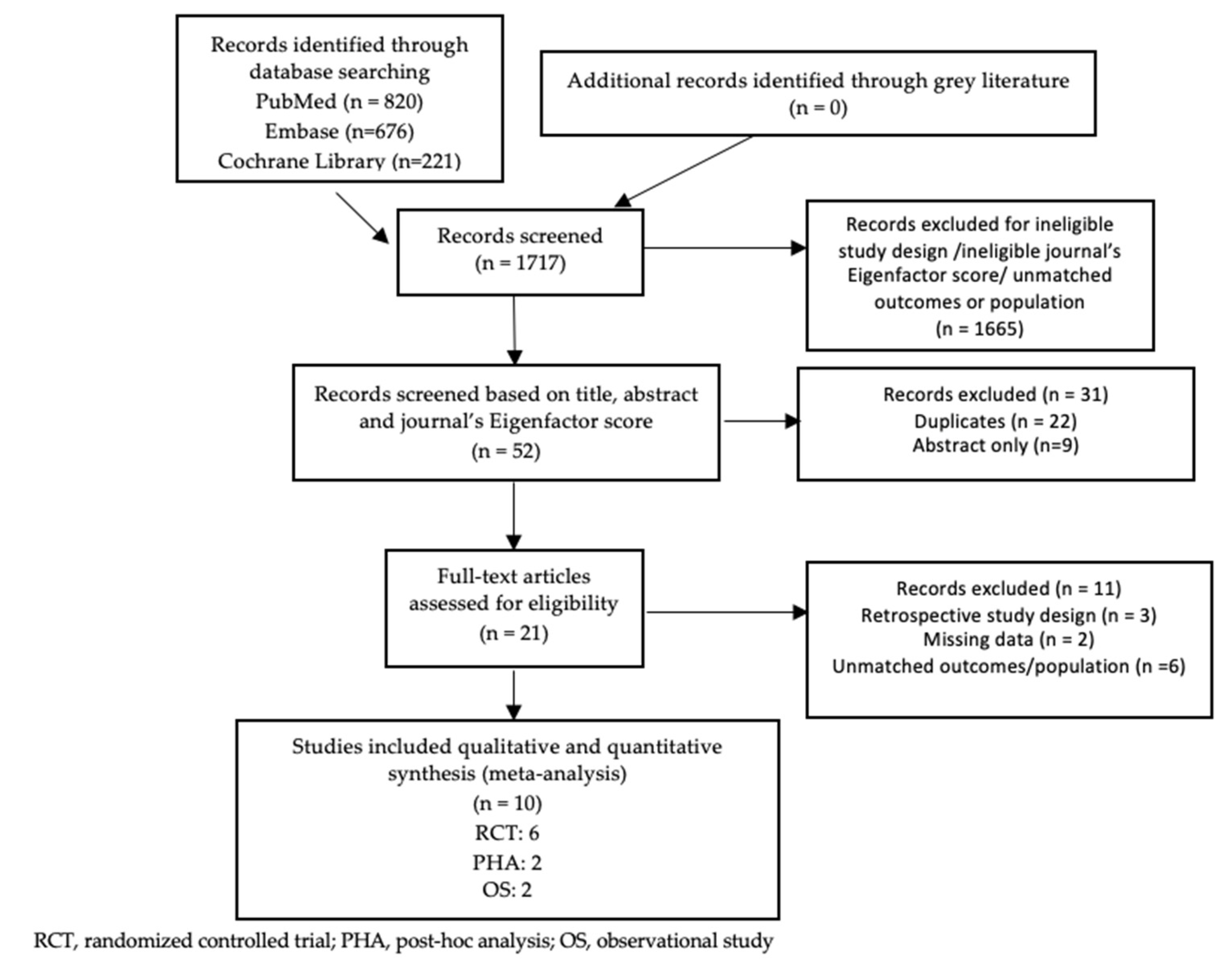
3.1. Study Selection
3.2. Study Characteristics
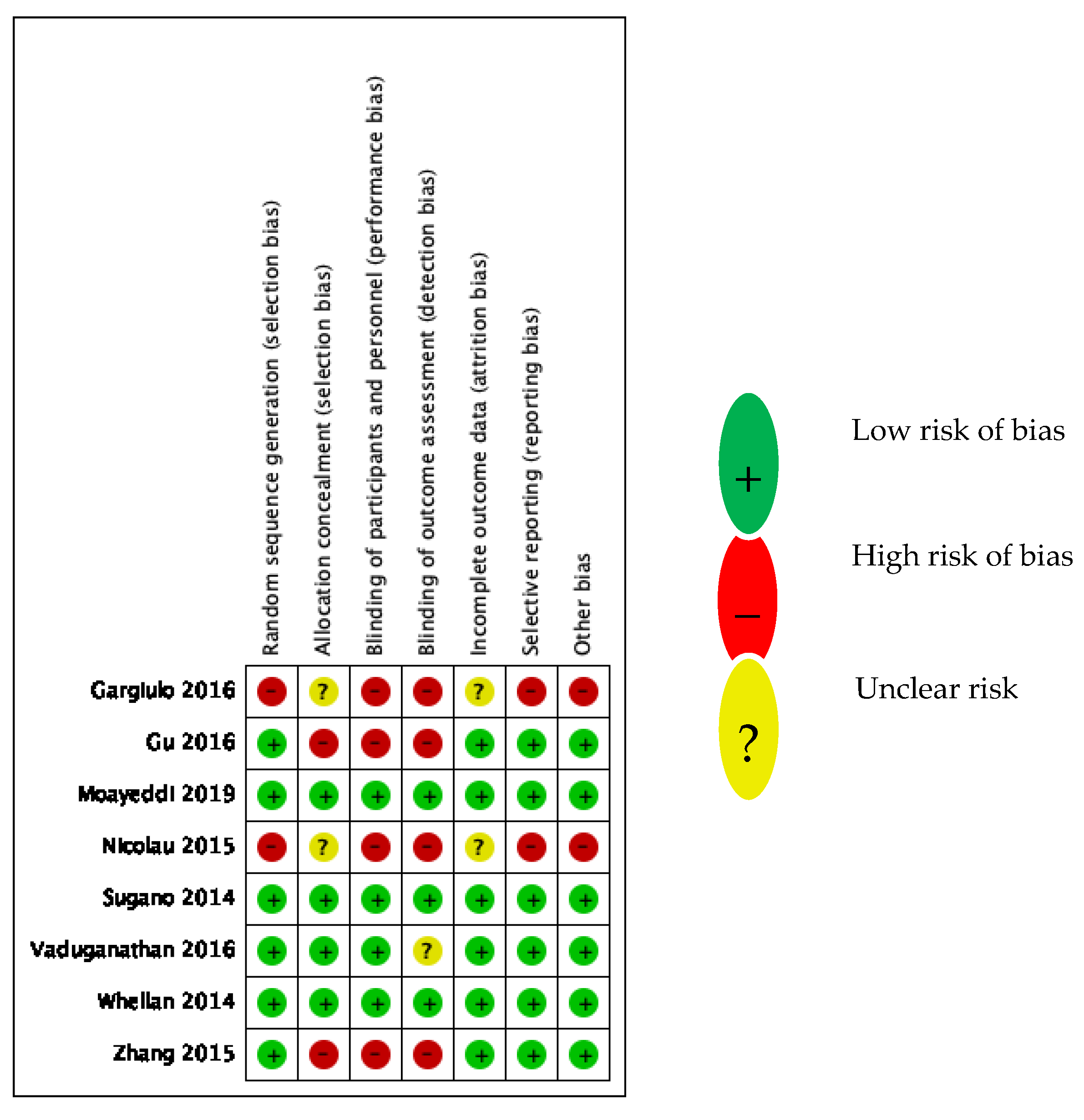
3.3. Study Quality Assessment
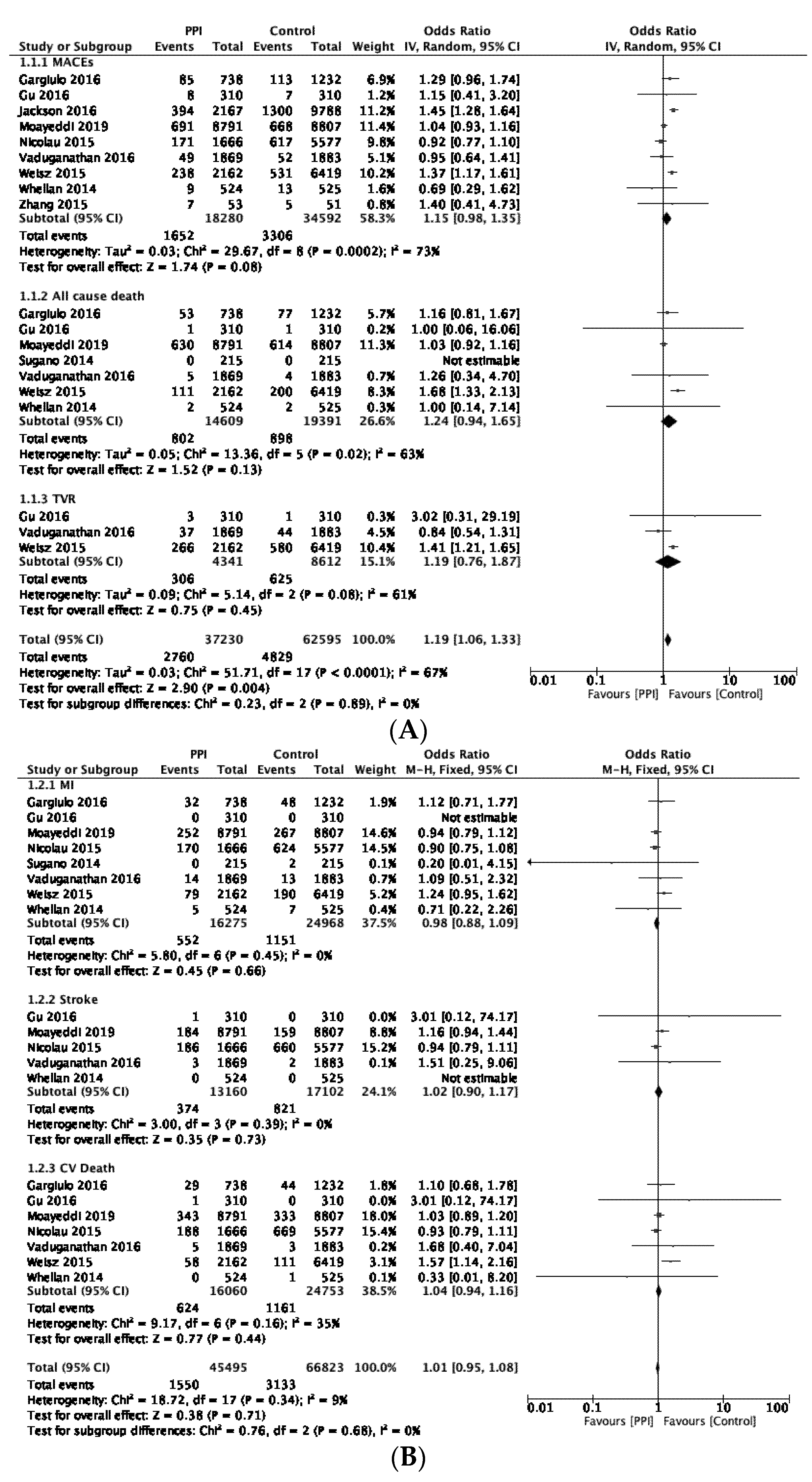
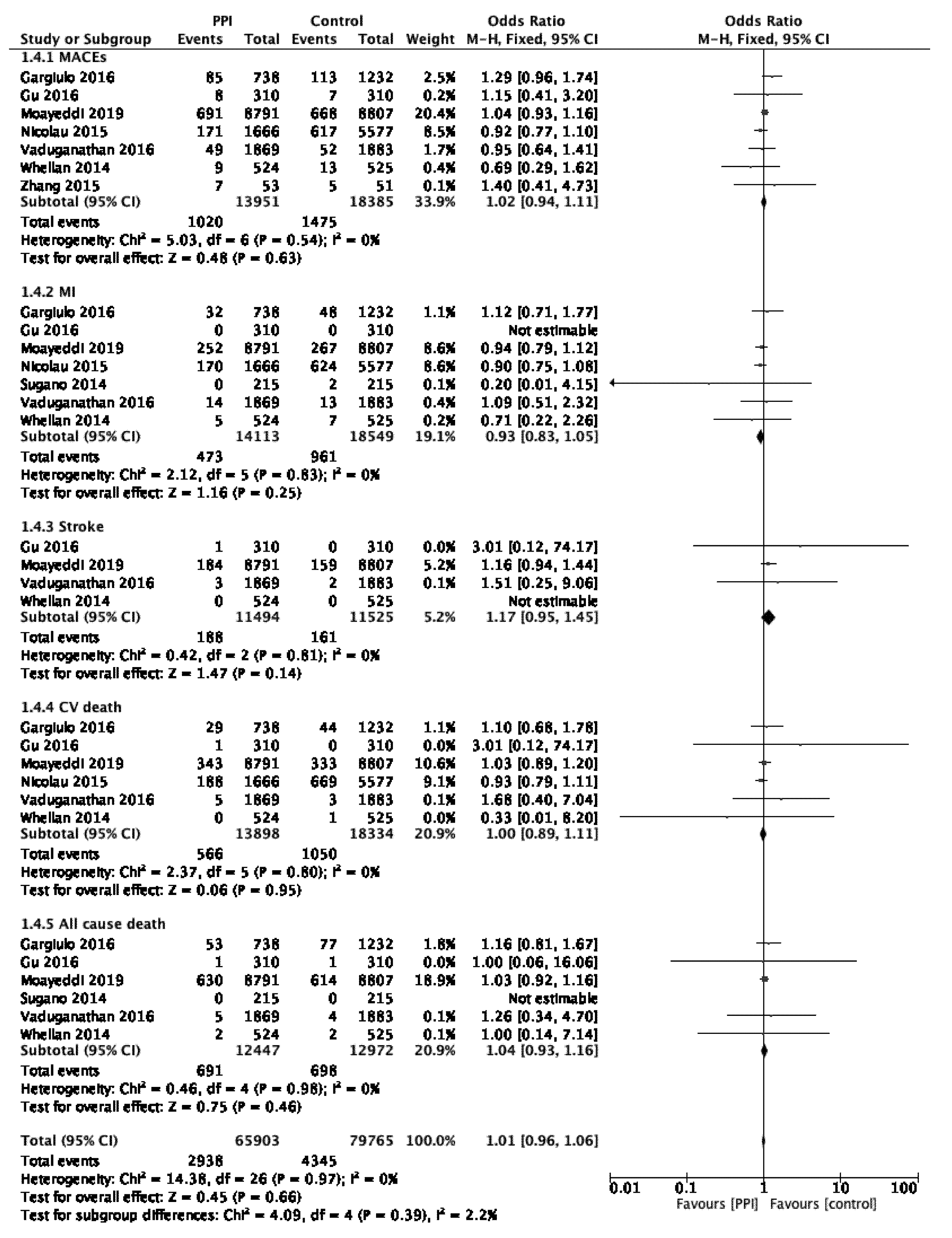
3.4. Primary Analysis
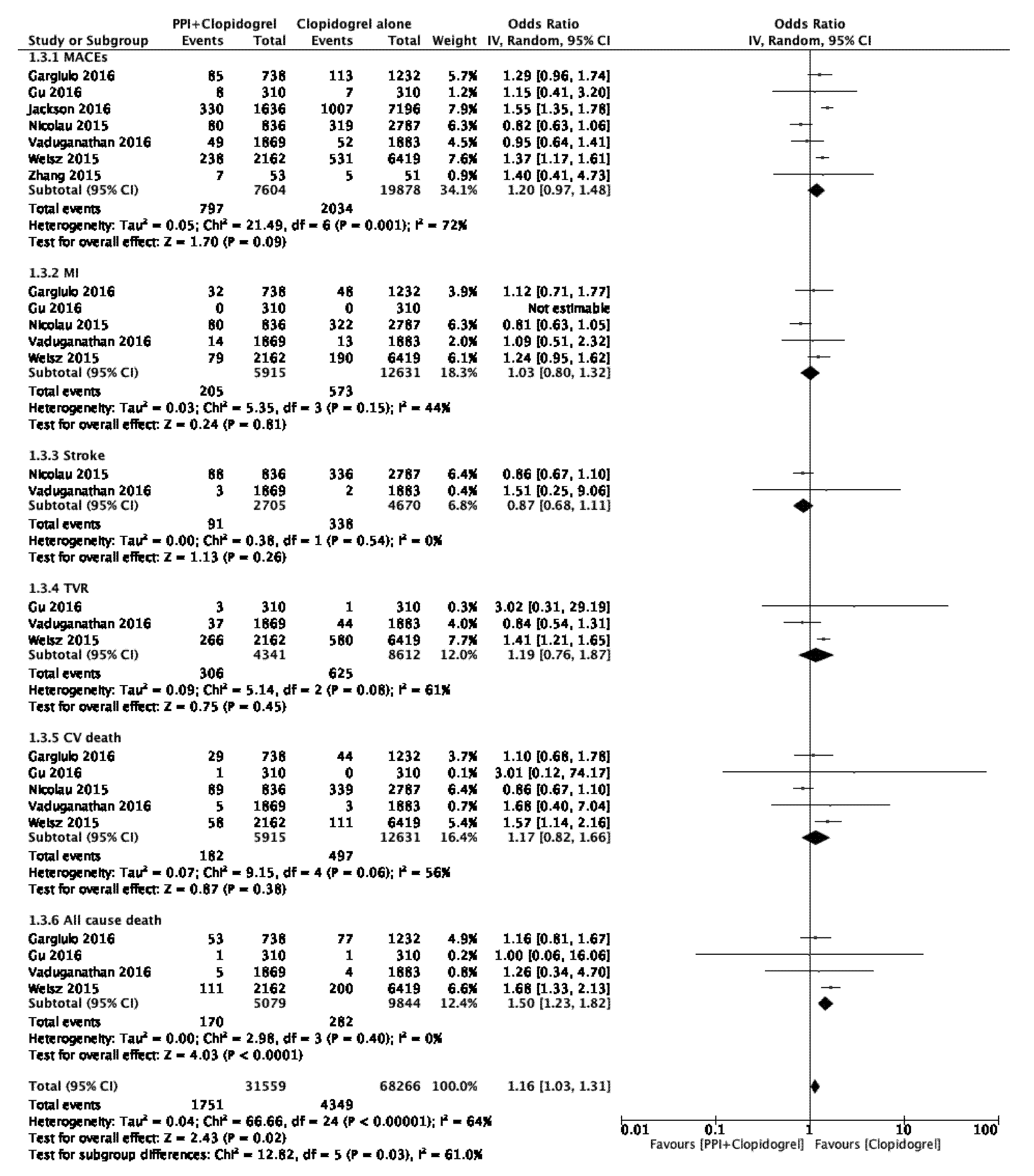
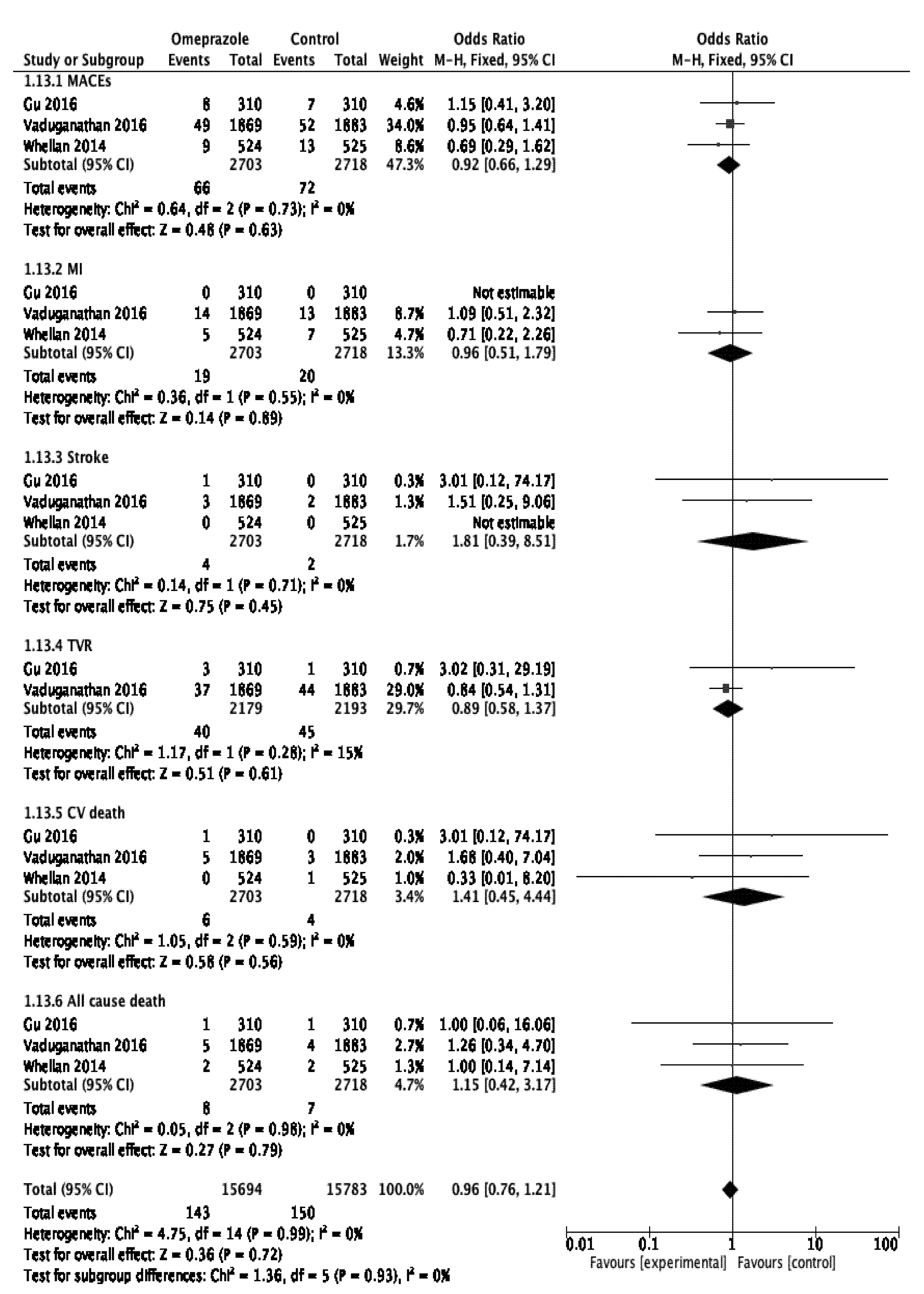
3.5. Subgroup Analysis
3.6. Heterogeneity and Sensitivity Analyses
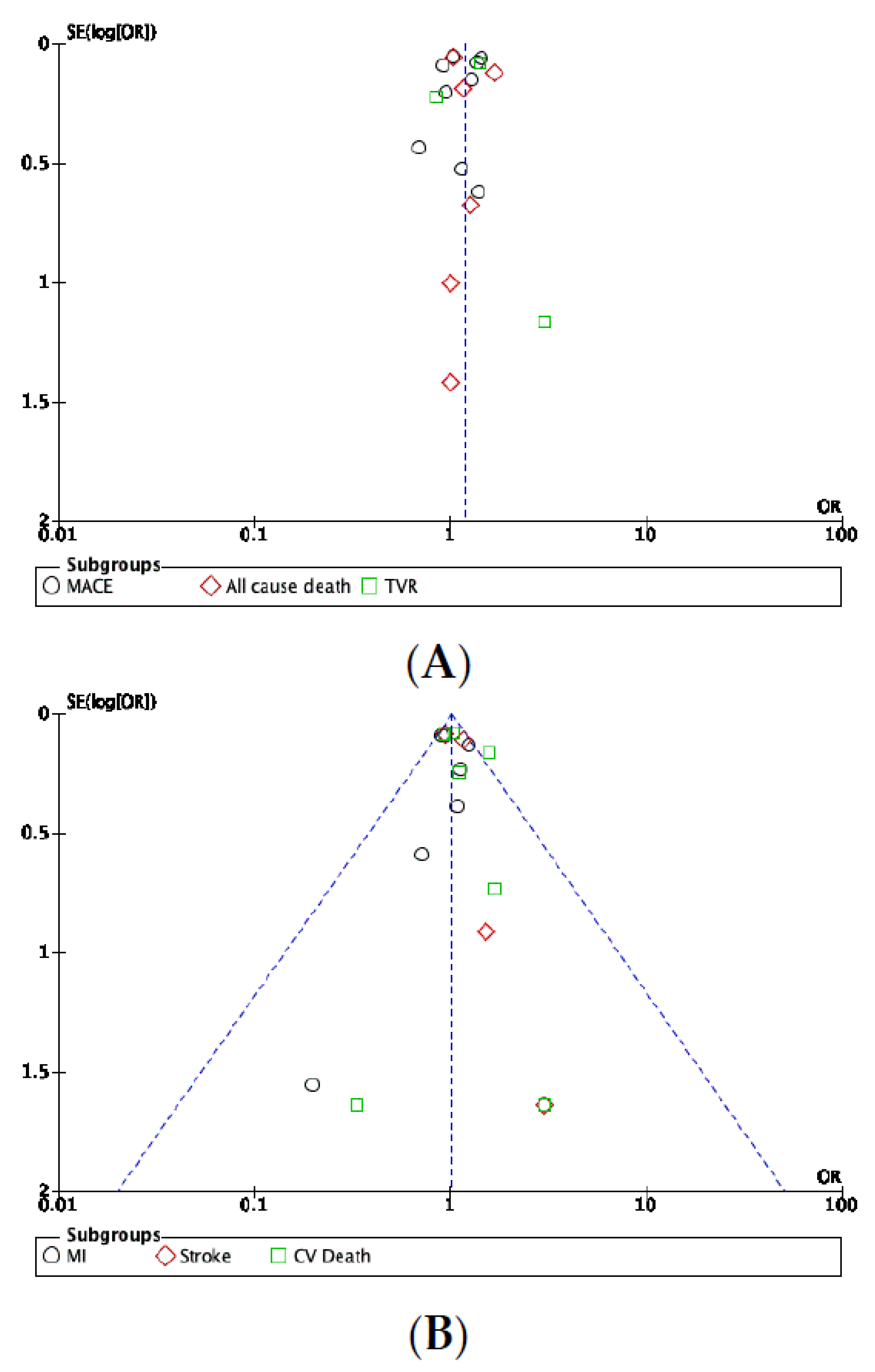
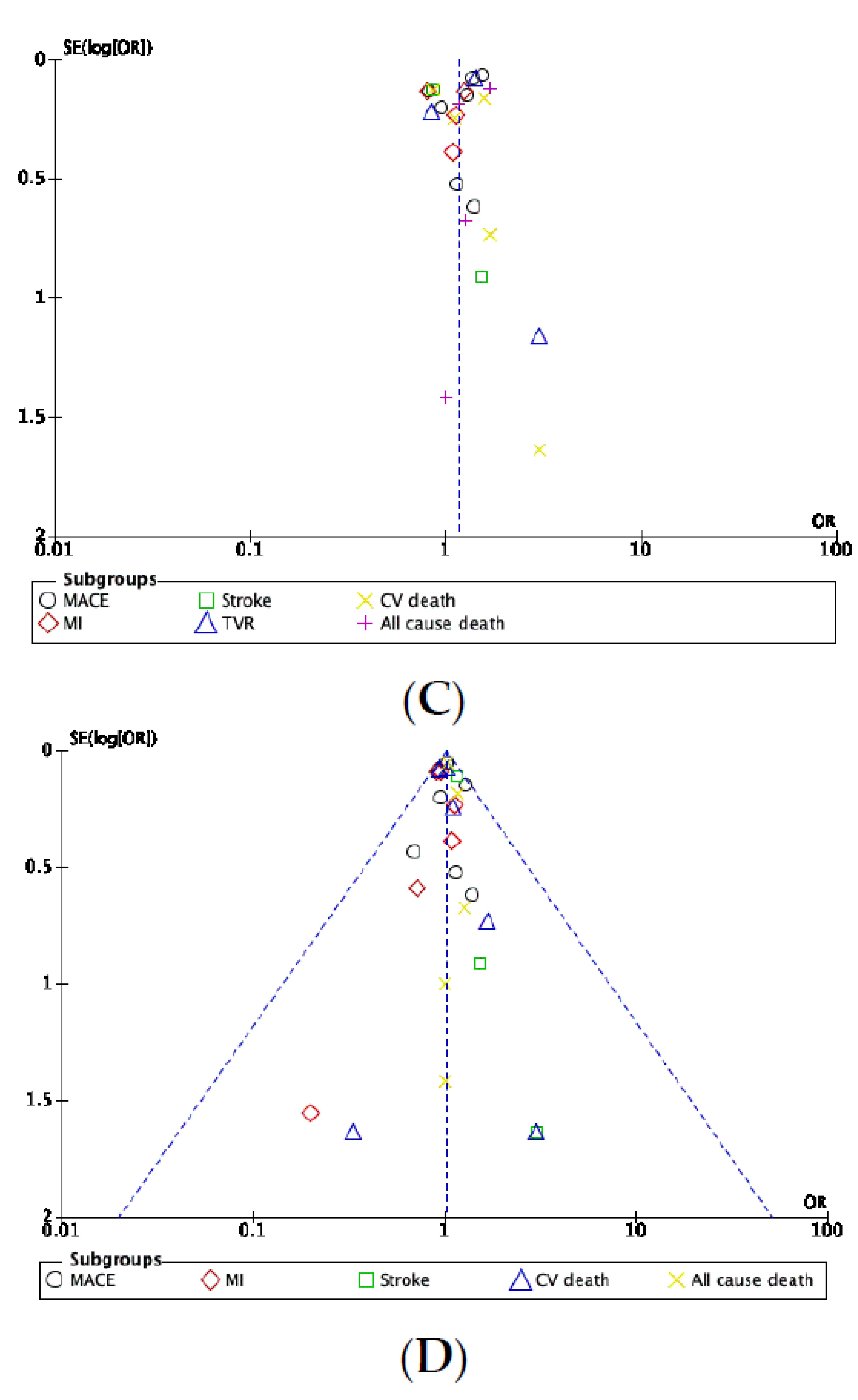
3.7. Publication Bias
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Li, R.; Zhang, J.; Zhang, F. Proton pump inhibitor utilisation and potentially inappropriate prescribing analysis: Insights from a single-centred retrospective study. BMJ Open 2020, 10, e040473. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.N.; Nakhla, N.R.; Tadrous, M. Further Evidence to Monitor Long-term Proton Pump Inhibitor Use. JAMA Netw. Open 2019, 2, e1916184. [Google Scholar] [CrossRef] [PubMed]
- Jaynes, M.; Kumar, A.B. The risks of long-term use of proton pump inhibitors: A critical review. Ther. Adv. Drug Saf. 2018, 10, 2042098618809927. [Google Scholar] [CrossRef] [PubMed]
- Hunfeld, N.G.M.; Geus, W.P.; Kuipers, E.J. Systematic review: Rebound acid hypersecretion after therapy with proton pump inhib-itors. Aliment Pharmacol. Ther. 2007, 25, 39–46. [Google Scholar] [CrossRef]
- Scarpignato, C.; Gatta, L.; Zullo, A.; Blandizzi, C. SIF-AIGO-FIMMG Group, Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases-A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016, 14, 179. [Google Scholar]
- Ghebremariam, Y.T.; LePendu, P.; Lee, J.C.; Erlanson, D.A.; Slaviero, A.; Shah, N.H.; Leiper, J.; Cooke, J.P. Unexpected Effect of Proton Pump Inhibitors: Elevation of the Cardiovascular Risk Factor Asymmetric Dimethylarginine. Circulation 2013, 128, 845–853. [Google Scholar] [CrossRef]
- Fossmark, R.; Martinsen, T.C.; Waldum, H.L. Adverse Effects of Proton Pump Inhibitors—Evidence and Plausibility. Int. J. Mol. Sci. 2019, 20, 5203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, D.; Du, J.; Qu, G.; Du, J.; Deng, S.; Liu, Y.; Cai, J.; She, Q. Efficacy of Clopidogrel and Clinical Outcome When Clopidogrel is Coad-ministered with Atorvastatin and Lansoprazole. Medicine 2015, 94. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5058921/ (accessed on 21 October 2020).
- Chung, K.C.; Swanson, J.A.; Schmitz, D.; Sullivan, D.; Rohrich, R.J. Introducing Evidence-Based Medicine to Plastic and Recon-structive Surgery. Plast. Reconstr. Surg. 2009, 123, 1385–1389. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook/current (accessed on 31 July 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Larissa Shamseer, J.M.T. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372:n71. [Google Scholar]
- Eigenfactor: The Eigenfactor Metrics. Available online: http://www.eigenfactor.org/projects/journalRank/journalsearch.php (accessed on 20 January 2022).
- Gu, R.-X.; Wang, X.-Z.; Li, J.; Deng, J.; Li, X.-X.; Wang, J. Effects of omeprazole or pantoprazole on platelet function in non-ST-segment elevation acute coronary syndrome patients receiving clopidogrel. Mil. Med. Res. 2016, 3. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5159972/ (accessed on 20 October 2020). [CrossRef] [PubMed]
- Nicolau, J.C.; Bhatt, D.L.; Roe, M.T.; Lokhnygina, Y.; Neely, B.; Corbalán, R.; Leiva-Pons, O.L.; Martinez, F.; Goodman, S.G.; Winters, K.J.; et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: Insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am. Heart J. 2015, 170, 683–694. [Google Scholar] [PubMed]
- Gargiulo, G.; Costa, F.; Ariotti, S.; Biscaglia, S.; Campo, G.; Esposito, G.; Leonardi, S.; Vranckx, P.; Windecker, S.W.; Valgimigli, M. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia ando trial. Am. Heart J. 2016, 174, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Eikelboom, J.W.; Bosch, J.; Connolly, S.J.; Dyal, L.; Shestakovska, O.; Leong, D.; Anand, S.S.; Störk, S.; Branch, K.R.; et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology 2019, 157, 682–691. [Google Scholar] [CrossRef]
- Sugano, K.; Choi, M.G.; Lin, J.T.; Goto, S.; Okada, Y.; Kinoshita, Y.; Miwa, H.; Chiang, C.E.; Chiba, T.; Hori, M.; et al. Multinational, double-blind, randomised, placebo-controlled, prospective study of esomeprazole in the prevention of recurrent peptic ulcer in low-dose acetylsalicylic acid users: The LAVENDER study. Gut 2014, 63, 1061–1068. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Bhatt, D.L.; Cryer, B.L.; Liu, Y.; Hsieh, W.H.; Doros, G.; Cohen, M.; Lanas, A.; Schnitzer, T.J.; Shook, T.L.; et al. Proton-Pump Inhibitors Reduce Gastrointestinal Events Regardless of Aspirin Dose in Patients Requiring Dual Antiplatelet Therapy. J. Am. Coll. Cardiol. 2016, 67, 1661–1671. [Google Scholar] [CrossRef]
- Jackson, L.R.; Peterson, E.D.; McCoy, L.A.; Ju, C.; Zettler, M.; Baker, B.A.; Messenger, J.C.; Faries, D.E.; Effron, M.B.; Cohen, D.J.; et al. Impact of Proton Pump Inhibitor Use on the Comparative Effectiveness and Safety of Prasugrel Versus Clopidogrel: Insights From the Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome (TRANSLATE-ACS) Study. J. Am. Heart Assoc. 2016, 5, e003824. [Google Scholar]
- Weisz, G.; Smilowitz, N.R.; Kirtane, A.J.; Rinaldi, M.J.; Parvataneni, R.; Xu, K.; Stuckey, T.D.; Maehara, A.; Witzenbichler, B.; Neumann, F.-J.; et al. Proton Pump Inhibitors, Platelet Reactivity, and Cardiovascular Outcomes After Drug-Eluting Stents in Clopidogrel-Treated Patients: The ADAPT-DES Study. Circ. Cardiovasc. Interv. 2015, 8, e001952. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions, version 6.3 (updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 4 December 2021).
- Whellan, D.J.; Goldstein, J.L.; Cryer, B.L.; Eisen, G.M.; Lanas, A.; Miller, A.B.; Scheiman, J.M.; Fort, J.G.; Zhang, Y.; O’Connor, C. PA32540 (a coordinated-delivery tablet of enter-ic-coated aspirin 325 mg and immediate-release omeprazole 40 mg) versus enteric-coated aspirin 325 mg alone in subjects at risk for aspirin-associated gastric ulcers: Results of two 6-month, phase 3 studies. Am. Heart J. 2014, 168, 495–502. [Google Scholar] [CrossRef]
- Lu, M. Report: Impact of drug combination of clopidogrel and pantoprazole In the prognosis of patients with transient ischemic attack. Pak. J. Pharm. Sci. 2017, 30, 217–221. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis; The Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 May 2022).
- Kenngott, S.; Olze, R.; Kollmer, M.; Bottheim, H.; Laner, A.; Holinski-Feder, E. Clopidogrel and proton pump inhibitor (PPI) interaction: Separate intake and a non-omeprazole PPI the solution? Eur. J. Med. Res. 2010, 15, 220–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harvey, A.; Modak, A.; Déry, U.; Roy, M.; Rinfret, S.; Bertrand, O.F.; Larose, É.; Rodés-Cabau, J.; Barbeau, G.; Gleeton, O.; et al. Changes in CYP2C19 enzyme activity evaluated by the [(13)C]-pantoprazole breath test after co-administration of clopidogrel and proton pump inhibitors following percutaneous coronary intervention and correlation to platelet reactivity. J. Breath Res. 2016, 10, 017104. [Google Scholar] [CrossRef]
- Furuta, T.; Iwaki, T.; Umemura, K. Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br. J. Clin. Pharmacol. 2010, 70, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Blume, H.; Donath, F.; Warnke, A.; Schug, B.S. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 2006, 29, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Melloni, C.; Washam, J.B.; Jones, W.S.; Halim, S.; Hasselblad, V.; Mayer, S.B.; Heidenfelder, B.L.; Dolor, R.J. Conflicting Results Between Randomized Trials and Observational Studies on the Impact of Proton Pump Inhibitors on Cardiovascular Events When Coadministered With Dual Antiplatelet Therapy: A Systematic Review. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 47–55. [Google Scholar] [CrossRef]
- Shi, W.; Yan, L.; Yang, J.; Yu, M. Ethnic variance on long term clinical outcomes of concomitant use of proton pump inhibitors and clopidogrel in patients with stent implantation. Medicine 2021, 100, e24366. [Google Scholar] [CrossRef]
- Cardoso, R.N.; Benjo, A.M.; DiNicolantonio, J.J.; Garcia, D.C.; Macedo, F.Y.B.; El-Hayek, G.; Nadkarni, G.N.; Gili, S.; Iannaccone, M.; Konstantinidis, I.; et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: An updated meta-analysis. Open Heart 2015, 2, e000248. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.S.; Kim, B.J.; Shin, S.Y.; Kim, D.B.; Ahn, H.S. Influence of individual proton pump inhibitors on clinical outcomes in patients receiving clopidogrel following percutaneous coronary intervention. Medicine 2021, 100, e27411. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Teeluck, A.R.; Bhurtu, A.; Huang, W.-Q. Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: A systematic review and me-ta-analysis of recently published studies (2012–2016). BMC Cardiovasc. Disord. 2017, 17, 3. [Google Scholar] [CrossRef]
- Kwok, C.S.; Jeevanantham, V.; Dawn, B.; Loke, Y.K. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: Meta-analysis. Int. J. Cardiol. 2013, 167, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-F.; Lin, P.-C.; Chang, C.-C.; Chang, W.-L.; Chu, F.-Y. Investigation of the interaction between proton pump inhibitors and clopidogrel using VerifyNow P2Y12 assay. Medicine 2020, 99, e23695. [Google Scholar] [CrossRef] [PubMed]
- Morath, T.; Stegherr, J.; Braun, S.; Vogt, W.; Hadamitzky, M.; Schömig, A.; Kastrati, A.; Von Beckerath, N.; Sibbing, D. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb. Haemost. 2009, 101, 714–719. [Google Scholar] [CrossRef]
- Hokimoto, S.; Mizobe, M.; Akasaka, T.; Arima, Y.; Kaikita, K.; Nakagawa, K.; Ogawa, H. Impact of CYP2C19 polymorphism and proton pump inhibitors on platelet reactivity to clopidogrel and clinical outcomes following stent implantation. Thromb. Res. 2014, 133, 599–605. [Google Scholar] [CrossRef]
- Biswas, M.; Rahaman, S.; Biswas, T.K.; Ibrahim, B. Risk of major adverse cardiovascular events for concomitant use of clopidogrel and proton pump inhibitors in patients inheriting CYP2C19 loss-of-function alleles: Meta-analysis. Int. J. Clin. Pharm. 2021, 43, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Sharma, S.P.; Kaur, J.; Anderson, B.J.; Singh, G. Efficacy and Safety of Proton Pump Inhibitors in the Long-Term Aspirin Users: A Meta-Analysis of Randomized Controlled Trials. Am. J. Ther. 2017, 24, e559–e569. [Google Scholar] [CrossRef]
- Zhai, Y.; Ye, X.; Hu, F.; Xu, J.; Guo, X.; Lin, Z.; Zhou, X.; Guo, Z.; Cao, Y.; He, J. Insights on Cardiac and Vascular Risks of Proton Pump Inhibitors: A Real-World Pharmacovigilance Study. Front. Cardiovasc. Med. 2022, 9, 767987. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease: From Marvel to Menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Ghebremariam, Y.T.; Cooke, J.P.; Khan, F.; Thakker, R.N.; Chang, P.; Shah, N.H.; Nead, K.T.; Leeper, N.J. Proton pump inhibitors and vascular function: A prospective cross-over pilot study: Vascular Medicine. Available online: http://journals.sagepub.com/doi/10.1177/1358863X14568444 (accessed on 1 May 2022).
| Study, Year | Country | Study Design | Centers | Total Patients (PPI/C) | Follow up Period | Intervention/PPI Type | Control | Outcomes | Age (y)PPI/C | Women (%) PPI/C | Body Mass Index (kg/m2) PPI/C | Hypertension (%) PPI/C | Dyslipidemia (%) PPI/C | Diabetes Mellitus (%)PPI/C | History of Smoking (%) PPI/CPPI/C | Clopidogrel (%) PPI/C | Aspirin (%) PPI/C | Prior Myocardial Infarction (%) PPI/C | Prior Stroke (%) PPI/C | CRBT (/7) or NOS (/9) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang et al., 2015 [9] | China | RCT | monocentric | 53/51 | 6 months | Lansoprazole | No PPI | MACEs | 64.5/61 | 55/43 | 21.9/22.1 | 51/49 | 40/39 | 19/27 | 40/41 | 100/100 | 100/100 | NA/NA | NA/NA | 4/7 |
| Gu et al., 2016 [14] | China | RCT | monocentric | 310/310 | 6 months | Omeprazole | Pantoprazole | MACEs, CVD, ACD, MI, Stroke, TVR | 59.2/58.8 | 31/29.3 | 25.6/25.5 | 65.7/61.2 | 44.6/41.1 | 27.7/27 | 56.1/56.3 | >98/>98 | >98/>98 | 15.8/15.1 | 8.9/8.9 | 4/7 |
| Nicolau et al., 2015 [15] | Multiple countries | RCT-PHA | multicentric | 1666/5577 | 30 months | Ome, panto, other PPIs | No PPI | MACEs, CVD, MI, Stroke, | 63/62 | 36.5/35.7 | NA/NA | 80,5/80,3 | 58,8/59 | 40.3/38.5 | 42.9/44.4 | 50.2/50 | 93.2/94.2 | 42.9/44.4 | 0/0 | 1/7 |
| Gargiulo et al., 2016 [16] | Italy | RCT-PHA | multicentric | 738/1232 | 2 years | Lanso 90.9%; panto 7.6%; ome, rabe, eso 0.5% each | No PPI | MACEs, CVD,ACD, MI | 71.2/68.1 | 27.5/20.8 | 26.2/26.9 | 72.5/71.3 | 53.8/55.3 | 23.3/24.8 | 22.6/24.4 | 99.9/99.8 | 100/100 | 27/26.1 | NA/NA | 1/7 |
| Moayyedi et al., 2019 [17] | 33 countries | RCT | multicentric | 8791/8807 | 3 years | Pantoprazole | Placebo | MACEs, CVD, ACD, MI, Stroke | 67.6/67.7 | 22/21 | 28.3/28.4 | 75.9/76.1 | 88.4/88.8 | 38/38 | 66.3/66.1 | NA/NA | NA/NA | 61.5/61 | 4/4 | 7/7 |
| Sugano et al., 2014 [18] | Japan, Korea, Taiwan | RCT | multicentric | 215/215 | 72 weeks | Esomeprazole | Placebo | ACD, MI | 66.1/68.1 | 19/21 | NA/NA | NA/NA | NA/NA | NA/NA | NA/NA | 0.5/2 | 100/100 | 66.5 */69.2 * | 66.5 */69.2 * | 7/7 |
| Vaduganathan et al., 2016 [19] | Multiple countries | RCT | multicentric | 1869/1883 | 6 months | Omeprazole | Placebo | MACEs, CVD, ACD MI, Stroke, TVR | 65.9/65.9 | 33.2/30.6 | 29.5/29.5 | 79.7/81 | 78.8/76.8 | 31.6/28.5 | 13.6/15.1 | 64.6/64.6 | 100/100 | 30.2/28.2 | 7.2/8 | 6.5/7 |
| Whellan et al., 2014 [23] | USA | RCT | multicentric | 524/525 | 6 months | Omeprazole | No PPI | CVD, ACD, MI,MACEs, Stroke | 66.3/65.7 | 28.4/28.8 | 31/31.1 | NA/NA | NA/NA | NA/NA | NA/NA | 21.2/21 | 100/100 | 40.8/37.9 | 19.5/21.5 | 7/7 |
| Jackson et al., 2016 [20] | USA | OS | multicentric | 2167/9788 | 12 months | PPIs | No PPI | MACEs | 63/59 | 34.1/26.6 | 30/29 | 76.1/64.8 | 73,1/63.9 | 32.4/25.2 | NA/NA | 75/74 | 97.9/98.3 | 24.5/18.4 | 7.7/4.9 | 7/9 |
| Weisz et al., 2015 [21] | USA, Germany | OS | multicentric | 2162/6419 | 2 years | PPIs | No PPI | CVD, ACD,MACEs, MI, TVR | 64.4/63.2 | 29.9/24.1 | 29.5/29.5 | 83.7/77.8 | 76.9/73.2 | 34.8/31.4 | 22.7/22.6 | 100/100 | 100/100 | 28.6/23.7 | NA/NA | 8/9 |
| Study | Selection (0 to 4 *) | Comparability (0 to 2 *) | Outcome (0 to 3 *) | Total | Quality Assessment |
|---|---|---|---|---|---|
| Jackson | 3 * | 2 * | 2 * | 7 * | Good |
| Weisz | 3 * | 2 * | 3 * | 8 * | Good |
| Meta-Analysis | Outcome | Removed Studies | Results |
|---|---|---|---|
| Primary Analysis | MACEs | [20,21] | OR 1.02, 95% CI 0.94–1.11, p = 0.62 |
| TVR | [19] | OR 1.42, 95% CI 1.22–1.65, p < 0.01 | |
| CVD | [21] | OR 1.00, 95% CI 0.89–1.11, p = 0.95 | |
| ACD | [21] | OR 1.04, 95% CI 0.93–1.16, p = 0.45 | |
| Clopidogrel Analysis | MACEs | [15,19] | OR 1.45, 95% CI 1.31–1.60, p < 0.001 |
| MI | [21] | OR 0.89, 95% CI 0.72–1.11, p = 0.29 | |
| TVR | [19] | OR 1.42, 95% CI 1.22–1.65, p < 0.001 | |
| CVD | [15] | OR 1.42, 95% CI 1.09–1.84, p = 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeridi, D.; Pellat, A.; Ginestet, C.; Assaf, A.; Hallit, R.; Corre, F.; Coriat, R. The Safety of Long-Term Proton Pump Inhibitor Use on Cardiovascular Health: A Meta-Analysis. J. Clin. Med. 2022, 11, 4096. https://doi.org/10.3390/jcm11144096
Jeridi D, Pellat A, Ginestet C, Assaf A, Hallit R, Corre F, Coriat R. The Safety of Long-Term Proton Pump Inhibitor Use on Cardiovascular Health: A Meta-Analysis. Journal of Clinical Medicine. 2022; 11(14):4096. https://doi.org/10.3390/jcm11144096
Chicago/Turabian StyleJeridi, Dalel, Anna Pellat, Claire Ginestet, Antoine Assaf, Rachel Hallit, Felix Corre, and Romain Coriat. 2022. "The Safety of Long-Term Proton Pump Inhibitor Use on Cardiovascular Health: A Meta-Analysis" Journal of Clinical Medicine 11, no. 14: 4096. https://doi.org/10.3390/jcm11144096
APA StyleJeridi, D., Pellat, A., Ginestet, C., Assaf, A., Hallit, R., Corre, F., & Coriat, R. (2022). The Safety of Long-Term Proton Pump Inhibitor Use on Cardiovascular Health: A Meta-Analysis. Journal of Clinical Medicine, 11(14), 4096. https://doi.org/10.3390/jcm11144096






