Precision Imaging Guidance in the Era of Precision Oncology: An Update of Imaging Tools for Interventional Procedures
Abstract
:1. Introduction
2. Vascular and Non-Vascular Approaches
3. Imaging Planning and Guidance
4. Imaging Modalities
4.1. US
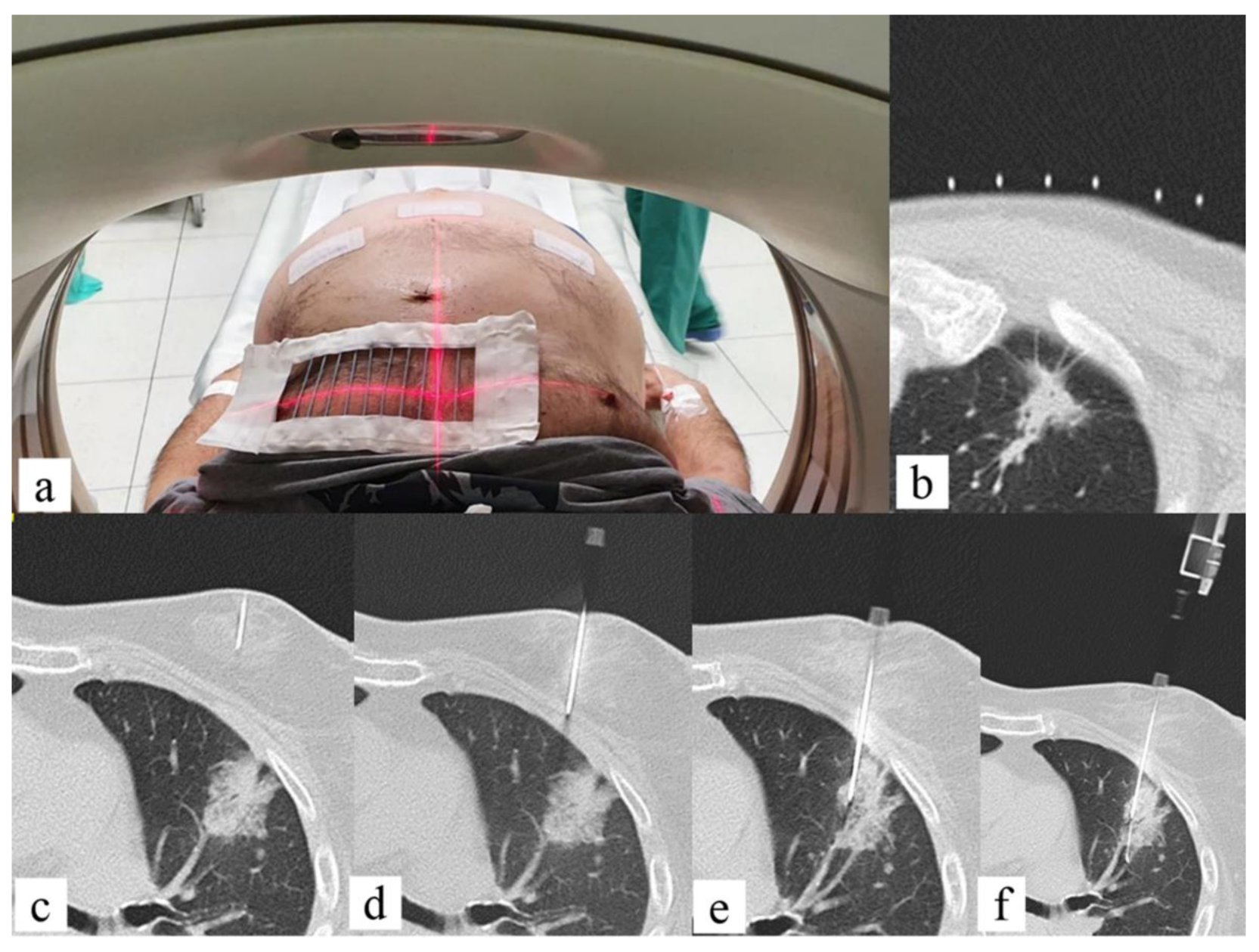
4.2. CT
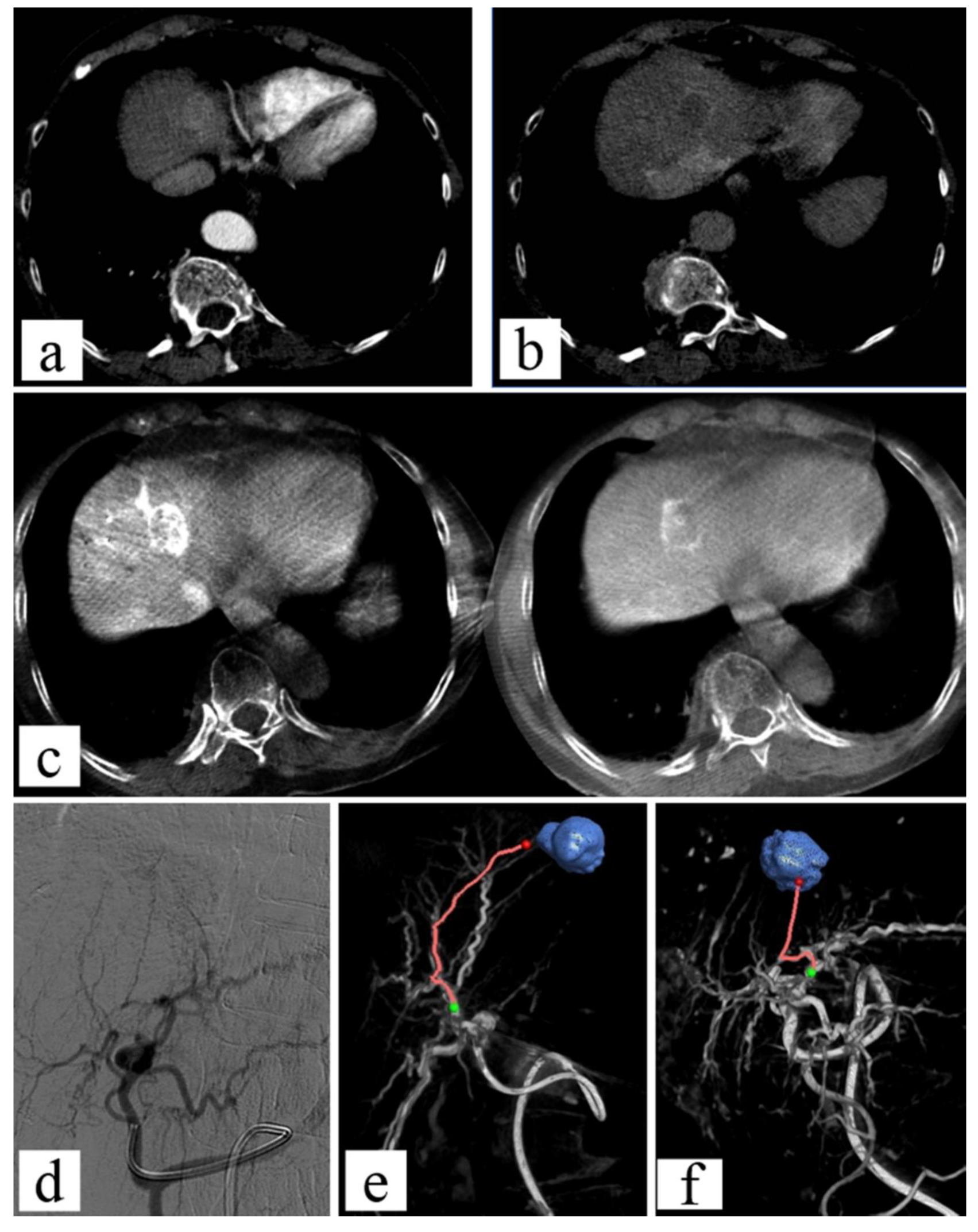
CT Angiography (CTA)
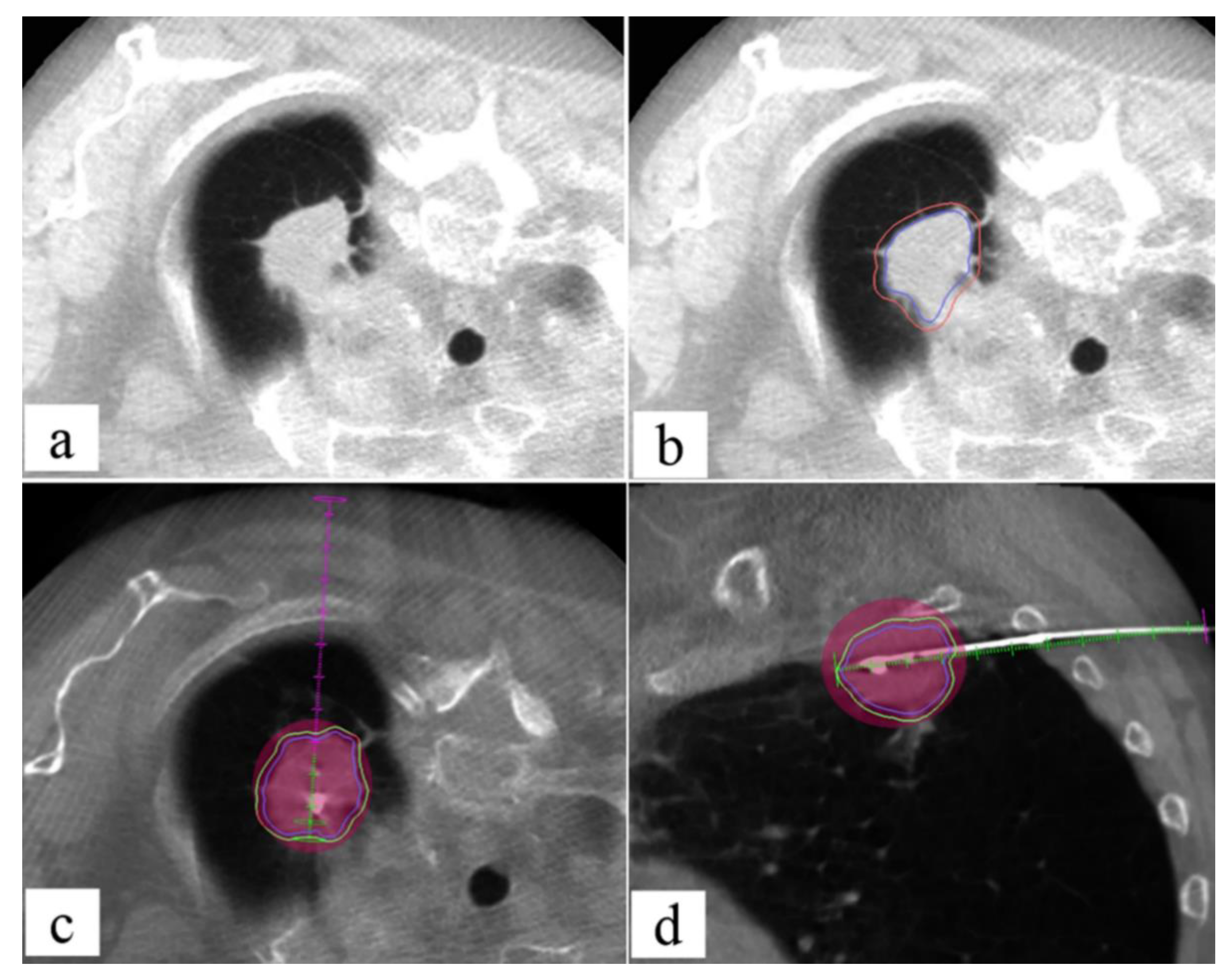
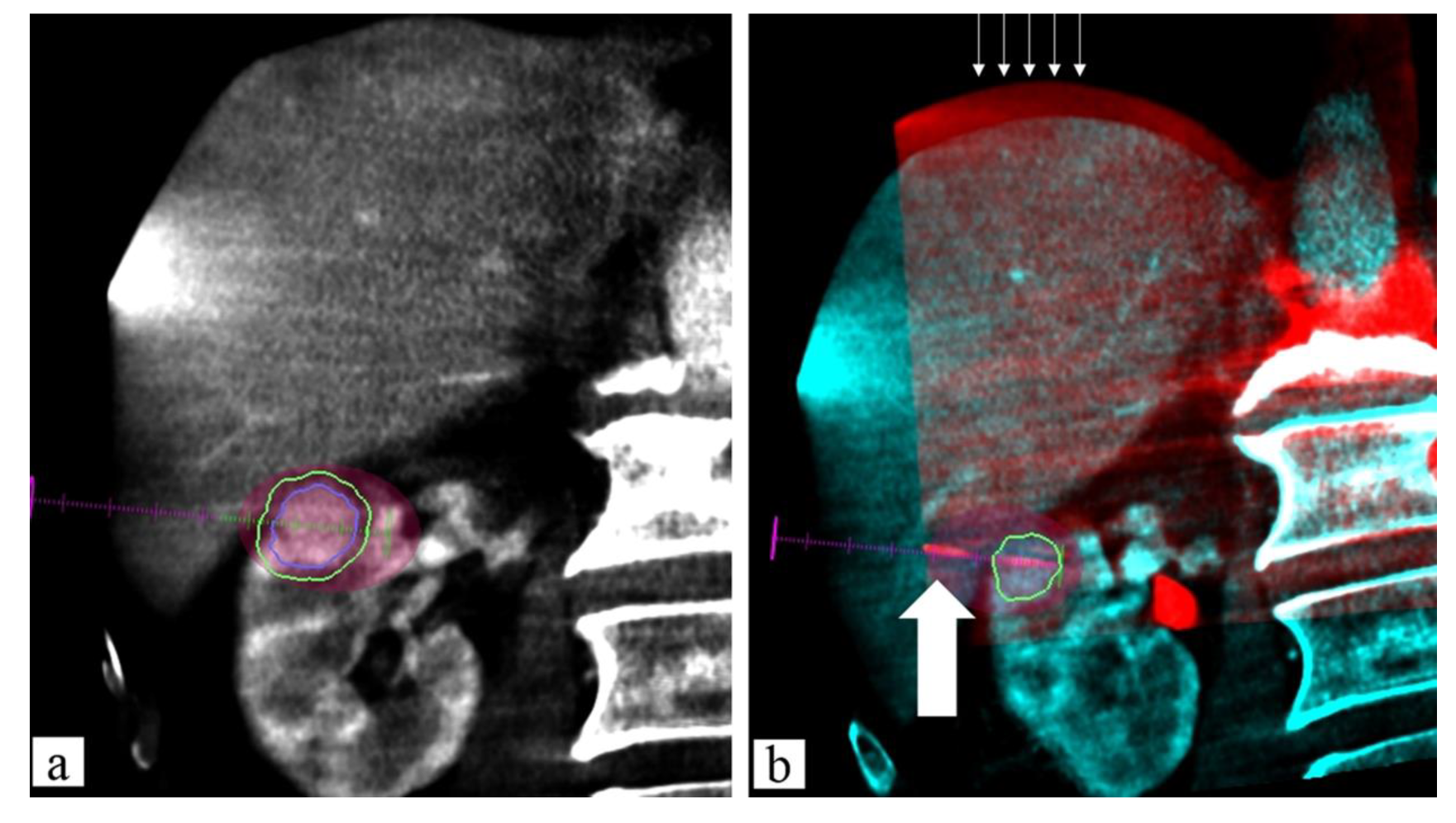
4.3. CBCT
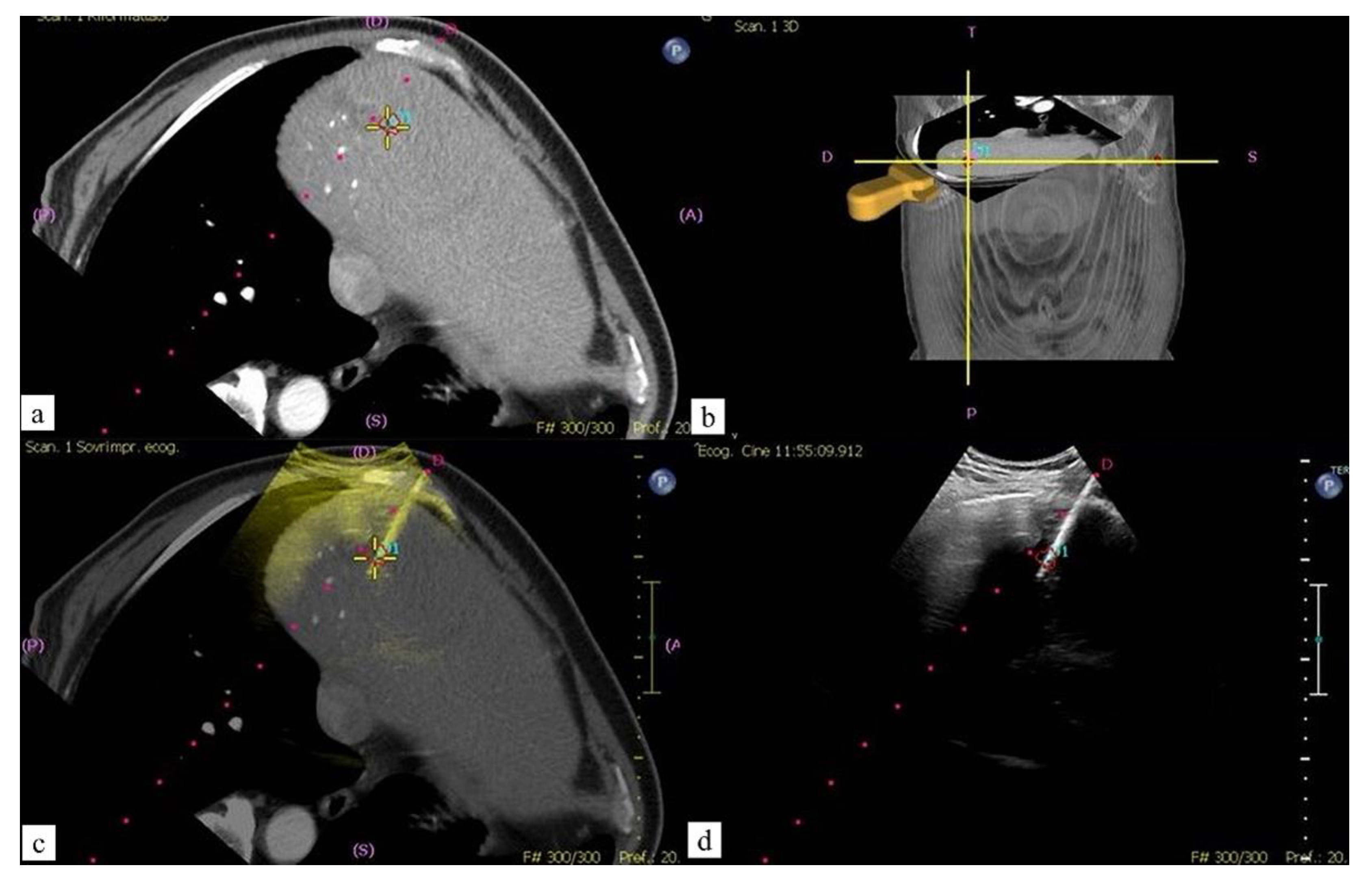
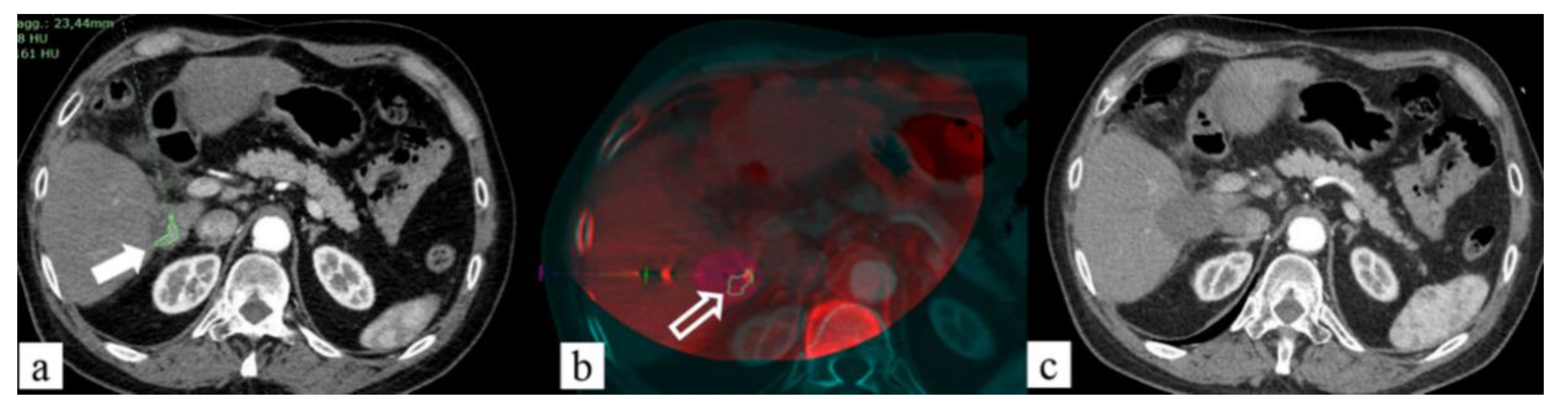
Fusion Technique
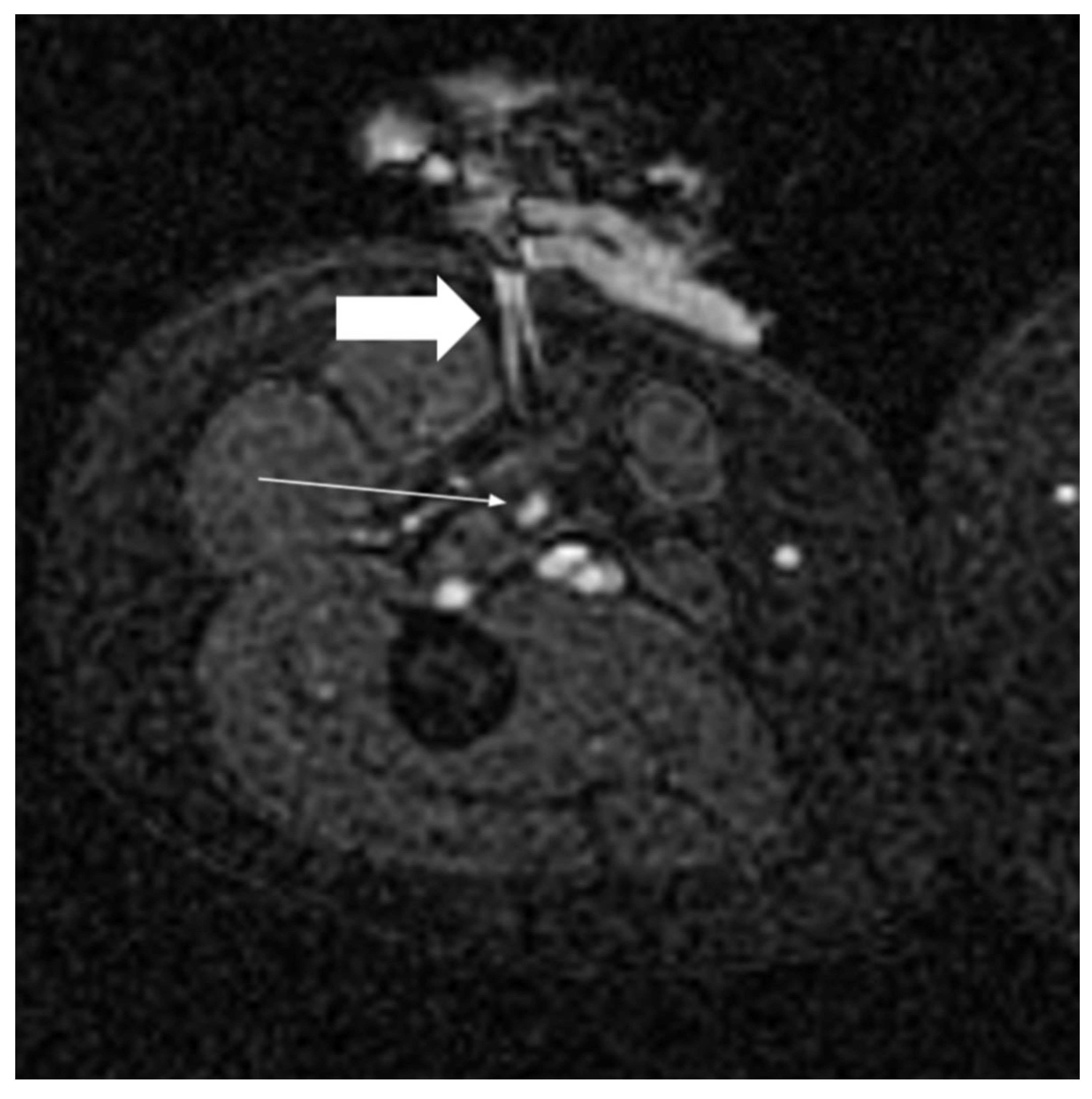
4.4. MRI
4.4.1. MRI Guidance
4.4.2. MRgFUS
5. New Frontiers
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rousseau, H.; Vernhet-Kovacsik, H.; Mouroz, P.R.; Otal, P.; Meyrignac, O.; Mokrane, F.Z. Avenir de La Radiologie Interventionnelle. Presse Med. 2019, 48, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Cannataci, C.; Cimo’, B.; Mamone, G.; Tuzzolino, F.; D’Amico, M.; Cortis, K.; Maruzzelli, L.; Miraglia, R. Portal Vein Puncture-Related Complications during Transjugular Intrahepatic Portosystemic Shunt Creation: Colapinto Needle Set vs Rösch-Uchida Needle Set. Radiol. Med. 2021, 126, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, A.M.; Wood, B.J.; Arrichiello, A.; Bottino, N.; Bracchi, L.; Forzenigo, L.; Andrisani, M.C.; Vespro, V.; Bonelli, C.; Amalou, A.; et al. Preparation of a Radiology Department in an Italian Hospital Dedicated to COVID-19 Patients. Radiol. Med. 2020, 125, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.; Vouche, M.; Sze, D.Y.; Hohlastos, E.; Collins, J.; Schirmang, T.; Memon, K.; Ryu, R.K.; Sato, K.; Chen, R.; et al. Cancer Concepts and Principles: Primer for the Interventional Oncologist—Part II. J. Vasc. Interv. Radiol. 2013, 24, 1167–1188. [Google Scholar] [CrossRef] [Green Version]
- Johnston, E.W.; von Stempel, C.; Singh, S.; Bandula, S.; Illing, R. Interventional Oncology. Br. J. Hosp. Med. 2016, 77, C114–C117. [Google Scholar] [CrossRef] [Green Version]
- Kovács, G.; Tagliaferri, L.; Valentini, V. Is an Interventional Oncology Center an advantage in the service of cancer patients or in the education? The Gemelli Hospital and INTERACTS experience. J. Contemp. Brachytherapy 2017, 9, 497–498. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Kovács, G.; Autorino, R.; Budrukkar, A.; Guinot, J.L.; Johansson, G.H.; Monge, R.M.; Meyer, J.E.; Niehoff, P.; Rovirosa, A.; et al. ENT COBRA (Consortium for Brachytherapy Data Analysis): Interdisciplinary standardized data collection system for head and neck patients treated with interventional radiotherapy (brachytherapy). J. Contemp. Brachytherapy 2016, 8, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Tagliaferri, L.; Pagliara, M.M.; Boldrini, L.; Caputo, C.G.; Azario, L.; Campitelli, M.; Gambacorta, M.A.; Smaniotto, D.; Deodato, V.F.; Morganti, A.G.; et al. INTERACTS (INTErventional Radiotherapy ACtive Teaching School) guidelines for quality assurance in choroidal melanoma interventional radiotherapy (brachytherapy) procedures. J. Contemp. Brachytherapy 2017, 9, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Hussaini, S.; Kang, J.; Madoff, D.C. The role of interventional oncology in the palliative care of cancer patients. Expert Rev. Qual. Life Cancer Care 2016, 1, 73–87. [Google Scholar] [CrossRef]
- Fairchild, A.H.; Rilling, W.S. Palliative Interventional Oncology. Cancer J. 2016, 22, 411–417. [Google Scholar] [CrossRef]
- Giurazza, F.; Corvino, F.; Silvestre, M.; Cangiano, G.; De Magistris, G.; Cavaglià, E.; Amodio, F.; Niola, R. Embolization of Peripheral Arteriovenous Malformations and Fistulas with Precipitating Hydrophobic Injectable Liquid (PHIL®). Radiol. Med. 2021, 126, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, F.A. Interventional Radiology in Pediatric Oncology. Eur. J. Radiol. 2005, 53, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Corvino, F.; Cavaglià, E.; Silvestre, M.; Cangiano, G.; Amodio, F.; De Magistris, G.; Niola, R. Emborrhoid in Patients with Portal Hypertension and Chronic Hemorrhoidal Bleeding: Preliminary Results in Five Cases with a New Coiling Release Fashion “Spaghetti Technique”. Radiol. Med. 2020, 125, 1008–1011. [Google Scholar] [CrossRef]
- Arnold, M.J.; Keung, J.J.; McCarragher, B. Interventional Radiology: Indications and Best Practices. Am. Fam. Physician 2019, 99, 547–556. [Google Scholar]
- Coldwell, D.M.; Sewell, P.E. The Expanding Role of Interventional Radiology in the Supportive Care of the Oncology Patient: From Diagnosis to Therapy. Semin. Oncol. 2005, 32, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Floridi, C.; Pesapane, F.; Angileri, S.A.; De Palma, D.; Fontana, F.; Caspani, F.; Barile, A.; Del Sole, A.; Masciocchi, C.; Lucignani, G.; et al. Yttrium-90 Radioembolization Treatment for Unresectable Hepatocellular Carcinoma: A Single-Centre Prognostic Factors Analysis. Med. Oncol. 2017, 34, 174. [Google Scholar] [CrossRef]
- Svarc, P.; Taudorf, M.; Nielsen, M.B.; Stroomberg, H.V.; Røder, M.A.; Lönn, L. Postembolization Syndrome after Prostatic Artery Embolization: A Systematic Review. Diagnostics 2020, 10, 659. [Google Scholar] [CrossRef]
- Marelli, L.; Stigliano, R.; Triantos, C.; Senzolo, M.; Cholongitas, E.; Davies, N.; Tibballs, J.; Meyer, T.; Patch, D.W.; Burroughs, A.K. Transarterial Therapy for Hepatocellular Carcinoma: Which Technique Is More Effective? A Systematic Review of Cohort and Randomized Studies. Cardiovasc. Interv. Radiol. 2007, 30, 6–25. [Google Scholar] [CrossRef]
- Tipaldi, M.A.; Ronconi, E.; Lucertini, E.; Krokidis, M.; Zerunian, M.; Polidori, T.; Begini, P.; Marignani, M.; Mazzuca, F.; Caruso, D.; et al. Hepatocellular Carcinoma Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE): Outcome Analysis Using a Model Based On Pre-Treatment CT Texture Features. Diagnostics 2021, 11, 956. [Google Scholar] [CrossRef]
- Orlacchio, A.; Chegai, F.; Roma, S.; Merolla, S.; Bosa, A.; Francioso, S. Degradable Starch Microspheres Transarterial Chemoembolization (DSMs-TACE) in Patients with Unresectable Hepatocellular Carcinoma (HCC): Long-Term Results from a Single-Center 137-Patient Cohort Prospective Study. Radiol. Med. 2020, 125, 98–106. [Google Scholar] [CrossRef]
- Petrillo, M.; Patella, F.; Pesapane, F.; Suter, M.B.; Ierardi, A.M.; Angileri, S.A.; Floridi, C.; de Filippo, M.; Carrafiello, G. Hypoxia and Tumor Angiogenesis in the Era of Hepatocellular Carcinoma Transarterial Loco-Regional Treatments. Futur. Oncol. 2018, 14, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Lungren, M.P.; Towbin, A.J.; Roebuck, D.J.; Monroe, E.J.; Gill, A.E.; Thakor, A.; Towbin, R.B.; Cahill, A.M.; Matthew Hawkins, C. Role of Interventional Radiology in Managing Pediatric Liver Tumors: Part 1: Endovascular Interventions. Pediatr. Radiol. 2018, 48, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, J.; Chevallier, O.; Manfredi, S.; Dygai-Cochet, I.; Tabouret-Viaud, C.; Nodari, G.; Ghiringhelli, F.; Riedinger, J.-M.; Popoff, R.; Vrigneaud, J.-M.; et al. Transarterial Radioembolization of Hepatocellular Carcinoma, Liver-Dominant Hepatic Colorectal Cancer Metastases, and Cholangiocarcinoma Using Yttrium90 Microspheres: Eight-Year Single-Center Real-Life Experience. Diagnostics 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Aberle, S.; Kenkel, D.; Becker, A.S.; Puippe, G.; Burger, I.; Schaefer, N.; Pfammatter, T. Outpatient Yttrium-90 Microsphere Radioembolization: Assessment of Radiation Safety and Quantification of Post-Treatment Adverse Events Causing Hospitalization. Radiol. Med. 2020, 125, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Veltri, A.; Bargellini, I.; Giorgi, L.; Almeida, P.A.M.S.; Akhan, O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB). Cardiovasc. Interv. Radiol. 2017, 40, 1501–1513. [Google Scholar] [CrossRef]
- Rigo, M.; Mazzola, R.; Napoli, G.; Giaj-Levra, N.; Figlia, V.; Nicosia, L.; Ricchetti, F.; Tomasini, D.; Bonù, M.L.; Cuccia, F.; et al. Post-HIFU Locally Relapsed Prostate Cancer: High-Dose Salvage Radiotherapy Guided by Molecular Imaging. Radiol. Med. 2020, 125, 491–499. [Google Scholar] [CrossRef]
- De Filippo, M.; Ziglioli, F.; Russo, U.; Pagano, P.; Brunese, L.; Bertelli, E.; Pagnini, F.; Maestroni, U. Radiofrequency Ablation (RFA) of T1a Renal Cancer with Externally Cooled Multitined Expandable Electrodes. Radiol. Med. 2020, 125, 790–797. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bruno, F.; Gianneramo, C.; Palumbo, P.; Zugaro, L.; Zoccali, C.; Barile, A.; Masciocchi, C. Evolution of the Imaging Features of Osteoid Osteoma Treated with RFA or MRgFUS during a Long-Term Follow-up: A Pictorial Review with Clinical Correlations. Radiol. Med. 2020, 125, 578–584. [Google Scholar] [CrossRef]
- Mimmo, A.; Pegoraro, F.; Rhaiem, R.; Montalti, R.; Donadieu, A.; Tashkandi, A.; Al-Sadairi, A.R.; Kianmanesh, R.; Piardi, T. Microwave Ablation for Colorectal Liver Metastases: A Systematic Review and Pooled Oncological Analyses. Cancers 2022, 14, 1305. [Google Scholar] [CrossRef]
- Saltiel, S.; Bize, P.E.; Goetti, P.; Gallusser, N.; Cherix, S.; Denys, A.; Becce, F.; Tsoumakidou, G. Cryoablation of Extra-Abdominal Desmoid Tumors: A Single-Center Experience with Literature Review. Diagnostics 2020, 10, 556. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Velonakis, G.; Kelekis, A.; Sofocleous, C.T. The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease. Diagnostics 2021, 11, 308. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Kang, C.-H.; Wang, H.-J.; Chen, C.-H.; Luo, H.-L.; Chen, Y.-T.; Cheng, Y.-T.; Chiang, P.-H. Comparison of Robot-Assisted Laparoscopic Partial Nephrectomy with Laparoscopic Cryoablation in the Treatment of Localised Renal Tumours: A Propensity Score-Matched Comparison of Long-Term Outcomes. Diagnostics 2021, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Catalucci, A.; Arrigoni, F.; Sucapane, P.; Cerone, D.; Cerrone, P.; Ricci, A.; Marini, C.; Masciocchi, C. An Experience-Based Review of HIFU in Functional Interventional Neuroradiology: Transcranial MRgFUS Thalamotomy for Treatment of Tremor. Radiol. Med. 2020, 125, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.B.; Silverman, S.G. Imaging in Interventional Oncology. Radiology 2010, 257, 624–640. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, W.; Xu, W.; Yu, Y. Asherman Syndrome in Adenomyosis Treated with Uterine Artery Embolization: Incidence Predictive Factors. Radiol. Med. 2020, 125, 437–443. [Google Scholar] [CrossRef]
- Ohashi, Y.; Takashima, H.; Ohmori, G.; Harada, K.; Chiba, A.; Numasawa, K.; Imai, T.; Hayasaka, S.; Itoh, A. Efficacy of Non-Rigid Registration Technique for Misregistration in 3D-CTA Fusion Imaging. Radiol. Med. 2020, 125, 618–624. [Google Scholar] [CrossRef]
- Mitani, H.; Naito, A.; Chosa, K.; Kodama, H.; Sumida, M.; Moriya, T.; Awai, K. Safety Margin for CT- and US-Guided Radiofrequency Ablation after TACE of HCC in the Hepatic Dome. Minim. Invasive Ther. Allied Technol. 2021, 30, 1–8. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Carnevale, A.; Cossu, A.; Coppola, A.; Fumarola, E.M.; Garanzini, E.; Silipigni, S.; Magenta Biasina, A.; Paolucci, A.; Giganti, M.; et al. Percutaneous Cervical Discectomy: Retrospective Comparison of Two Different Techniques. Radiol. Med. 2020, 125, 569–577. [Google Scholar] [CrossRef]
- Sabatino, V.; Russo, U.; D’Amuri, F.; Bevilacqua, A.; Pagnini, F.; Milanese, G.; Gentili, F.; Nizzoli, R.; Tiseo, M.; Pedrazzi, G.; et al. Pneumothorax and Pulmonary Hemorrhage after CT-Guided Lung Biopsy: Incidence, Clinical Significance and Correlation. Radiol. Med. 2021, 126, 170–177. [Google Scholar] [CrossRef]
- Venturini, M.; Angeli, E.; Maffi, P.; Losio, C.; Pozzi, P.; Paties, C.; Cellina, M.; De Cobelli, F.; Fiorina, P.; Secchi, A.; et al. Liver Focal Fatty Changes at Ultrasound after Islet Transplantation: An Early Sign of Altered Graft Function? Diabet. Med. 2010, 27, 960–964. [Google Scholar] [CrossRef]
- Çildağ, M.B.; Gök, M.; Abdullayev, O. Pre-Procedural Shear Wave Elastography on Prediction of Hemorrhage after Percutaneous Real-Time Ultrasound-Guided Renal Biopsy. Radiol. Med. 2020, 125, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; McLeod, G.; Huang, Z.; Zhu, X.; Jiao, Y.; Li, X.; Shen, Z.; Cui, Y. Localization Accuracy of Ultrasound-Actuated Needle with Color Doppler Imaging. Diagnostics 2020, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, S.; Numata, K.; Ogushi, K.; Chuma, M.; Tanaka, R.; Chiba, S.; Otani, M.; Inayama, Y.; Nakano, M.; Maeda, S. Liver Injury and Use of Contrast-Enhanced Ultrasound for Evaluating Intrahepatic Recurrence in a Case of TACE-Refractory Hepatocellular Carcinoma Receiving Atezolizumab-Bevacizumab Combination Therapy: A Case Report. Diagnostics 2021, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Ing, E. Temporal Artery Biopsy versus Imaging in Patients with Cranial Giant Cell Arteritis. Radiol. Med. 2020, 125, 902–903. [Google Scholar] [CrossRef] [PubMed]
- Perera Molligoda Arachchige, A.S. What Must Be Done in Case of a Dense Collection? Radiol. Med. 2021, 126, 1657–1658. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.D.; Esola, C.C.; Memel, D.S.; Ghiatas, A.A.; Chintapalli, K.N.; Paulson, E.K.; Nelson, R.C.; Ferris, J.V.; Baron, R.L. Sonography: The Undiscovered Jewel of Interventional Radiology. RadioGraphics 1996, 16, 1271–1288. [Google Scholar] [CrossRef] [Green Version]
- Meo, D.; Falsaperla, D.; Modica, A.; Calcagno, M.C.; Libra, F.; Desiderio, C.; Palmucci, S.; Foti, P.V.; Musumeci, A.G.; Basile, A. Proximal and Distal Radial Artery Approaches for Endovascular Percutaneous Procedures: Anatomical Suitability by Ultrasound Evaluation. Radiol. Med. 2020, 126, 630. [Google Scholar] [CrossRef]
- Coppola, M.; Giurazza, F.; Corvino, F.; Pane, F.; Silvestre, M.; Niola, R. Severe Metrorrhagia in Patients with Advanced Gynecologic Cancer: Endovascular Treatment Benefits in Acute and Chronic Setting. Radiol. Med. 2021, 126, 277–282. [Google Scholar] [CrossRef]
- Huang, D.Y.; Yusuf, G.T.; Daneshi, M.; Husainy, M.A.; Ramnarine, R.; Sellars, M.E.K.; Sidhu, P.S. Contrast-Enhanced US–Guided Interventions: Improving Success Rate and Avoiding Complications Using US Contrast Agents. RadioGraphics 2017, 37, 652–664. [Google Scholar] [CrossRef] [Green Version]
- Iijima, M.; Arisaka, T.; Yamamiya, A.; Tominaga, K.; Nagashima, K.; Kanamori, A.; Masuyama, S.; Majima, Y.; Goda, K.; Ishida, K.; et al. Feasibility and Safety of Transjugular Liver Biopsy for Japanese Patients with Chronic Liver Diseases. Diagnostics 2021, 11, 131. [Google Scholar] [CrossRef]
- Carriero, S.; Della Pepa, G.; Monfardini, L.; Vitale, R.; Rossi, D.; Masperi, A.; Mauri, G. Role of Fusion Imaging in Image-Guided Thermal Ablations. Diagnostics 2021, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Argalia, G.; Tarantino, G.; Ventura, C.; Campioni, D.; Tagliati, C.; Guardati, P.; Kostandini, A.; Marzioni, M.; Giuseppetti, G.M.; Giovagnoni, A. Shear Wave Elastography and Transient Elastography in HCV Patients after Direct-Acting Antivirals. Radiol. Med. 2021, 126, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Bolboacă, S.D.; Elec, F.I.; Elec, A.D.; Muntean, A.M.; Socaciu, M.A.; Iacob, G.; Zaro, R.; Andrieș, A.-I.; Bădulescu, R.M.; Ignat, R.M.; et al. Shear-Wave Elastography Variability Analysis and Relation with Kidney Allograft Dysfunction: A Single-Center Study. Diagnostics 2020, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Minami, Y.; Kudo, M. Ultrasound Fusion Imaging Technologies for Guidance in Ablation Therapy for Liver Cancer. J. Med. Ultrason. 2020, 47, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.S.; Greif, V.; Crispin, A.; Burgard, C.; Forbrig, R.; Liebig, T.; Trumm, C.G.; Stahl, R. CT-Guided Drainage of Fluid Collections Following Liver Resection: Technical and Clinical Outcome of 143 Patients during a 14-Year Period. Diagnostics 2021, 11, 826. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-Guided Percutaneous Drainage of Abdominopelvic Collections: A Pictorial Essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Sánchez, Y.; Anvari, A.; Samir, A.E.; Arellano, R.S.; Prabhakar, A.M.; Uppot, R.N. Navigational Guidance and Ablation Planning Tools for Interventional Radiology. Curr. Probl. Diagn. Radiol. 2017, 46, 225–233. [Google Scholar] [CrossRef]
- Fuhrmann, I.; Probst, U.; Wiggermann, P.; Beyer, L. Navigation Systems for Treatment Planning and Execution of Percutaneous Irreversible Electroporation. Technol. Cancer Res. Treat. 2018, 17, 153303381879179. [Google Scholar] [CrossRef]
- IJzerman, M.J.; de Boer, J.; Azad, A.; Degeling, K.; Geoghegan, J.; Hewitt, C.; Hollande, F.; Lee, B.; To, Y.H.; Tothill, R.W.; et al. Towards Routine Implementation of Liquid Biopsies in Cancer Management: It Is Always Too Early, until Suddenly It Is Too Late. Diagnostics 2021, 11, 103. [Google Scholar] [CrossRef]
- Abi-Jaoudeh, N.; Kobeiter, H.; Xu, S.; Wood, B.J. Image Fusion During Vascular and Nonvascular Image-Guided Procedures. Tech. Vasc. Interv. Radiol. 2013, 16, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Mauri, G.; Cova, L.; De Beni, S.; Ierace, T.; Tondolo, T.; Cerri, A.; Goldberg, S.N.; Solbiati, L. Real-Time US-CT/MRI Image Fusion for Guidance of Thermal Ablation of Liver Tumors Undetectable with US: Results in 295 Cases. Cardiovasc. Interv. Radiol. 2015, 38, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.R.; Vaidya, S.S.; Su, D.K.; Moe, K.S.; Kim, L.J.; Sekhar, L.N.; Hallam, D.K.; Ghodke, B.V. The “Triple-Overlay” Technique for Percutaneous Diagnosis and Treatment of Lesions of the Head and Neck: Combined Three-Dimensional Guidance with Magnetic Resonance Imaging, Cone-Beam Computed Tomography, and Fluoroscopy. World Neurosurg. 2013, 79, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hein, L.; Tekbas, A.; Seitel, A.; Pianka, F.; Müller, S.A.; Satzl, S.; Schawo, S.; Radeleff, B.; Tetzlaff, R.; Franz, A.M.; et al. In Vivo Accuracy Assessment of a Needle-Based Navigation System for CT-Guided Radiofrequency Ablation of the Liver. Med. Phys. 2008, 35, 5385–5396. [Google Scholar] [CrossRef]
- Altintas, G.; Akduman, I.; Janjic, A.; Yilmaz, T. A Novel Approach on Microwave Hyperthermia. Diagnostics 2021, 11, 493. [Google Scholar] [CrossRef]
- Engstrand, J.; Toporek, G.; Harbut, P.; Jonas, E.; Nilsson, H.; Freedman, J. Stereotactic CT-Guided Percutaneous Microwave Ablation of Liver Tumors With the Use of High-Frequency Jet Ventilation: An Accuracy and Procedural Safety Study. Am. J. Roentgenol. 2017, 208, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penzkofer, T.; Bruners, P.; Isfort, P.; Schoth, F.; Günther, R.W.; Schmitz-Rode, T.; Mahnken, A.H. Free-Hand CT-Based Electromagnetically Guided Interventions: Accuracy, Efficiency and Dose Usage. Minim. Invasive Ther. Allied Technol. 2011, 20, 226–233. [Google Scholar] [CrossRef]
- Schaible, J.; Lürken, L.; Wiggermann, P.; Verloh, N.; Einspieler, I.; Zeman, F.; Schreyer, A.G.; Bale, R.; Stroszczynski, C.; Beyer, L. Primary Efficacy of Percutaneous Microwave Ablation of Malignant Liver Tumors: Comparison of Stereotactic and Conventional Manual Guidance. Sci. Rep. 2020, 10, 18835. [Google Scholar] [CrossRef]
- Ringe, K.I.; Pöhler, G.H.; Rabeh, H.; Wacker, F. Electromagnetic Navigation System-Guided Microwave Ablation of Hepatic Tumors: A Matched Cohort Study. Cardiovasc. Interv. Radiol. 2021, 44, 500–506. [Google Scholar] [CrossRef]
- Heerink, W.J.; Ruiter, S.J.S.; Pennings, J.P.; Lansdorp, B.; Vliegenthart, R.; Oudkerk, M.; de Jong, K.P. Robotic versus Freehand Needle Positioning in CT-Guided Ablation of Liver Tumors: A Randomized Controlled Trial. Radiology 2019, 290, 826–832. [Google Scholar] [CrossRef]
- Inaba, Y.; Hitachi, S.; Watanuki, M.; Chida, K. Radiation Eye Dose for Physicians in CT Fluoroscopy-Guided Biopsy. Tomography 2022, 8, 438–446. [Google Scholar] [CrossRef]
- Braak, S.J.; van Strijen, M.J.L.; van Es, H.W.; Nievelstein, R.A.J.; van Heesewijk, J.P.M. Effective Dose during Needle Interventions: Cone-Beam CT Guidance Compared with Conventional CT Guidance. J. Vasc. Interv. Radiol. 2011, 22, 455–461. [Google Scholar] [CrossRef]
- Faiella, E.; Messina, L.; Castiello, G.; Bernetti, C.; Pacella, G.; Altomare, C.; Andresciani, F.; Sarli, M.; Longo, F.; Crucitti, P.; et al. Augmented Reality 3D Navigation System for Percutaneous CT-Guided Pulmonary Ground-Glass Opacity Biopsies: A Comparison with the Standard CT-Guided Technique. J. Thorac. Dis. 2022, 14, 247–256. [Google Scholar] [CrossRef]
- Narsule, C.K.; dos Santos, R.S.; Gupta, A.; Ebright, M.I.; Rivas, R.; Daly, B.D.T.; Fernando, H.C. The Efficacy of Electromagnetic Navigation to Assist with Computed Tomography–Guided Percutaneous Thermal Ablation of Lung Tumors. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2012, 7, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Asvadi, N.H.; Anvari, A.; Uppot, R.N.; Thabet, A.; Zhu, A.X.; Arellano, R.S. CT-Guided Percutaneous Microwave Ablation of Tumors in the Hepatic Dome: Assessment of Efficacy and Safety. J. Vasc. Interv. Radiol. 2016, 27, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Yamakado, K.; Nakatsuka, A.; Yamada, T.; Uraki, J.; Kashima, M.; Yamanaka, T.; Shiraki, K.; Takei, Y.; Takeda, K. Computed Tomography Fluoroscopy-Guided Radiofrequency Ablation Following Intra-Arterial Iodized-Oil Injection for Hepatocellular Carcinomas Invisible on Ultrasonographic Images. Int. J. Clin. Oncol. 2013, 18, 46–53. [Google Scholar] [CrossRef]
- Cozzi, D.; Moroni, C.; Cavigli, E.; Bindi, A.; Caviglioli, C.; Nazerian, P.; Vanni, S.; Miele, V.; Bartolucci, M. Prognostic Value of CT Pulmonary Angiography Parameters in Acute Pulmonary Embolism. Radiol. Med. 2021, 126, 1030–1036. [Google Scholar] [CrossRef]
- Taiji, R.; Lin, E.Y.; Lin, Y.-M.; Yevich, S.; Avritscher, R.; Sheth, R.A.; Ruiz, J.R.; Jones, A.K.; Chintalapani, G.; Nishiofuku, H.; et al. Combined Angio-CT Systems: A Roadmap Tool for Precision Therapy in Interventional Oncology. Radiol. Imaging Cancer 2021, 3, e210039. [Google Scholar] [CrossRef]
- Hansen, K.L.; Carlsen, J.F. New Trends in Vascular Imaging. Diagnostics 2021, 11, 112. [Google Scholar] [CrossRef]
- Tsochatzis, A.; Mazioti, A.; Iliadis, G.; Velonakis, G.; Efthymiou, E.; Kelekis, A.; Kelekis, N.; Filippiadis, D. Percutaneous Microwave Ablation of Liver Lesions: Differences on the Sphericity Index of the Ablation Zone between Cirrhotic and Healthy Liver Parenchyma. Diagnostics 2021, 11, 655. [Google Scholar] [CrossRef]
- Shin, N.; Choi, J.A.; Choi, J.M.; Cho, E.-S.; Kim, J.H.; Chung, J.-J.; Yu, J.-S. Sclerotic Changes of Cavernous Hemangioma in the Cirrhotic Liver: Long-Term Follow-up Using Dynamic Contrast-Enhanced Computed Tomography. Radiol. Med. 2020, 125, 1225–1232. [Google Scholar] [CrossRef]
- Alsafi, A.; Jackson, J.E. Re: Safety and Efficacy of Transcatheter Embolization in Patients with Massive Haemoptysis Due to Intercostal Pulmonary Venous Shunts. Radiol. Med. 2021, 126, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Lionberg, A.; Nijhawan, K.; Navuluri, R.; Zangan, S.; Van Ha, T.; Funaki, B.; Ahmed, O. Hybrid Angiography-CT for Transarterial Radioembolization: A Pictorial Essay. Abdom. Radiol. 2021, 46, 2850–2854. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, F.; Li, X.; Guan, Y.; Wang, M. Angio-CT-Guided Transarterial Chemoembolization Immediately in Combination with Radiofrequency Ablation for Large Hepatocellular Carcinoma. Acad. Radiol. 2019, 26, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Schembri, V.; Piron, L.; Le Roy, J.; Hermida, M.; Lonjon, J.; Escal, L.; Pierredon, M.-A.; Belgour, A.; Cassinotto, C.; Guiu, B. Percutaneous Ablation of Obscure Hypovascular Liver Tumours in Challenging Locations Using Arterial CT-Portography Guidance. Diagn. Interv. Imaging 2020, 101, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Piron, L.; Le Roy, J.; Cassinotto, C.; Delicque, J.; Belgour, A.; Allimant, C.; Beregi, J.-P.; Greffier, J.; Molinari, N.; Guiu, B. Radiation Exposure During Transarterial Chemoembolization: Angio-CT Versus Cone-Beam CT. Cardiovasc. Interv. Radiol. 2019, 42, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Jones, A.K.; Chintalapani, G.; Jeng, Z.S.; Ensor, J.; Odisio, B.C. Comparative Analysis of Intra-Arterial Cone-Beam Versus Conventional Computed Tomography During Hepatic Arteriography for Transarterial Chemoembolization Planning. Cardiovasc. Interv. Radiol. 2019, 42, 591–600. [Google Scholar] [CrossRef]
- Erinjeri, J.P.; Doustaly, R.; Avignon, G.; Bendet, A.; Petre, E.N.; Ziv, E.; Yarmohammadi, H.; Solomon, S.B. Utilization of Integrated Angiography-CT Interventional Radiology Suites at a Tertiary Cancer Center. BMC Med. Imaging 2020, 20, 114. [Google Scholar] [CrossRef]
- Floridi, C.; Radaelli, A.; Abi-Jaoudeh, N.; Grass, M.; De Lin, M.; Chiaradia, M.; Geschwind, J.-F.; Kobeiter, H.; Squillaci, E.; Maleux, G.; et al. C-Arm Cone-Beam Computed Tomography in Interventional Oncology: Technical Aspects and Clinical Applications. Radiol. Med. 2014, 119, 521–532. [Google Scholar] [CrossRef]
- Tacher, V.; Radaelli, A.; Lin, M.; Geschwind, J.-F. How I Do It: Cone-Beam CT during Transarterial Chemoembolization for Liver Cancer. Radiology 2015, 274, 320–334. [Google Scholar] [CrossRef]
- Lin, M.; Loffroy, R.; Noordhoek, N.; Taguchi, K.; Radaelli, A.; Blijd, J.; Balguid, A.; Geschwind, J.-F. Evaluating Tumors in Transcatheter Arterial Chemoembolization (TACE) Using Dual-Phase Cone-Beam CT. Minim. Invasive Ther. Allied Technol. 2011, 20, 276–281. [Google Scholar] [CrossRef]
- Refai, M.; Andolfi, M.; Barbisan, F.; Roncon, A.; Guiducci, G.M.; Xiumè, F.; Salati, M.; Tiberi, M.; Giovagnoni, A.; Paci, E. Computed Tomography-Guided Microcoil Placement for Localizing Small Pulmonary Nodules before Uniportal Video-Assisted Thoracoscopic Resection. Radiol. Med. 2020, 125, 24–30. [Google Scholar] [CrossRef]
- Shellikeri, S.; Setser, R.M.; Vatsky, S.; Srinivasan, A.; Krishnamurthy, G.; Zhu, X.; Keller, M.S.; Cahill, A.M. Prospective Evaluation of MR Overlay on Real-Time Fluoroscopy for Percutaneous Extremity Biopsies of Bone Lesions Visible on MRI but Not on CT in Children in the Interventional Radiology Suite. Pediatr. Radiol. 2018, 48, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Floridi, C.; Radaelli, A.; Pesapane, F.; Fumarola, E.M.; Lecchi, M.; Agostini, A.; Giovagnoni, A.; Carrafiello, G.; Wood, B. Clinical Impact of Cone Beam Computed Tomography on Iterative Treatment Planning during Ultrasound-Guided Percutaneous Ablation of Liver Malignancies. Med. Oncol. 2017, 34, 113. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, R.L.; Buy, X.; Alberti, N.; Fonck, M.; Grasso, R.F.; Palussière, J. Flat-Panel Cone-Beam Ct-Guided Radiofrequency Ablation of Very Small (≤1.5 cm) Liver Tumors: Technical Note on a Preliminary Experience. Cardiovasc. Interv. Radiol. 2015, 38, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Monfardini, L.; Orsi, F.; Caserta, R.; Sallemi, C.; Della Vigna, P.; Bonomo, G.; Varano, G.; Solbiati, L.; Mauri, G. Ultrasound and Cone Beam CT Fusion for Liver Ablation: Technical Note. Int. J. Hyperth. 2018, 35, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Sutter, O.; Fihri, A.; Ourabia-Belkacem, R.; Sellier, N.; Diallo, A.; Seror, O. Real-Time 3D Virtual Target Fluoroscopic Display for Challenging Hepatocellular Carcinoma Ablations Using Cone Beam CT. Technol. Cancer Res. Treat. 2018, 17, 153303381878963. [Google Scholar] [CrossRef]
- Solbiati, M.; Passera, K.M.; Goldberg, S.N.; Rotilio, A.; Ierace, T.; Pedicini, V.; Poretti, D.; Solbiati, L. A Novel CT to Cone-Beam CT Registration Method Enables Immediate Real-Time Intraprocedural Three-Dimensional Assessment of Ablative Treatments of Liver Malignancies. Cardiovasc. Interv. Radiol. 2018, 41, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Pung, L.; Ahmad, M.; Mueller, K.; Rosenberg, J.; Stave, C.; Hwang, G.L.; Shah, R.; Kothary, N. The Role of Cone-Beam CT in Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2017, 28, 334–341. [Google Scholar] [CrossRef]
- Lee, I.J.; Chung, J.W.; Yin, Y.H.; Kim, H.-C.; Kim, Y.I.; Jae, H.J.; Park, J.H. Cone-Beam Computed Tomography (CBCT) Hepatic Arteriography in Chemoembolization for Hepatocellular Carcinoma: Performance Depicting Tumors and Tumor Feeders. Cardiovasc. Interv. Radiol. 2015, 38, 1218–1230. [Google Scholar] [CrossRef]
- Yu, M.H.; Kim, J.H.; Yoon, J.-H.; Kim, H.-C.; Chung, J.W.; Han, J.K.; Choi, B.-I. Role of C-Arm CT for Transcatheter Arterial Chemoembolization of Hepatocellular Carcinoma: Diagnostic Performance and Predictive Value for Therapeutic Response Compared with Gadoxetic Acid–Enhanced MRI. Am. J. Roentgenol. 2013, 201, 675–683. [Google Scholar] [CrossRef]
- Deschamps, F.; Solomon, S.B.; Thornton, R.H.; Rao, P.; Hakime, A.; Kuoch, V.; de Baere, T. Computed Analysis of Three-Dimensional Cone-Beam Computed Tomography Angiography for Determination of Tumor-Feeding Vessels During Chemoembolization of Liver Tumor: A Pilot Study. Cardiovasc. Interv. Radiol. 2010, 33, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, Y.; Yagyu, Y.; Murakami, T.; Kudo, M. Tracking Navigation Imaging of Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma Using Three-Dimensional Cone-Beam CT Angiography. Liver Cancer 2014, 3, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Iwazawa, J.; Ohue, S.; Hashimoto, N.; Mitani, T. Accuracy of Software-Assisted Detection of Tumour Feeders in Transcatheter Hepatic Chemoembolization Using Three Target Definition Protocols. Clin. Radiol. 2014, 69, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Ikuno, M.; Okumura, K.; Yoshida, M. Ultraselective Transcatheter Arterial Chemoembolization for Small Hepatocellular Carcinoma Guided by Automated Tumor-Feeders Detection Software: Technical Success and Short-Term Tumor Response. Abdom. Imaging 2014, 39, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Ronot, M.; Abdel-Rehim, M.; Hakimé, A.; Kuoch, V.; Roux, M.; Chiaradia, M.; Vilgrain, V.; de Baere, T.; Deschamps, F. Cone-Beam CT Angiography for Determination of Tumor-Feeding Vessels During Chemoembolization of Liver Tumors: Comparison of Conventional and Dedicated-Software Analysis. J. Vasc. Interv. Radiol. 2016, 27, 32–38. [Google Scholar] [CrossRef]
- Sohail, A.H.; Musa, A.; Khan, M.S.; Hashmi, H.R.; Salam, B.; Brathwaite, C.E.M. Hepatocellular Carcinoma with Extrahepatic Blood Supply from Right Renal Artery. J. Surg. Case Reports 2021, 10, 1–3. [Google Scholar] [CrossRef]
- Ridouani, F.; Doustaly, R.; Yarmohammadi, H.; Solomon, S.B.; Gonzalez-Aguirre, A.J. Retrospective Use of Breathing Motion Compensation Technology (MCT) Enhances Vessel Detection Software Performance. Cardiovasc. Interv. Radiol. 2021, 44, 619–624. [Google Scholar] [CrossRef]
- Cui, Z.; Shukla, P.A.; Habibollahi, P.; Park, H.S.; Fischman, A.; Kolber, M.K. A Systematic Review of Automated Feeder Detection Software for Locoregional Treatment of Hepatic Tumors. Diagn. Interv. Imaging 2020, 101, 439–449. [Google Scholar] [CrossRef]
- Derbel, H.; Kobeiter, H.; Pizaine, G.; Ridouani, F.; Luciani, A.; Radaelli, A.; Van der Sterren, W.; Chiaradia, M.; Tacher, V. Accuracy of a Cone-Beam CT Virtual Parenchymal Perfusion Algorithm for Liver Cancer Targeting during Intra-Arterial Therapy. J. Vasc. Interv. Radiol. 2018, 29, 254–261. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, K.A.; Choi, W.; Kwon, Y.; Cho, S.B. Usefulness of Cone-Beam CT-Based Liver Perfusion Mapping for Evaluating the Response of Hepatocellular Carcinoma to Conventional Transarterial Chemoembolization. J. Clin. Med. 2021, 10, 713. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, D.; Si, G.; Han, X.; Zhang, W.; Li, Y.; Zhou, X.; Liu, J.; Li, J.; Liu, Z. Making Timely Remedial Measures after TACE Based on the Results of Cone-Beam CT Liver Perfusion. Int. J. Hyperth. 2021, 38, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Jiao, D.-C.; Si, G.; Zhou, X.; Li, Y.; Liu, J.; Han, X. Clinical Outcomes After Selective Renal Artery Embolization Combined With DynaCT-Guided Microwave Ablation for T1a Renal-Cell Carcinoma: Case Series. Clin. Genitourin. Cancer 2021, 19, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiao, D.; Han, X.; Si, G.; Li, Y.; Liu, J.; Xu, Y.; Zheng, B.; Zhang, X. Transcatheter Arterial Chemoembolization Combined with Simultaneous DynaCT-Guided Microwave Ablation in the Treatment of Small Hepatocellular Carcinoma. Cancer Imaging 2020, 20, 13. [Google Scholar] [CrossRef] [Green Version]
- De Marini, P.; Cazzato, R.L.; Garnon, J.; Shaygi, B.; Koch, G.; Auloge, P.; Tricard, T.; Lang, H.; Gangi, A. Percutaneous MR-Guided Prostate Cancer Cryoablation Technical Updates and Literature Review. BJR Open 2019, 1, 20180043. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Placidi, L.; Teodoli, S.; Boldrini, L.; Greco, F.; Longo, S.; Cellini, F.; Dinapoli, N.; Valentini, V.; De Spirito, M.; et al. On the Accuracy of Bulk Synthetic CT for MR-Guided Online Adaptive Radiotherapy. Radiol. Med. 2020, 125, 157–164. [Google Scholar] [CrossRef]
- Samtani, S.; Burotto, M.; Roman, J.C.; Cortes-Herrera, D.; Walton-Diaz, A. MRI and Targeted Biopsy Essential Tools for an Accurate Diagnosis and Treatment Decision Making in Prostate Cancer. Diagnostics 2021, 11, 1551. [Google Scholar] [CrossRef]
- Assadsangabi, R.; Babaei, R.; Songco, C.; Ivanovic, V.; Bobinski, M.; Chen, Y.J.; Nabavizadeh, S.A. Multimodality Oncologic Evaluation of Superficial Neck and Facial Lymph Nodes. Radiol. Med. 2021, 126, 1074–1084. [Google Scholar] [CrossRef]
- De Marini, P.; Cazzato, R.L.; Garnon, J.; Dalili, D.; Leonard-Lorant, I.; Leclerc, L.; Autrusseau, P.-A.; Auloge, P.; Weiss, J.; Tricard, T.; et al. Safety and Oncologic Efficacy of Percutaneous MRI-Guided Cryoablation of Intraparenchymal Renal Cancers. Diagn. Interv. Imaging 2021, 102, 531–538. [Google Scholar] [CrossRef]
- Cazzato, R.L.; De Marini, P.; Auloge, P.; Leclerc, L.; Tricard, T.; Linder, V.; Jost, M.; Ramamurthy, N.; Lang, H.; Garnon, J.; et al. Diagnostic Accuracy and Safety of Percutaneous MRI-Guided Biopsy of Solid Renal Masses: Single-Center Results after 4.5 Years. Eur. Radiol. 2021, 31, 580–590. [Google Scholar] [CrossRef]
- Petralia, G.; Summers, P.E.; Agostini, A.; Ambrosini, R.; Cianci, R.; Cristel, G.; Calistri, L.; Colagrande, S. Dynamic Contrast-Enhanced MRI in Oncology: How We Do It. Radiol. Med. 2020, 125, 1288–1300. [Google Scholar] [CrossRef]
- Santone, A.; Brunese, M.C.; Donnarumma, F.; Guerriero, P.; Mercaldo, F.; Reginelli, A.; Miele, V.; Giovagnoni, A.; Brunese, L. Radiomic Features for Prostate Cancer Grade Detection through Formal Verification. Radiol. Med. 2021, 126, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Liu, M.; Lu, R.; Wang, L.; Xu, Y.; He, X.; Blanco, R.; Li, C. Magnetic Resonance-Guided Ablation of Liver Tumors: A Systematic Review and Pooled Analysis. J. Cancer Res. Ther. 2020, 16, 1093. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.J.; King, A.J.; Patel, N.; Lockyer, R.; Hayes, M. Image-Guided Cryoablation for Sporadic Renal Cell Carcinoma: Three- and 5-Year Outcomes in 220 Patients with Biopsy-Proven Renal Cell Carcinoma. Radiology 2018, 289, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Takahama, N.; Tozaki, M.; Ohgiya, Y. Current status of MRI-guided vacuum-assisted breast biopsy in Japan. Breast Cancer 2021, 28, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Papalouka, V.; Kilburn-Toppin, F.; Gaskarth, M.; Gilbert, F. MRI-guided breast biopsy: A review of technique, indications, and radiological-pathological correlations. Clin Radiol. 2018, 73, 908.e17–908.e25. [Google Scholar] [CrossRef]
- McGrath, A.L.; Price, E.R.; Eby, P.R.; Rahbar, H. MRI-guided breast interventions. J. Magn. Reson. Imaging 2017, 46, 631–645. [Google Scholar] [CrossRef]
- Lilly, A.J.; Johnson, M.; Kuzmiak, C.M.; Ollila, D.W.; O’Connor, S.M.; Hertel, J.D.; Calhoun, B.C. MRI-guided core needle biopsy of the breast: Radiology-pathology correlation and impact on clinical management. Ann. Diagn. Pathology. 2020, 48, 151563. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bruno, F.; Palumbo, P.; Zugaro, L.; Zoccali, C.; Barile, A.; Masciocchi, C. Magnetic Resonance-Guided Focused Ultrasound Surgery Treatment of Non-Spinal Intra-Articular Osteoblastoma: Feasibility, Safety, and Outcomes in a Single-Center Retrospective Analysis. Int. J. Hyperth. 2019, 36, 767–774. [Google Scholar] [CrossRef]
- Louis Hinshaw, J.; Lubner, M.G.; Ziemlewicz, T.J.; Lee, F.T.; Brace, C.L. Percutaneous Tumor Ablation Tools: Microwave, Radiofrequency, or Cryoablation-What Should You Use and Why? Radiographics 2014, 34, 1344–1362. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, S.; Wang, J.; Pang, Z. Efficacy and Safety of Magnetic Resonance-Guided Focused Ultrasound Surgery (MRgFUS) Ablation in the Management of Abnormal Uterine Bleeding Due to Uterine Leiomyoma or Adenomyosis. Am. J. Transl. Res. 2022, 14, 656–663. [Google Scholar]
- Anzidei, M.; Marincola, B.C.; Bezzi, M.; Brachetti, G.; Nudo, F.; Cortesi, E.; Berloco, P.; Catalano, C.; Napoli, A. Magnetic resonance-guided high-intensity focused ultrasound treatment of locally advanced pancreatic adenocarcinoma: Preliminary experience for pain palliation and local tumor control. Invest Radiol. 2014, 49, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Dababou, S.; Marrocchio, C.; Rosenberg, J.; Bitton, R.; Pauly, K.B.; Napoli, A.; Hwang, J.H.; Ghanouni, P. A meta-analysis of palliative treatment of pancreatic cancer with high intensity focused ultrasound. J. Ther. Ultrasound 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, E.; Coppola, F.; Miele, V.; Bibbolino, C.; Grassi, R. Artificial Intelligence: Who Is Responsible for the Diagnosis? Radiol. Med. 2020, 125, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A Deep Look into Radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.P.; Neri, E. Artificial Intelligence in Radiology—Ethical Considerations. Diagnostics 2020, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Letzen, B.; Wang, C.J.; Chapiro, J. The Role of Artificial Intelligence in Interventional Oncology: A Primer. J. Vasc. Interv. Radiol. 2019, 30, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Zhang, K.; Liu, Y.; Cui, J.; Tao, J.; Wang, Y.; Wang, S. Invasive Ductal Breast Cancer: Preoperative Predict Ki-67 Index Based on Radiomics of ADC Maps. Radiol. Med. 2020, 125, 109–116. [Google Scholar] [CrossRef]
- Reginelli, A.; Nardone, V.; Giacobbe, G.; Belfiore, M.P.; Grassi, R.; Schettino, F.; Del Canto, M.; Grassi, R.; Cappabianca, S. Radiomics as a New Frontier of Imaging for Cancer Prognosis: A Narrative Review. Diagnostics 2021, 11, 1796. [Google Scholar] [CrossRef]
- Seah, J.; Boeken, T.; Sapoval, M.; Goh, G.S. Prime Time for Artificial Intelligence in Interventional Radiology. Cardiovasc. Interv. Radiol. 2022, 45, 283–289. [Google Scholar] [CrossRef]
- Gao, Y.; Song, Y.; Yin, X.; Wu, W.; Zhang, L.; Chen, Y.; Shi, W. Deep Learning-Based Digital Subtraction Angiography Image Generation. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1775–1784. [Google Scholar] [CrossRef]
- Koç, A.; Sezgin, Ö.S.; Kayıpmaz, S. Comparing Different Planimetric Methods on Volumetric Estimations by Using Cone Beam Computed Tomography. Radiol. Med. 2020, 125, 398–405. [Google Scholar] [CrossRef]
- Nielsen, C.A.-B.; Lönn, L.; Konge, L.; Taudorf, M. Simulation-Based Virtual-Reality Patient-Specific Rehearsal Prior to Endovascular Procedures: A Systematic Review. Diagnostics 2020, 10, 500. [Google Scholar] [CrossRef]
- Meek, R.D.; Lungren, M.P.; Gichoya, J.W. Machine Learning for the Interventional Radiologist. Am. J. Roentgenol. 2019, 213, 782–784. [Google Scholar] [CrossRef]
- Compagnone, G.; Padovani, R.; D’Ercole, L.; Orlacchio, A.; Bernardi, G.; D’Avanzo, M.A.; Grande, S.; Palma, A.; Campanella, F.; Rosi, A. Provision of Italian Diagnostic Reference Levels for Diagnostic and Interventional Radiology. Radiol. Med. 2021, 126, 99–105. [Google Scholar] [CrossRef]
- Grasso, R.F.; Andresciani, F.; Altomare, C.; Pacella, G.; Castiello, G.; Carassiti, M.; Quattrocchi, C.C.; Faiella, E.; Beomonte Zobel, B. Lung Thermal Ablation: Comparison between an Augmented Reality Computed Tomography (CT) 3D Navigation System (SIRIO) and Standard CT-Guided Technique. Biology 2021, 10, 646. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floridi, C.; Cellina, M.; Irmici, G.; Bruno, A.; Rossini, N.; Borgheresi, A.; Agostini, A.; Bruno, F.; Arrigoni, F.; Arrichiello, A.; et al. Precision Imaging Guidance in the Era of Precision Oncology: An Update of Imaging Tools for Interventional Procedures. J. Clin. Med. 2022, 11, 4028. https://doi.org/10.3390/jcm11144028
Floridi C, Cellina M, Irmici G, Bruno A, Rossini N, Borgheresi A, Agostini A, Bruno F, Arrigoni F, Arrichiello A, et al. Precision Imaging Guidance in the Era of Precision Oncology: An Update of Imaging Tools for Interventional Procedures. Journal of Clinical Medicine. 2022; 11(14):4028. https://doi.org/10.3390/jcm11144028
Chicago/Turabian StyleFloridi, Chiara, Michaela Cellina, Giovanni Irmici, Alessandra Bruno, Nicolo’ Rossini, Alessandra Borgheresi, Andrea Agostini, Federico Bruno, Francesco Arrigoni, Antonio Arrichiello, and et al. 2022. "Precision Imaging Guidance in the Era of Precision Oncology: An Update of Imaging Tools for Interventional Procedures" Journal of Clinical Medicine 11, no. 14: 4028. https://doi.org/10.3390/jcm11144028
APA StyleFloridi, C., Cellina, M., Irmici, G., Bruno, A., Rossini, N., Borgheresi, A., Agostini, A., Bruno, F., Arrigoni, F., Arrichiello, A., Candelari, R., Barile, A., Carrafiello, G., & Giovagnoni, A. (2022). Precision Imaging Guidance in the Era of Precision Oncology: An Update of Imaging Tools for Interventional Procedures. Journal of Clinical Medicine, 11(14), 4028. https://doi.org/10.3390/jcm11144028