Combined Trifocal and Microsurgical Testicular Sperm Extraction Enhances Sperm Retrieval Rate in Low-Chance Retrieval Non-Obstructive Azoospermia
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- -
- Azoospermia confirmed on 2 specimens according to WHO guidelines [18];
- -
- Reduced testicular volume (<10 mL);
- -
- Increased serum FSH (>12.4 IU/L);
- -
- Patients who underwent a M-TeSE or combined trifocal/M-TeSE.
- -
- Re-do surgical sperm retrieval procedures;
- -
- Genetic disorders related to azoospermia;
- -
- Varicocele or prior varicocele repair.
2.2. Surgical Technique
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Stephen, E.H.; Chandra, A. Declining estimates of infertility in the United States: 1982–2002. Fertil. Steril. 2006, 86, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.F.; Thoma, M.E.; Sørensen, D.N.; McLain, A.; King, R.B.; Sundaram, R.; Keiding, N.; Louis, G.B. The prevalence of couple infertility in the United States from a male perspective: Evidence from a nationally representative sample. Andrology 2013, 1, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.E.; McLain, A.; Louis, J.F.; King, R.B.; Trumble, A.C.; Sundaram, R.; Louis, G.B. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013, 99, 1324–1331.e1. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Jarow, J.P.; Espeland, M.A.; Lipshultz, L.I. Evaluation of the Azoospermic Patient. J. Urol. 1989, 142, 62–65. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Zhang, H.; Sun, J.; Sun, Y.; Wang, Z.; Liu, J.; Ding, Q.; Lu, S.; Shi, R.; et al. A Genome-wide Association Study Reveals that Variants within the HLA Region Are Associated with Risk for Nonobstructive Azoospermia. Am. J. Hum. Genet. 2012, 90, 900–906. [Google Scholar] [CrossRef]
- Pantke, P.; Diemer, T.; Marconi, M.; Bergmann, M.; Steger, K.; Schuppe, H.-C.; Weidner, W. Testicular Sperm Retrieval in Azoospermic Men. Eur. Urol. Suppl. 2008, 7, 703–714. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Su, L.M. Physiological consequences of testicular sperm extraction. Hum. Reprod. 1997, 12, 1688–1692. [Google Scholar] [CrossRef]
- Schlegel, P.N. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum. Reprod. 1999, 14, 131–135. [Google Scholar] [CrossRef]
- Maglia, E.; Boeri, L.; Fontana, M.; Gallioli, A.; De Lorenzis, E.; Palmisano, F.; Zanetti, S.; Sampogna, G.; Restelli, L.; Somigliana, E.; et al. Clinical comparison between conventional and microdissection testicular sperm extraction for non-obstructive azoospermia: Understanding which treatment works for which patient. Arch. Ital. Urol. E Androl. 2018, 90, 130–135. [Google Scholar] [CrossRef]
- Ostad, M.; Liotta, D.; Ye, Z.; Schlegel, P.N. Testicular sperm extraction for nonobstructive azoospermia: Results of a multibiopsy approach with optimized tissue dispersion. Urology 1998, 52, 692–696. [Google Scholar] [CrossRef]
- Boeri, L.; Bebi, C.; Dente, D.; Greco, E.; Turetti, M.; Capece, M.; Cocci, A.; Cito, G.; Preto, M.; Pescatori, E.; et al. Outcomes and predictive factors of successful salvage microdissection testicular sperm extraction (mTESE) after failed classic TESE: Results from a multicenter cross-sectional study. Int. J. Impot. Res. 2021, 99, 136–140. [Google Scholar] [CrossRef]
- Boeri, L.; Capogrosso, P.; Ortensi, I.; Miacola, C.; Cai, T.; Verze, P.; Salonia, A.; Giammusso, B.; Palmieri, A. Diagnostic and therapeutic workup of male infertility: Results from a Delphi consensus panel. Int. J. Impot. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Bromage, S.J.; Falconer, D.A.; Lieberman, B.A.; Sangar, V.; Payne, S.R. Sperm Retrieval Rates in Subgroups of Primary Azoospermic Males. Eur. Urol. 2007, 51, 534–540. [Google Scholar] [CrossRef]
- Tüttelmann, F.; Werny, F.; Cooper, T.G.; Kliesch, S.; Simoni, M.; Nieschlag, E. Clinical experience with azoospermia: Aetiology and chances for spermatozoa detection upon biopsy. Int. J. Androl. 2010, 34, 291–298. [Google Scholar] [CrossRef]
- Marconi, M.; Keudel, A.; Diemer, T.; Bergmann, M.; Steger, K.; Schuppe, H.-C.; Weidner, W. Combined Trifocal and Microsurgical Testicular Sperm Extraction Is the Best Technique for Testicular Sperm Retrieval in “Low-Chance” Nonobstructive Azoospermia. Eur. Urol. 2012, 62, 713–719. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010; p. 252. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Ghanem, M.; Bakr, N.I.; Elgayaar, M.A.; El Mongy, S.; Fathy, H.; Ibrahim, A.-H.A. Comparison of the outcome of intracytoplasmic sperm injection in obstructive and non-obstructive azoospermia in the first cycle: A report of case series and meta-analysis. Int. J. Androl. 2005, 28, 16–21. [Google Scholar] [CrossRef]
- Boeri, L.; Palmisano, F.; Preto, M.; Sibona, M.; Capogrosso, P.; Franceschelli, A.; Ruiz-Castañé, E.; Sarquella-Geli, J.; Bassas-Arnau, L.; Scroppo, F.I.; et al. Sperm retrieval rates in non-mosaic Klinefelter patients undergoing testicular sperm extraction: What expectations do we have in the real-life setting? Andrology 2020, 8, 680–687. [Google Scholar] [CrossRef]
- Bernie, A.M.; Mata, D.A.; Ramasamy, R.; Schlegel, P.N. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: A systematic review and meta-analysis. Fertil. Steril. 2015, 104, 1099–1103.e3. [Google Scholar] [CrossRef]
- Craft, I.; Bennett, V.; Nicholson, N. Fertilising ability of testicular spermatozoa. Lancet 1993, 342, 864. [Google Scholar] [CrossRef]
- Schoysman, R.; Vanderzwalmen, P.; Nijs, M.; Segal, L.; Segal-Bertin, G.; Geerts, L.; Van Roosendaal, E. Pregnancy after fertilisation with human testicular spermatozoa. Lancet 1993, 342, 1237. [Google Scholar] [CrossRef]
- Tsujimura, A.; Matsumiya, K.; Miyagawa, Y.; Takao, T.; Fujita, K.; Koga, M.; Takeyama, M.; Fujioka, H.; Okuyama, A. Prediction of successful outcome of microdissection testicular sperm extraction in men with idiopathic nonobstructive azoospermia. J. Urol. 2004, 172, 1944–1947. [Google Scholar] [CrossRef]
- Eliveld, J.; van Wely, M.; Meißner, A.; Repping, S.; Van Der Veen, F.; Van Pelt, A.M.M. The risk of TESE-induced hypogonadism: A systematic review and meta-analysis. Hum. Reprod. Updat. 2018, 24, 442–454. [Google Scholar] [CrossRef]
- Deruyver, Y.; Vanderschueren, D.; Van Der Aa, F. Outcome of microdissection TESE compared with conventional TESE in non-obstructive azoospermia: A systematic review. Andrology 2014, 2, 20–24. [Google Scholar] [CrossRef]
- Tsujimura, A.; Matsumiya, K.; Miyagawa, Y.; Tohda, A.; Miura, H.; Nishimura, K.; Koga, M.; Takeyama, M.; Fujioka, H.; Okuyama, A. Conventional multiple or microdissection testicular sperm extraction: A comparative study. Hum. Reprod. 2002, 17, 2924–2929. [Google Scholar] [CrossRef] [PubMed]
- Turunç, T.; Gul, U.; Haydardedeoglu, B.; Bal, N.; Kuzgunbay, B.; Peskircioğlu, L.; Özkardeş, H. Conventional testicular sperm extraction combined with the microdissection technique in nonobstructive azoospermic patients: A prospective comparative study. Fertil. Steril. 2010, 94, 2157–2160. [Google Scholar] [CrossRef]
- Corona, G.; Minhas, S.; Giwercman, A.; Bettocchi, C.; Dinkelman-Smit, M.; Dohle, G.; Fusco, F.; Kadioglou, A.; Kliesch, S.; Kopa, Z.; et al. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: A systematic review and meta-analysis. Hum. Reprod. Updat. 2019, 25, 733–757. [Google Scholar] [CrossRef] [PubMed]
- Gilany, K.; Jafarzadeh, N.; Mani-Varnosfaderani, A.; Minai-Tehrani, A.; Sadeghi, M.R.; Darbandi, M.; Darbandi, S.; Amini, M.; Arjmand, B.; Pahlevanzadeh, Z. Metabolic Fingerprinting of Seminal Plasma from Non-obstructive Azoospermia Patients: Positive Versus Negative Sperm Retrieval. J. Reprod. Infertil. 2018, 19, 109–114. [Google Scholar] [PubMed]
- Achermann, A.P.P.; Pereira, T.A.; Esteves, S.C. Microdissection testicular sperm extraction (micro-TESE) in men with infertility due to nonobstructive azoospermia: Summary of current literature. Int. Urol. Nephrol. 2021, 53, 2193–2210. [Google Scholar] [CrossRef]
- Dabaja, A.A.; Schlegel, P.N. Microdissection testicular sperm extraction: An update. Asian J. Androl. 2013, 15, 35–39. [Google Scholar] [CrossRef]
- Raheem, A.A.; Garaffa, G.; Rushwan, N.; De Luca, F.; Zacharakis, E.; Raheem, T.A.; Freeman, A.; Serhal, P.; Harper, J.C.; Ralph, D. Testicular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermia. Br. J. Urol. 2012, 111, 492–499. [Google Scholar] [CrossRef]
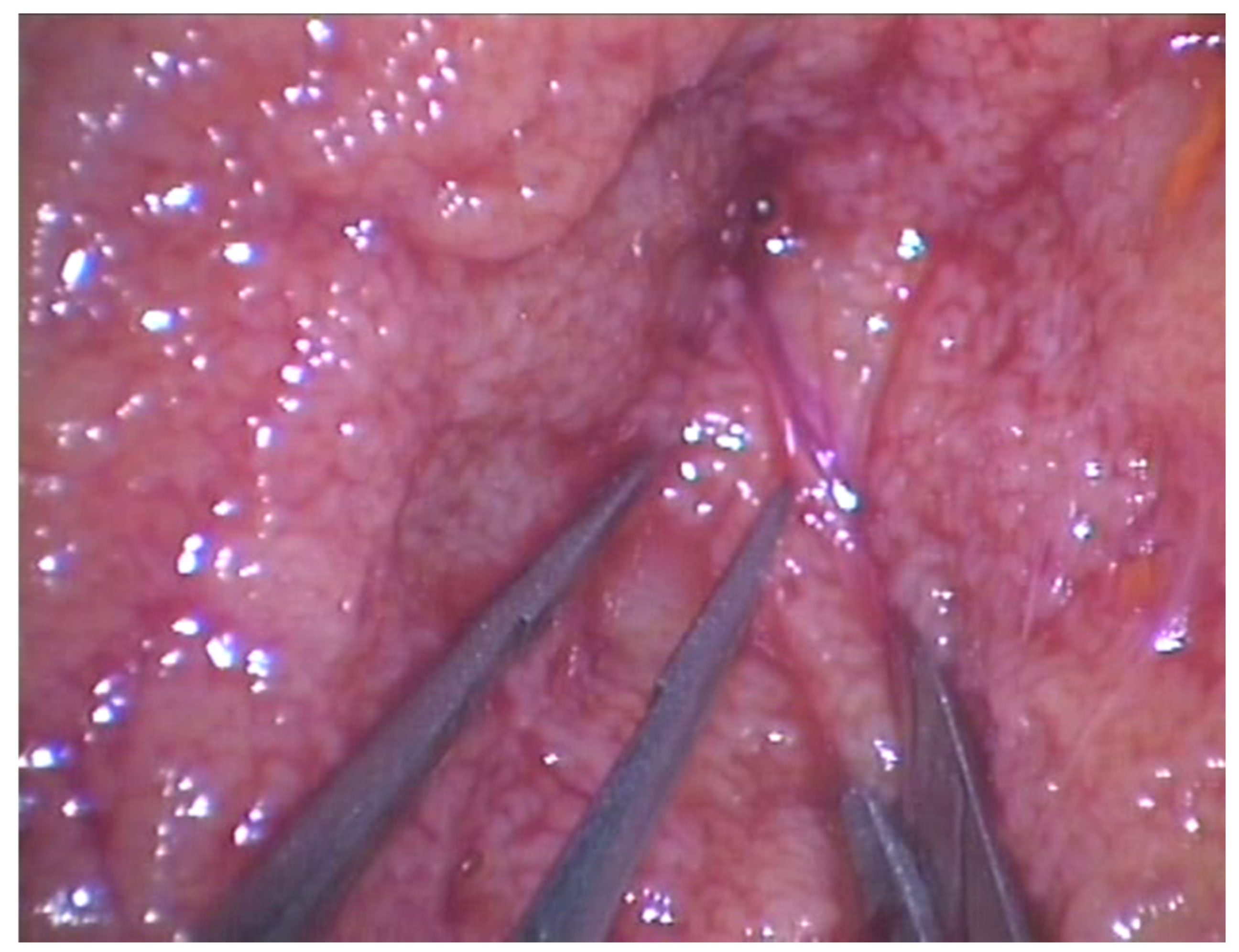
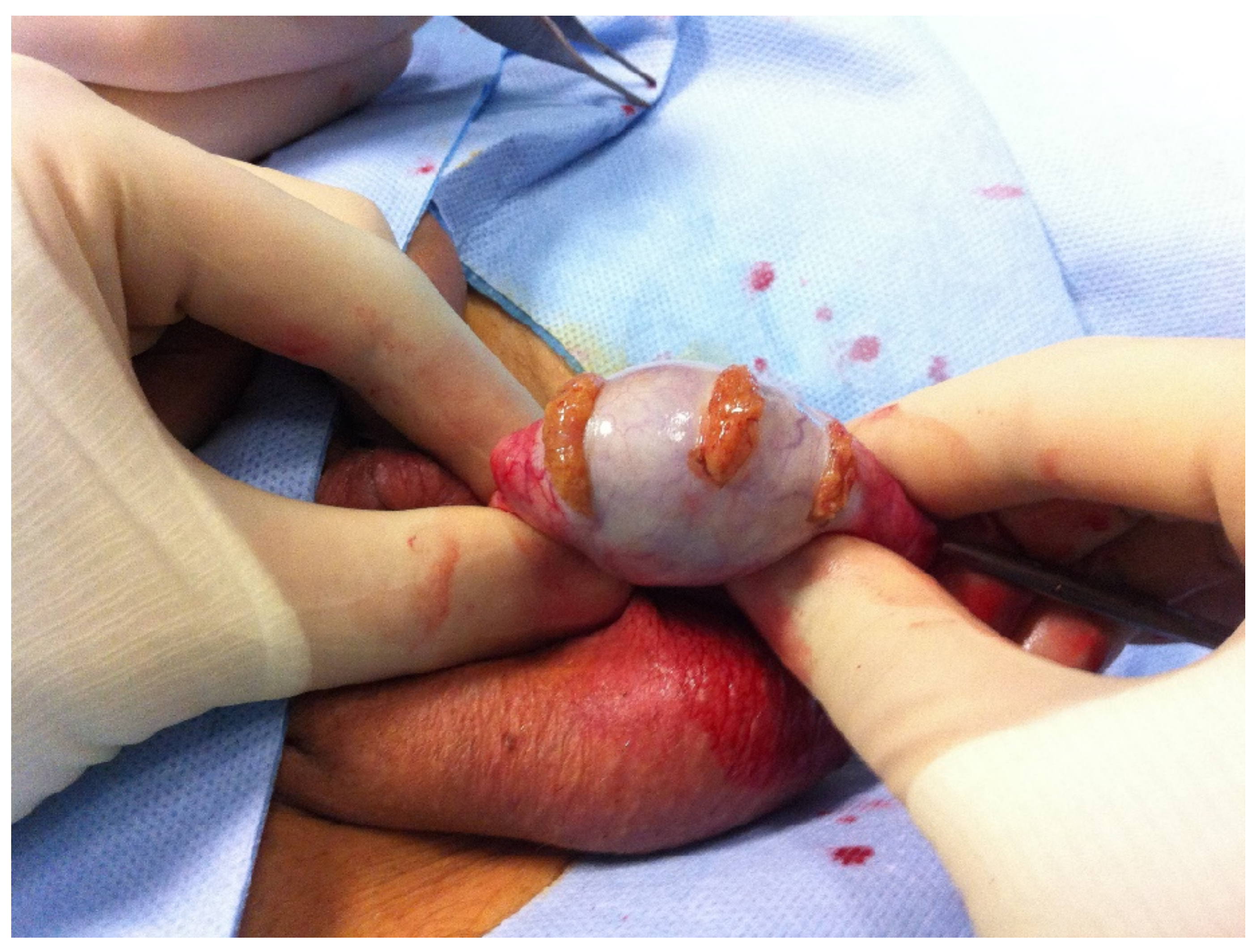
| Figures and Tables | Groups | |||
|---|---|---|---|---|
| Variables | Total | Micro-TeSE | Trifocal Micro-TeSE | p-Value |
| Number of patients, n (%) | 80 | 60 (75) | 20 (25) | |
| Mean age, years (SD) | 35 (8.2) | 35 (8.7) | 38 (7.4) | 0.2 |
| Smoking habit, n (%) | 26 (32.5) | 19 (31.6) | 7 (35) | 0.08 |
| Mean testicular volume, cc (SD) | ||||
| Left side | 6.3 (3) | 7.2 (4) | 6.1 (5) | 0.1 |
| Right side | 6.8 (2.5) | 7.1 (5.7) | 6.5 (4.1) | |
| Mean FSH, UI/L (SD) | 27.5 (13) | 26 (14.2) | 28.2 (13.4) | 0.9 |
| Positive sperm retrieval, n (%) | 22 (27.5) | 16 (26.7) | 6 (30) | 0.03 |
| Histology, n (%) | ||||
| Hypospermatogenesis | 2 (2.5) | 2 (3.3) | 0 (0) | 0.07 |
| Spermatogenic arrest | 9 (11.2) | 7 (11.6) | 2 (10) | 0.1 |
| Sertoli cell-only syndrome | 69 (86.3) | 51 (85) | 18 (90) | 0.09 |
| Mean Johnsen score, n (SD) | 3 (1.6) | 2.6 (1.7) | 3.2 (2) | 0.3 |
| Mean sperm vials stored, n (SD) | 4 (1.9) | 4.5 (1.7) | 4 (1.9) | 0.4 |
| Groups | |||
|---|---|---|---|
| Variables | Positive SR | Negative SR | p-Value |
| Mean age, years (SD) | 35 (6) | 35 (9) | 0.98 |
| Mean testicular volume, cc (SD) | |||
| Left side | 6.2 (3.4) | 6.6 (3.1) | 0.86 |
| Right side | 7.3 (4.1) | 7.2 (5.2) | 0.09 |
| Mean FSH, UI/L (SD) | 24.7 (11.2) | 26 (13) | 0.74 |
| Histology, n (%) | |||
| Hypospermatogenesis | 2 (9,1) | 0 (0) | 0.73 |
| Spermatogenic arrest | 5 (38.5) | 4 (6.9) | 0.105 |
| Sertoli cell-only syndrome | 15 (68.2) | 54 (93.1) | 0.08 |
| Surgery n (%) | |||
| Micro-TeSE | 9 (40.9) | 51 (87.9) | 0.02 |
| Combined Trifocal + Micro-TeSE | 13 (59.1) | 7 (12.1) | <0.01 |
| Variables | Odds Ratio | p Value | [95% Conf. Interval] |
|---|---|---|---|
| Age | 1.02 | 0.64 | 0.91–1.13 |
| FSH | 1.06 | 0.14 | 0.98–1.15 |
| Testicular volume | 1.15 | 0.27 | 0.89–1.5 |
| Hypospermatogenesis | 0.43 | 0.004 | 0.008–0.5 |
| Spermatogenic arrest | 0.12 | 0.007 | 0.01–0.25 |
| Sertoli cell-only syndrome | 0.05 | 0.013 | 0.006–0.12 |
| Johnsen Score | 1.59 | 0.53 | 0.98–2.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcone, M.; Boeri, L.; Timpano, M.; Cirigliano, L.; Preto, M.; Russo, G.I.; Peretti, F.; Ferro, I.; Plamadeala, N.; Gontero, P. Combined Trifocal and Microsurgical Testicular Sperm Extraction Enhances Sperm Retrieval Rate in Low-Chance Retrieval Non-Obstructive Azoospermia. J. Clin. Med. 2022, 11, 4058. https://doi.org/10.3390/jcm11144058
Falcone M, Boeri L, Timpano M, Cirigliano L, Preto M, Russo GI, Peretti F, Ferro I, Plamadeala N, Gontero P. Combined Trifocal and Microsurgical Testicular Sperm Extraction Enhances Sperm Retrieval Rate in Low-Chance Retrieval Non-Obstructive Azoospermia. Journal of Clinical Medicine. 2022; 11(14):4058. https://doi.org/10.3390/jcm11144058
Chicago/Turabian StyleFalcone, Marco, Luca Boeri, Massimiliano Timpano, Lorenzo Cirigliano, Mirko Preto, Giorgio I. Russo, Federica Peretti, Ilaria Ferro, Natalia Plamadeala, and Paolo Gontero. 2022. "Combined Trifocal and Microsurgical Testicular Sperm Extraction Enhances Sperm Retrieval Rate in Low-Chance Retrieval Non-Obstructive Azoospermia" Journal of Clinical Medicine 11, no. 14: 4058. https://doi.org/10.3390/jcm11144058
APA StyleFalcone, M., Boeri, L., Timpano, M., Cirigliano, L., Preto, M., Russo, G. I., Peretti, F., Ferro, I., Plamadeala, N., & Gontero, P. (2022). Combined Trifocal and Microsurgical Testicular Sperm Extraction Enhances Sperm Retrieval Rate in Low-Chance Retrieval Non-Obstructive Azoospermia. Journal of Clinical Medicine, 11(14), 4058. https://doi.org/10.3390/jcm11144058







