Auxological and Endocrinological Features in Children and Adolescents with Cystic Fibrosis
Abstract
1. Introduction
2. Cystic Fibrosis-Related Diabetes (CFRD)
2.1. Epidemiology
2.2. Pathophysiology
2.3. Diagnosis
2.4. Therapy
2.5. Complications
3. Cystic-Fibrosis-Related Bone Disease
3.1. Epidemiology & Pathogenesis
3.2. Screening
3.3. Therapy and Management
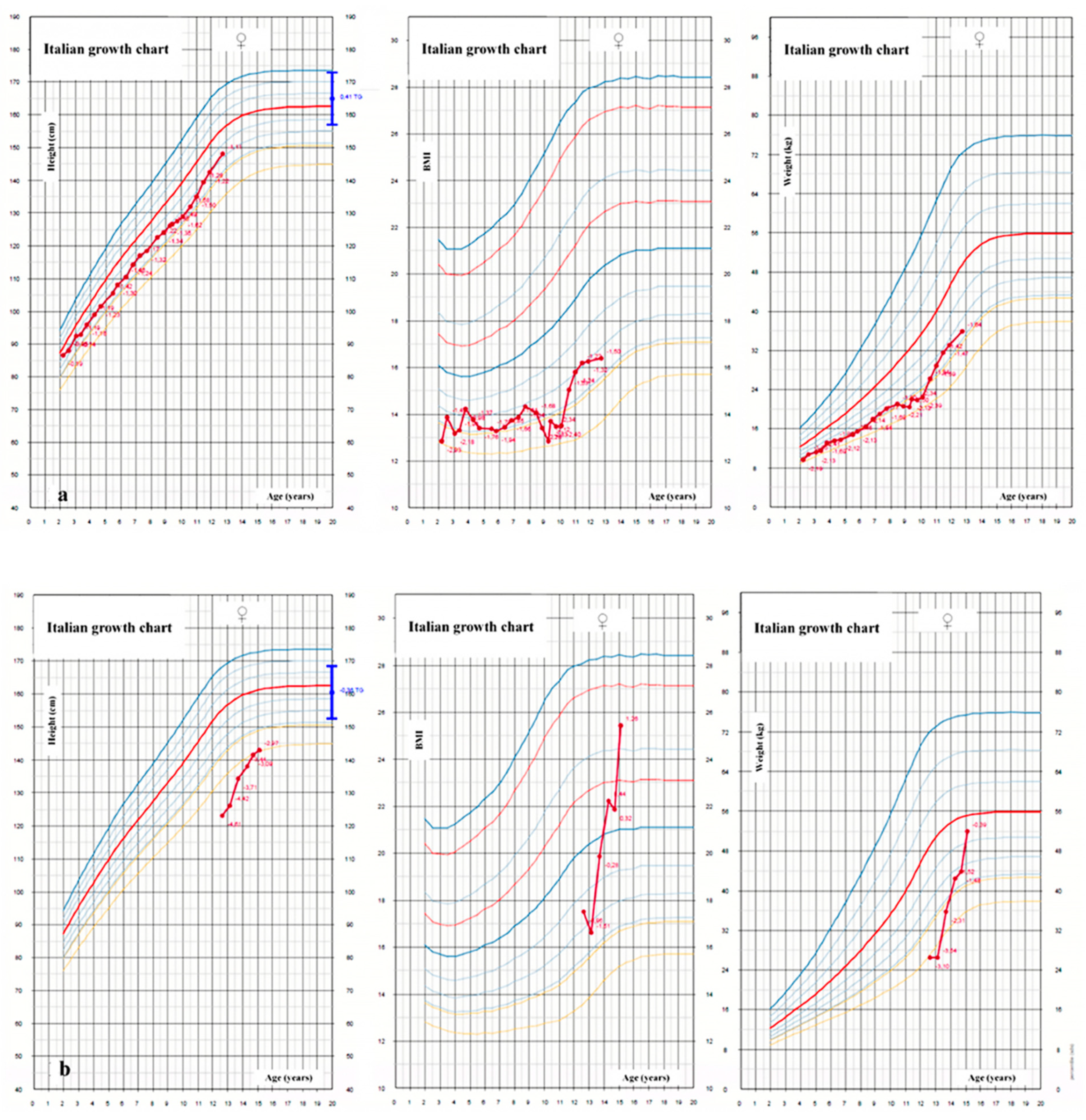
4. Growth Failure
4.1. Epidemiology and Pathogenesis
4.2. Recommendations
4.3. Therapy
5. Delayed Puberty
5.1. Diagnosis and Pathogenesis
5.2. Treatment
6. Male Hypogonadism and Infertility
7. Thyroid Function
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheppard, D.N.; Welsh, M.J. Structure and function of the CFTR chloride channel. Physiol. Rev. 1999, 79, S23–S45. [Google Scholar] [CrossRef] [PubMed]
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef]
- Rommens, J.M.; Iannuzzi, M.C.; Kerem, B.; Drumm, M.L.; Melmer, G.; Dean, M.; Rozmahel, R.; Cole, J.L.; Kennedy, D.; Hidaka, N.; et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 1989, 245, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Orenti, A.; Zolin, A.; Jung, A.; van Rens, J.; Fox, A.; Krasnyk, M.; Daneau, G.; Hatziagorou, E.; Mei-Zahav, M.; Naehrlich, L.; et al. ECFSPR Annual Report 2019. European Cystic Fibrosis Society 2021. Available online: https://www.ecfs.eu/sites/default/files/general-content-files/working-groups/ecfs-patient-registry/ECFSPR_Report_2019_v1_16Feb2022.pdf (accessed on 10 June 2022).
- Terlizzi, V.; Claut, L.; Tosco, A.; Colombo, C.; Raia, V.; Fabrizzi, B.; Lucarelli, M.; Angeloni, A.; Cimino, G.; Castaldo, A.; et al. A survey of the prevalence, management and outcome of infants with an inconclusive diagnosis following newborn bloodspot screening for cystic fibrosis (CRMS/CFSPID) in six Italian centres. J. Cyst. Fibros. 2021, 20, 828–834. [Google Scholar] [CrossRef]
- Sims, E.J.; Clark, A.; McCormick, J.; Mehta, G.; Connett, G.; Mehta, A.; United Kingdom Cystic Fibrosis Database Steering Committee. Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatrics 2007, 119, 19–28. [Google Scholar] [CrossRef]
- Dijk, F.N.; McKay, K.; Barzi, F.; Gaskin, K.J.; Fitzgerald, D.A. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch. Dis. Child. 2011, 96, 1118–1123. [Google Scholar] [CrossRef]
- Taccetti, G.; Botti, M.; Terlizzi, V.; Cavicchi, M.C.; Neri, A.S.; Galici, V.; Mergni, G.; Centrone, C.; Peroni, D.G.; Festini, F. Clinical and Genotypical Features of False-Negative Patients in 26 Years of Cystic Fibrosis Neonatal Screening in Tuscany, Italy. Diagnostics 2020, 10, 446. [Google Scholar] [CrossRef]
- Orenstein, D.M.; Winnie, G.B.; Altman, H. Cystic fibrosis: A 2002 update. J. Pediatr. 2002, 140, 156–164. [Google Scholar] [CrossRef]
- Corriveau, S.; Sykes, J.; Stephenson, A.L. Cystic fibrosis survival: The changing epidemiology. Curr. Opin. Pulm. Med. 2018, 24, 574–578. [Google Scholar] [CrossRef]
- Ode, K.L.; Chan, C.L.; Granandos, A.; Putman, M.; Moheet, A. Endocrine Complications of Cystic Fibrosis: A Multisystem Disease of the Endocrine Organs. Semin. Respir. Crit. Care Med. 2019, 40, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.; Moran, A. CF-related diabetes: Containing the metabolic miscreant of cystic fibrosis. Pediatr. Pulmonol. 2017, 52, S37–S43. [Google Scholar] [CrossRef]
- Terliesner, N.; Vogel, M.; Steighardt, A.; Gausche, R.; Henn, C.; Hentschel, J.; Kapellen, T.; Klamt, S.; Gebhardt, J.; Kiess, W.; et al. Cystic-fibrosis related-diabetes (CFRD) is preceded by and associated with growth failure and deteriorating lung function. J. Pediatr. Endocrinol. Metab. 2017, 30, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Becker, D.; Casella, S.J.; Gottlieb, P.A.; Kirkman, M.S.; Marshall, B.C.; Slovis, B.; CFRD Consensus Conference Committee. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: A technical review. Diabetes Care 2010, 33, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Olesen, H.V.; Drevinek, P.; Gulmans, V.A.; Hatziagorou, E.; Jung, A.; Mei-Zahav, M.; Stojnic, N.; Thomas, M.; Zolin, A.; ECFSPR Steering Group. Cystic fibrosis related diabetes in Europe: Prevalence, risk factors and outcome. J. Cyst. Fibros. 2020, 19, 321–327. [Google Scholar] [CrossRef]
- Yi, Y.; Norris, A.W.; Wang, K.; Sun, X.; Uc, A.; Moran, A.; Engelhardt, J.F.; Ode, K.L. Abnormal Glucose Tolerance in Infants and Young Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 194, 974–980. [Google Scholar] [CrossRef]
- Ode, K.L.; Frohnert, B.; Laguna, T.; Phillips, J.; Holme, B.; Regelmann, W.; Thomas, W.; Moran, A. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr. Diabetes 2010, 11, 487–492. [Google Scholar] [CrossRef]
- Lewis, C.; Blackman, S.M.; Nelson, A.; Oberdorfer, E.; Wells, D.; Dunitz, J.; Thomas, W.; Moran, A. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am. J. Respir. Crit. Care Med. 2015, 191, 194–200. [Google Scholar] [CrossRef]
- Blackman, S.M.; Commander, C.W.; Watson, C.; Arcara, K.M.; Strug, L.J.; Stonebraker, J.R.; Wright, F.A.; Rommens, J.M.; Sun, L.; Pace, R.G.; et al. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes 2013, 62, 3627–3635. [Google Scholar] [CrossRef]
- Gottlieb, P.A.; Yu, L.; Babu, S.; Wenzlau, J.; Bellin, M.; Frohnert, B.I.; Moran, A. No relation between cystic fibrosis-related diabetes and type 1 diabetes autoimmunity. Diabetes Care 2012, 35, e57. [Google Scholar] [CrossRef]
- Hart, N.J.; Aramandla, R.; Poffenberger, G.; Fayolle, C.; Thames, A.H.; Bautista, A.; Spigelman, A.F.; Babon, J.A.B.; DeNicola, M.E.; Dadi, P.K.; et al. Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight 2018, 3, e98240–e98260. [Google Scholar] [CrossRef] [PubMed]
- Soejima, K.; Landing, B.H. Pancreatic islets in older patients with cystic fibrosis with and without diabetes mellitus: Morphometric and immunocytologic studies. Pediatr. Pathol. 1986, 6, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Bogdani, M.; Blackman, S.M.; Ridaura, C.; Bellocq, J.P.; Powers, A.C.; Aguilar-Bryan, L. Structural abnormalities in islets from very young children with cystic fibrosis may contribute to cystic fibrosis-related diabetes. Sci. Rep. 2017, 7, 17231–17242. [Google Scholar] [CrossRef]
- Edlund, A.; Esguerra, J.L.; Wendt, A.; Flodström-Tullberg, M.; Eliasson, L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Stalvey, M.S.; Muller, C.; Schatz, D.A.; Wasserfall, C.H.; Campbell-Thompson, M.L.; Theriaque, D.W.; Flotte, T.R.; Atkinson, M.A. Cystic fibrosis transmembrane conductance regulator deficiency exacerbates islet cell dysfunction after beta-cell injury. Diabetes 2006, 55, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Blackman, S.M.; Hsu, S.; Ritter, S.E.; Naughton, K.M.; Wright, F.A.; Drumm, M.L.; Knowles, M.R.; Cutting, G.R. A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia 2009, 52, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Brunzell, C.; Cohen, R.C.; Katz, M.; Marshall, B.C.; Onady, G.; Robinson, K.A.; Sabadosa, K.A.; Stecenko, A.; Slovis, B.; et al. Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010, 33, 2697–2708. [Google Scholar] [CrossRef]
- Moran, A.; Pekow, P.; Grover, P.; Zorn, M.; Slovis, B.; Pilewski, J.; Tullis, E.; Liou, T.G.; Allen, H.; Cystic Fibrosis Related Diabetes Therapy Study Group. Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: Results of the cystic fibrosis related diabetes therapy trial. Diabetes Care 2009, 32, 1783–1788. [Google Scholar] [CrossRef]
- Moran, A.; Pillay, K.; Becker, D.; Granados, A.; Hameed, S.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. 27), 64–74. [Google Scholar] [CrossRef]
- Hardin, D.S.; Rice, J.; Rice, M.; Rosenblatt, R. Use of the insulin pump in treat cystic fibrosis related diabetes. J. Cyst. Fibros. 2009, 8, 174–178. [Google Scholar] [CrossRef]
- Sunni, M.; Bellin, M.D.; Moran, A. Exogenous insulin requirements do not differ between youth and adults with cystic fibrosis related diabetes. Pediatr. Diabetes 2013, 14, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Colomba, J.; Boudreau, V.; Lehoux-Dubois, C.; Desjardins, K.; Coriati, A.; Tremblay, F.; Rabasa-Lhoret, R. The main mechanism associated with progression of glucose intolerance in older patients with cystic fibrosis is insulin resistance and not reduced insulin secretion capacity. J. Cyst. Fibros. 2019, 18, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, N.; Seggelke, S.; Gibbs, J.; Hawkins, R.M.; Casciano, M.L.; Cohlmia, E.; Taylor-Cousar, J.; Wang, C.; Pereira, R.; Hsia, E.; et al. Cystic fibrosis-related diabetes in adults: Inpatient management of 121 patients during 410 admissions. J. Diabetes Sci. Technol. 2012, 6, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.; Stevens, J.E.; Russo, A.; Maddox, A.; Wishart, J.M.; Jones, K.L.; Greville, H.; Hetzel, D.; Chapman, I.; Horowitz, M.; et al. Gastric emptying, incretin hormone secretion, and postprandial glycemia in cystic fibrosis—Effects of pancreatic enzyme supplementation. J. Clin. Endocrinol. Metab. 2011, 96, E851–E855. [Google Scholar] [CrossRef]
- Geyer, M.C.; Sullivan, T.; Tai, A.; Morton, J.M.; Edwards, S.; Martin, A.J.; Perano, S.J.; Gagliardi, L.; Rayner, C.K.; Horowitz, M.; et al. Exenatide corrects postprandial hyperglycaemia in young people with cystic fibrosis and impaired glucose tolerance: A randomized crossover trial. Diabetes Obes. Metab. 2019, 21, 700–704. [Google Scholar] [CrossRef]
- Kelly, A.; De Leon, D.D.; Sheikh, S.; Camburn, D.; Kubrak, C.; Peleckis, A.J.; Stefanovski, D.; Hadjiliadis, D.; Rickels, M.R.; Rubenstein, R.C. Islet Hormone and Incretin Secretion in Cystic Fibrosis after Four Months of Ivacaftor Therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 342–351. [Google Scholar] [CrossRef]
- Bellin, M.D.; Laguna, T.; Leschyshyn, J.; Regelmann, W.; Dunitz, J.; Billings, J.; Moran, A. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: A small pilot study. Pediatr. Diabetes 2013, 14, 417–421. [Google Scholar] [CrossRef]
- Mauch, R.M.; Kmit, A.H.; Marson, F.A.; Levy, C.E.; Barros-Filho, A.A.; Ribeiro, J.D. Association of growth and nutritional parameters with pulmonary function in cystic fibrosis: A literature review. Rev. Paul. Pediatr. 2016, 34, 503–509. [Google Scholar] [CrossRef]
- Van Sambeek, L.; Cowley, E.S.; Newman, D.K.; Kato, R. Sputum glucose and glycemic control in cystic fibrosis-related diabetes: A cross-sectional study. PLoS ONE 2015, 10, e0119938. [Google Scholar] [CrossRef]
- Garnett, J.P.; Kalsi, K.K.; Sobotta, M.; Bearham, J.; Carr, G.; Powell, J.; Brodlie, M.; Ward, C.; Tarran, R.; Baines, D.L. Hyperglycaemia and Pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate-H+ secretion. Sci. Rep. 2016, 6, 37955–37968. [Google Scholar] [CrossRef]
- Simon, S.L.; Vigers, T.; Campbell, K.; Pyle, L.; Branscomb, R.; Nadeau, K.J.; Chan, C.L. Reduced insulin sensitivity is correlated with impaired sleep in adolescents with cystic fibrosis. Pediatr. Diabetes 2018, 19, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Putman, M.S.; Milliren, C.E.; Derrico, N.; Uluer, A.; Sicilian, L.; Lapey, A.; Sawicki, G.; Gordon, C.M.; Bouxsein, M.L.; Finkelstein, J.S. Compromised bone microarchitecture and estimated bone strength in young adults with cystic fibrosis. J. Clin. Endocrinol. Metab. 2014, 99, 3399–3407. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Zeboulon, N.; Combescure, C.; Gossec, L.; Cortet, B. The prevalence of osteoporosis, osteopenia, and fractures among adults with cystic fibrosis: A systematic literature review with meta-analysis. Calcif. Tissue Int. 2010, 86, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Laine, C.M.; Laine, T. Diagnosis of Osteoporosis in Children and Adolescents. Eur. Endocrinol. 2013, 9, 141–144. [Google Scholar] [PubMed]
- Dif, F.; Marty, C.; Baudoin, C.; de Vernejoul, M.C.; Levi, G. Severe osteopenia in CFTR-null mice. Bone 2004, 35, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, J.; Delion, M.; Gangloff, S.; Braux, J.; Velard, F. Bone disease in cystic fibrosis: New pathogenic insights opening novel therapies. Osteoporos. Int. 2016, 27, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Gaudelus, I.; Bianchi, M.L.; Garabédian, M.; Aris, R.M.; Morton, A.; Hardin, D.S.; Elkin, S.L.; Compston, J.E.; Conway, S.P.; Castanet, M.; et al. European cystic fibrosis bone mineralisation guidelines. J. Cyst. Fibros. 2011, 10 (Suppl. 2), S16–S23. [Google Scholar] [CrossRef]
- Sharma, S.; Jaksic, M.; Fenwick, S.; Byrnes, C.; Cundy, T. Accrual of Bone Mass in Children and Adolescents with Cystic Fibrosis. J. Clin. Endocrinol. Metab. 2017, 102, 1734–1739. [Google Scholar] [CrossRef]
- Adami, G.; Saag, K.G. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos. Int. 2019, 30, 1145–1156. [Google Scholar] [CrossRef]
- Mazziotti, G.; Giustina, A. Glucocorticoids and the regulation of growth hormone secretion. Nat. Rev. Endocrinol. 2013, 9, 265–276. [Google Scholar] [CrossRef]
- Shead, E.F.; Haworth, C.S.; Barker, H.; Bilton, D.; Compston, J.E. Osteoclast function, bone turnover and inflammatory cytokines during infective exacerbations of cystic fibrosis. J. Cyst. Fibros. 2010, 9, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mora Vallellano, J.; Delgado Pecellín, C.; Delgado Pecellín, I.; Quintana Gallego, E.; López-Campos, J.L. Evaluation of bone metabolism in children with cystic fibrosis. Bone 2021, 147, 115929. [Google Scholar] [CrossRef]
- Ishii, M.; Kikuta, J.; Shimazu, Y.; Meier-Schellersheim, M.; Germain, R.N. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 2010, 207, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, J.; Kawamura, S.; Okiji, F.; Shirazaki, M.; Sakai, S.; Saito, H.; Ishii, M. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc. Natl. Acad. Sci. USA 2013, 110, 7009–7013. [Google Scholar] [CrossRef]
- Worgall, T.S.; Veerappan, A.; Sung, B.; Kim, B.I.; Weiner, E.; Bholah, R.; Silver, R.B.; Jiang, X.C.; Worgall, S. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci. Transl. Med. 2013, 5, 186ra67. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Krause, A.; Limberis, M.; Worgall, T.S.; Worgall, S. Low sphingosine-1-phosphate impairs lung dendritic cells in cystic fibrosis. Am. J. Respir. Cell. Mol. Biol. 2013, 48, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Le Heron, L.; Guillaume, C.; Velard, F.; Braux, J.; Touqui, L.; Moriceau, S.; Sermet-Gaudelus, I.; Laurent-Maquin, D.; Jacquot, J. Cystic fibrosis transmembrane conductance regulator (CFTR) regulates the production of osteoprotegerin (OPG) and prostaglandin (PG) E2 in human bone. J. Cyst. Fibros. 2010, 9, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Aris, R.M.; Merkel, P.A.; Bachrach, L.K.; Borowitz, D.S.; Boyle, M.P.; Elkin, S.L.; Guise, T.A.; Hardin, D.S.; Haworth, C.S.; Holick, M.F.; et al. Guide to bone health and disease in cystic fibrosis. J. Clin. Endocrinol. Metab. 2005, 90, 1888–1896. [Google Scholar] [CrossRef]
- Sermet-Gaudelus, I.; Castanet, M.; Souberbielle, J.C.; Mallet, E.; Le Groupe de Travail sur Minéralisation Osseuse et Mucoviscidose de la Fédération Française des Centres de Ressource et de Compétence en Mucoviscidose. Minéralisation osseuse et mucoviscidose [Bone health in cystic fibrosis]. Arch. Pediatr. 2009, 16, 616–618. [Google Scholar] [CrossRef]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef]
- Schulze, K.J.; O’brien, K.O.; Germain-Lee, E.L.; Baer, D.J.; Leonard, A.L.; Rosenstein, B.J. Endogenous fecal losses of calcium compromise calcium balance in pancreatic-insufficient girls with cystic fibrosis. J. Pediatr. 2003, 143, 765–771. [Google Scholar] [CrossRef]
- Aris, R.M.; Lester, G.E.; Dingman, S.; Ontjes, D.A. Altered calcium homeostasis in adults with cystic fibrosis. Osteoporos. Int. 1999, 10, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lyytikäinen, A.; Kröger, H.; Lamberg-Allardt, C.; Alén, M.; Koistinen, A.; Wang, Q.J.; Suuriniemi, M.; Suominen, H.; Mahonen, A.; et al. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10-12-y-old girls: A 2-y randomized trial. Am. J. Clin. Nutr. 2005, 82, 1115–1126. [Google Scholar] [CrossRef]
- Maurel, D.B.; Boisseau, N.; Benhamou, C.L.; Jaffre, C. Alcohol and bone: Review of dose effects and mechanisms. Osteoporos. Int. 2012, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V.; Kelly, A.; Stephenson, A.; Maguiness, K.; Enders, J.; Robinson, K.A.; Marshall, B.C.; Borowitz, D.; Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: Evidence-based recommendations from the Cystic Fibrosis Foundation. J. Clin. Endocrinol. Metab. 2012, 97, 1082–1093. [Google Scholar] [CrossRef]
- Rashid, M.; Durie, P.; Andrew, M.; Kalnins, D.; Shin, J.; Corey, M.; Tullis, E.; Pencharz, P.B. Prevalence of vitamin K deficiency in cystic fibrosis. Am. J. Clin. Nutr. 1999, 70, 378–382. [Google Scholar] [CrossRef][Green Version]
- Conway, S.P.; Wolfe, S.P.; Brownlee, K.G.; White, H.; Oldroyd, B.; Truscott, J.G.; Harvey, J.M.; Shearer, M.J. Vitamin K status among children with cystic fibrosis and its relationship to bone mineral density and bone turnover. Pediatrics 2005, 115, 1325–1331. [Google Scholar] [CrossRef]
- Aris, R.M.; Ontjes, D.A.; Brown, S.A.; Chalermskulrat, W.; Neuringer, I.; Lester, G.E. Carboxylated osteocalcin levels in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 1129. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Benden, C.; Williams, J.E.; Chomtho, S.; Ginty, F.; Nigdikar, S.V.; Jaffe, A. Undercarboxylated osteocalcin and bone mass in 8-12 year old children with cystic fibrosis. J. Cyst. Fibros. 2008, 7, 307–312. [Google Scholar] [CrossRef]
- Jagannath, V.A.; Fedorowicz, Z.; Thaker, V.; Chang, A.B. Vitamin K supplementation for cystic fibrosis. Cochrane Database Syst. Rev. 2015, 1, CD008482. [Google Scholar]
- Putman, M.S.; Anabtawi, A.; Le, T.; Tangpricha, V.; Sermet-Gaudelus, I. Cystic fibrosis bone disease treatment: Current knowledge and future directions. J. Cyst. Fibros. 2019, 18 (Suppl. 2), S56–S65. [Google Scholar] [CrossRef] [PubMed]
- Conwell, L.S.; Chang, A.B. Bisphosphonates for osteoporosis in people with cystic fibrosis. Cochrane Database Syst. Rev. 2012, 4, CD002010. [Google Scholar]
- Lai, H.C.; Kosorok, M.R.; Sondel, S.A.; Chen, S.T.; FitzSimmons, S.C.; Green, C.G.; Shen, G.; Walker, S.; Farrell, P.M. Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry data: Evaluation of various criteria used to identify malnutrition. J. Pediatr. 1998, 132, 478–485. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry. Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2016; Available online: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-Patient-Registry-Annual-Data-Report.pdf (accessed on 10 June 2022).
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry. Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2017; Available online: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2017-Patient-Registry-Annual-Data-Report.pdf (accessed on 10 June 2022).
- Leung, D.H.; Heltshe, S.L.; Borowitz, D.; Gelfond, D.; Kloster, M.; Heubi, J.E.; Stalvey, M.; Ramsey, B.W.; Baby Observational and Nutrition Study (BONUS) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Effects of Diagnosis by Newborn Screening for Cystic Fibrosis on Weight and Length in the First Year of Life. JAMA Pediatr. 2017, 171, 546–554. [Google Scholar] [CrossRef]
- Karlberg, J.; Kjellmer, I.; Kristiansson, B. Linear growth in children with cystic fibrosis. I. Birth to 8 years of age. Acta Paediatr. Scand. 1991, 80, 508–514. [Google Scholar] [CrossRef]
- Blackman, S.M.; Tangpricha, V. Endocrine Disorders in Cystic Fibrosis. Pediatr. Clin. N. Am. 2016, 63, 699–708. [Google Scholar] [CrossRef]
- Haeusler, G.; Frisch, H.; Waldhör, T.; Götz, M. Perspectives of longitudinal growth in cystic fibrosis from birth to adult age. Eur. J. Pediatr. 1994, 153, 158–163. [Google Scholar] [CrossRef]
- Laursen, E.M.; Lanng, S.; Rasmussen, M.H.; Koch, C.; Skakkebaek, N.E.; Müller, J. Normal spontaneous and stimulated GH levels despite decreased IGF-I concentrations in cystic fibrosis patients. Eur. J. Endocrinol. 1999, 140, 315–321. [Google Scholar] [CrossRef]
- Hodges, C.A.; Grady, B.R.; Palmert, M.R.; Drumm, M.L. Loss of cftr function in neurons results in poor growth and possible endocrine dysfunction. Pediatr. Pulmonol. 2010, 45, 290–291. [Google Scholar]
- Pagani, S.; Bozzola, E.; Acquafredda, G.; Terlizzi, V.; Raia, V.; Majo, F.; Villani, A.; Bozzola, M. GH-IGF-1 Axis in Children with Cystic Fibrosis. Clin. Med. Res. 2019, 17, 82–89. [Google Scholar] [CrossRef]
- Hawkes, C.P.; Grimberg, A. Insulin-Like Growth Factor-I is a Marker for the Nutritional State. Pediatr. Endocrinol. Rev. 2015, 13, 499–511. [Google Scholar] [PubMed]
- Cirillo, F.; Lazzeroni, P.; Sartori, C.; Street, M.E. Inflammatory Diseases and Growth: Effects on the GH-IGF Axis and on Growth Plate. Int. J. Mol. Sci. 2017, 18, 1878. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.B. Growth suppression by glucocorticoid therapy. Endocrinol. Metab. Clin. N. Am. 1996, 25, 699–717. [Google Scholar] [CrossRef]
- Ripa, P.; Robertson, I.; Cowley, D.; Harris, M.; Masters, I.B.; Cotterill, A.M. The relationship between insulin secretion, the insulin-like growth factor axis and growth in children with cystic fibrosis. Clin. Endocrinol. 2002, 56, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.S.; Bridges, N.A.; Prasad, S.A.; Francis, J.; Carr, S.B.; Suri, R.; Balfour-Lynn, I.M. Growth in children with cystic fibrosis-related diabetes. Pediatr. Pulmonol. 2009, 44, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- Ziegler, E.E. 4.2 The CDC and Euro Growth Charts. World Rev. Nutr. Diet. 2015, 113, 295–307. [Google Scholar]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef]
- Thaker, V.; Haagensen, A.L.; Carter, B.; Fedorowicz, Z.; Houston, B.W. Recombinant growth hormone therapy for cystic fibrosis in children and young adults. Cochrane Database Syst. Rev. 2015, 5, CD008901. [Google Scholar] [CrossRef]
- Phung, O.J.; Coleman, C.I.; Baker, E.L.; Scholle, J.M.; Girotto, J.E.; Makanji, S.S.; Chen, W.T.; Talati, R.; Kluger, J.; White, C.M. Recombinant human growth hormone in the treatment of patients with cystic fibrosis. Pediatrics 2010, 126, e1211–e1226. [Google Scholar] [CrossRef]
- Stalvey, M.S.; Pace, J.; Niknian, M.; Higgins, M.N.; Tarn, V.; Davis, J.; Heltshe, S.L.; Rowe, S.M. Growth in Prepubertal Children With Cystic Fibrosis Treated With Ivacaftor. Pediatrics 2017, 139, e20162522. [Google Scholar] [CrossRef] [PubMed]
- Goldsweig, B.; Kaminski, B.; Sidhaye, A.; Blackman, S.M.; Kelly, A. Puberty in cystic fibrosis. J. Cyst. Fibros. 2019, 18 (Suppl. 2), S88–S94. [Google Scholar] [CrossRef] [PubMed]
- Welt, C.K.; Chan, J.L.; Bullen, J.; Murphy, R.; Smith, P.; DePaoli, A.M.; Karalis, A.; Mantzoros, C.S. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 2004, 351, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Frisch, R.E.; Revelle, R. Height and weight at menarche and a hypothesis of menarche. Arch. Dis. Child. 1971, 46, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Dobie, R.; Altowati, M.A.; Werther, G.A.; Farquharson, C.; Ahmed, S.F. Growth and the Growth Hormone-Insulin Like Growth Factor 1 Axis in Children With Chronic Inflammation: Current Evidence, Gaps in Knowledge, and Future Directions. Endocr. Rev. 2016, 37, 62–110. [Google Scholar] [CrossRef]
- Mitchell-Heggs, P.; Mearns, M.; Batten, J.C. Cystic fibrosis in adolescents and adults. Q. J. Med. 1976, 45, 479–504. [Google Scholar]
- Landon, C.; Rosenfeld, R.G. Short stature and pubertal delay in male adolescents with cystic fibrosis. Androgen treatment. Am. J. Dis. Child. 1984, 138, 388–391. [Google Scholar] [CrossRef]
- Johannesson, M.; Gottlieb, C.; Hjelte, L. Delayed puberty in girls with cystic fibrosis despite good clinical status. Pediatrics 1997, 99, 29–34. [Google Scholar] [CrossRef]
- Landon, C.; Kerner, J.A.; Castillo, R.; Adams, L.; Whalen, R.; Lewiston, N.J. Oral correction of essential fatty acid deficiency in cystic fibrosis. JPEN J. Parenter. Enteral. Nutr. 1981, 5, 501–504. [Google Scholar] [CrossRef]
- Jin, R.; Hodges, C.A.; Drumm, M.L.; Palmert, M.R. The cystic fibrosis transmembrane conductance regulator (Cftr) modulates the timing of puberty in mice. J. Med. Genet. 2006, 43, e29. [Google Scholar] [CrossRef]
- Weyler, R.T.; Yurko-Mauro, K.A.; Rubenstein, R.; Kollen, W.J.; Reenstra, W.; Altschuler, S.M.; Egan, M.; Mulberg, A.E. CFTR is functionally active in GnRH-expressing GT1-7 hypothalamic neurons. Am. J. Physiol. 1999, 277, C563–C571. [Google Scholar] [CrossRef] [PubMed]
- Buntain, H.M.; Greer, R.M.; Wong, J.C.; Schluter, P.J.; Batch, J.; Lewindon, P.; Bell, S.C.; Wainwright, C.E. Pubertal development and its influences on bone mineral density in Australian children and adolescents with cystic fibrosis. J. Paediatr. Child Health 2005, 41, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Schall, J.I.; Stallings, V.A.; Zemel, B.S. Deficits in bone mineral content in children and adolescents with cystic fibrosis are related to height deficits. J. Clin. Densitom. 2008, 11, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Bournez, M.; Bellis, G.; Huet, F. Growth during puberty in cystic fibrosis: A retrospective evaluation of a French cohort. Arch. Dis. Child. 2012, 97, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.O.; Stern, R.C.; Root, A.W. The reproductive endocrine system in cystic fibrosis. I. Basal gonadotropin and sex steroid levels. Am. J. Dis. Child. 1981, 135, 422–426. [Google Scholar] [CrossRef]
- Allan, J.L.; Townley, R.R.; Phelan, P.D. Family response to cystic fibrosis. Aust. Paediatr. J. 1974, 10, 136–146. [Google Scholar] [CrossRef]
- Torres-Santiago, L.; Mericq, V.; Taboada, M.; Unanue, N.; Klein, K.O.; Singh, R.; Hossain, J.; Santen, R.J.; Ross, J.L.; Mauras, N. Metabolic effects of oral versus transdermal 17β-estradiol (E2): A randomized clinical trial in girls with Turner syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 2716–2724. [Google Scholar] [CrossRef]
- Wei, C.; Crowne, E.C. Recent advances in the understanding and management of delayed puberty. Arch. Dis. Child. 2016, 101, 481–488. [Google Scholar] [CrossRef]
- Thaker, V.; Carter, B.; Putman, M. Recombinant growth hormone therapy for cystic fibrosis in children and young adults. Cochrane Database Syst. Rev. 2021, 8, CD008901. [Google Scholar]
- Ahmad, A.; Ahmed, A.; Patrizio, P. Cystic fibrosis and fertility. Curr. Opin. Obstet. Gynecol. 2013, 25, 167–172. [Google Scholar] [CrossRef]
- Patrizio, P.; Zielenski, J. Congenital absence of the vas deferens: A mild form of cystic fibrosis. Mol. Med. Today 1996, 2, 24–31. [Google Scholar] [CrossRef]
- Brugman, S.M.; Taussig, L.M. The reproductive system. In Cystic Fibrosis; Taussig, L.M., Ed.; Theime-Stratton: New York, NY, USA, 1984; pp. 323–337. [Google Scholar]
- Leifke, E.; Friemert, M.; Heilmann, M.; Puvogel, N.; Smaczny, C.; von zur Muhlen, A.; Brabant, G. Sex steroids and body composition in men with cystic fibrosis. Eur. J. Endocrinol. 2003, 148, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, P.; Salameh, W.A. Expression of the cystic fibrosis transmembrane conductance regulator (CFTR) mRNA in normal and pathological adult human epididymis. J. Reprod. Fertil. Suppl. 1998, 53, 261–270. [Google Scholar]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Devuyst, O.; Golstein, P.E.; Sanches, M.V.; Piontek, K.; Wilson, P.D.; Guggino, W.B.; Dumont, J.E.; Beauwens, R. Expression of CFTR in human and bovine thyroid epithelium. Am. J. Physiol. 1997, 272, C1299–C1308. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ganta, S.; Fong, P. Altered ion transport by thyroid epithelia from CFTR(-/-) pigs suggests mechanisms for hypothyroidism in cystic fibrosis. Exp. Physiol. 2010, 95, 1132–1144. [Google Scholar] [CrossRef]
- Dolan, T.F., Jr.; Gibson, L.E. Complications of iodide therapy in patients with cystic fibrosis. J. Pediatr. 1971, 79, 684–687. [Google Scholar]
- Azizi, F.; Bentley, D.; Vagenakis, A.; Portnay, G.; Bush, J.E.; Shwachman, H.; Ingbar, S.H.; Braverman, L.E. Abnormal thyroid function and response to iodides in patients with cystic fibrosis. Trans. Assoc. Am. Physicians 1974, 87, 111–119. [Google Scholar]
- Zimmermann, M.B.; Köhrle, J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: Biochemistry and relevance to public health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef]
- Kauf, E.; Dawczynski, H.; Jahreis, G.; Janitzky, E.; Winnefeld, K. Sodium selenite therapy and thyroid-hormone status in cystic fibrosis and congenital hypothyroidism. Biol. Trace Elem. Res. 1994, 40, 247–253. [Google Scholar] [CrossRef]
- Segall-Blank, M.; Vagenakis, A.G.; Shwachman, H.; Ingbar, S.H.; Braverman, L.E. Thyroid gland function and pituitary TSH reserve in patients with cystic fibrosis. J. Pediatr. 1981, 98, 218–222. [Google Scholar] [CrossRef]
- De Luca, F.; Trimarchi, F.; Sferlazzas, C.; Benvenga, S.; Costante, G.; Mami, C.; Di Pasquale, G.; Magazzu, G. Thyroid function in children with cystic fibrosis. Eur. J. Pediatr. 1982, 138, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Volta, C.; Street, M.E.; Ziveri, M.A.; Bonelli, P.; Spaggiari, C.; Grzincich, G.L.; Bernasconi, S. Thyroid function, cytokine and IGF-IGFBP interactions in cystic fibrosis patients. Horm. Res. 2005, 63, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Naehrlich, L.; Dörr, H.G.; Bagheri-Behrouzi, A.; Rauh, M. Iodine deficiency and subclinical hypothyroidism are common in cystic fibrosis patients. J. Trace Elem. Med. Biol. 2013, 27, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chesdachai, S.; Lee, M.J.; He, X.M.; Tangpricha, V.; Braverman, L.E. Thyroid Function in Patients with Cystic Fibrosis: No Longer a Concern? Thyroid 2016, 26, 875–879. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, V.; Terlizzi, V.; Stagi, S. Auxological and Endocrinological Features in Children and Adolescents with Cystic Fibrosis. J. Clin. Med. 2022, 11, 4041. https://doi.org/10.3390/jcm11144041
Ferrari V, Terlizzi V, Stagi S. Auxological and Endocrinological Features in Children and Adolescents with Cystic Fibrosis. Journal of Clinical Medicine. 2022; 11(14):4041. https://doi.org/10.3390/jcm11144041
Chicago/Turabian StyleFerrari, Vittorio, Vito Terlizzi, and Stefano Stagi. 2022. "Auxological and Endocrinological Features in Children and Adolescents with Cystic Fibrosis" Journal of Clinical Medicine 11, no. 14: 4041. https://doi.org/10.3390/jcm11144041
APA StyleFerrari, V., Terlizzi, V., & Stagi, S. (2022). Auxological and Endocrinological Features in Children and Adolescents with Cystic Fibrosis. Journal of Clinical Medicine, 11(14), 4041. https://doi.org/10.3390/jcm11144041







