Dynamics of Cognitive Function in Patients with Heart Failure Following Transcatheter Mitral Valve Repair
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Assessment of the Cognitive Function Using the Montreal Cognitive Assessment Test
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural Outcome
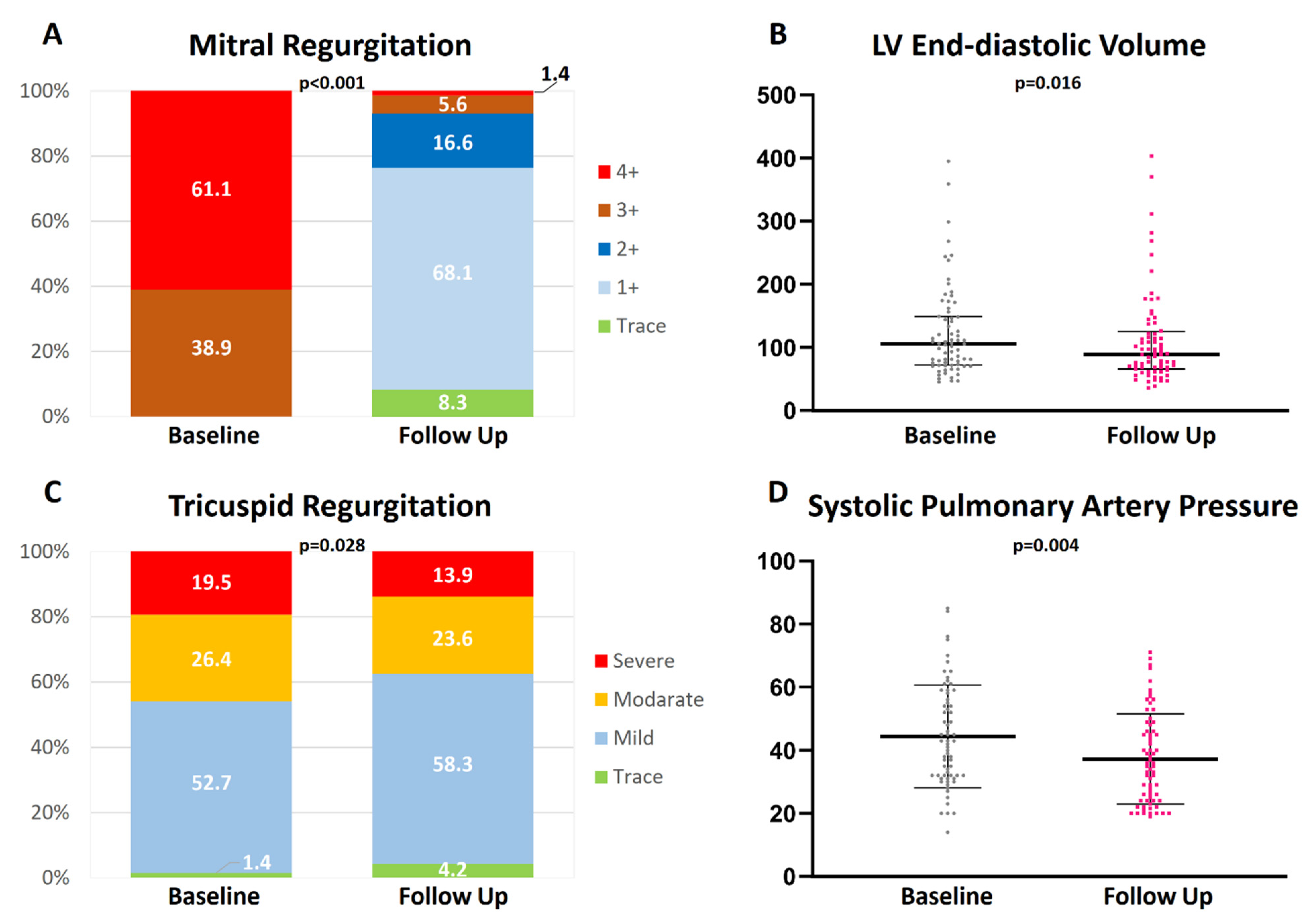
3.3. Echocardiography Results
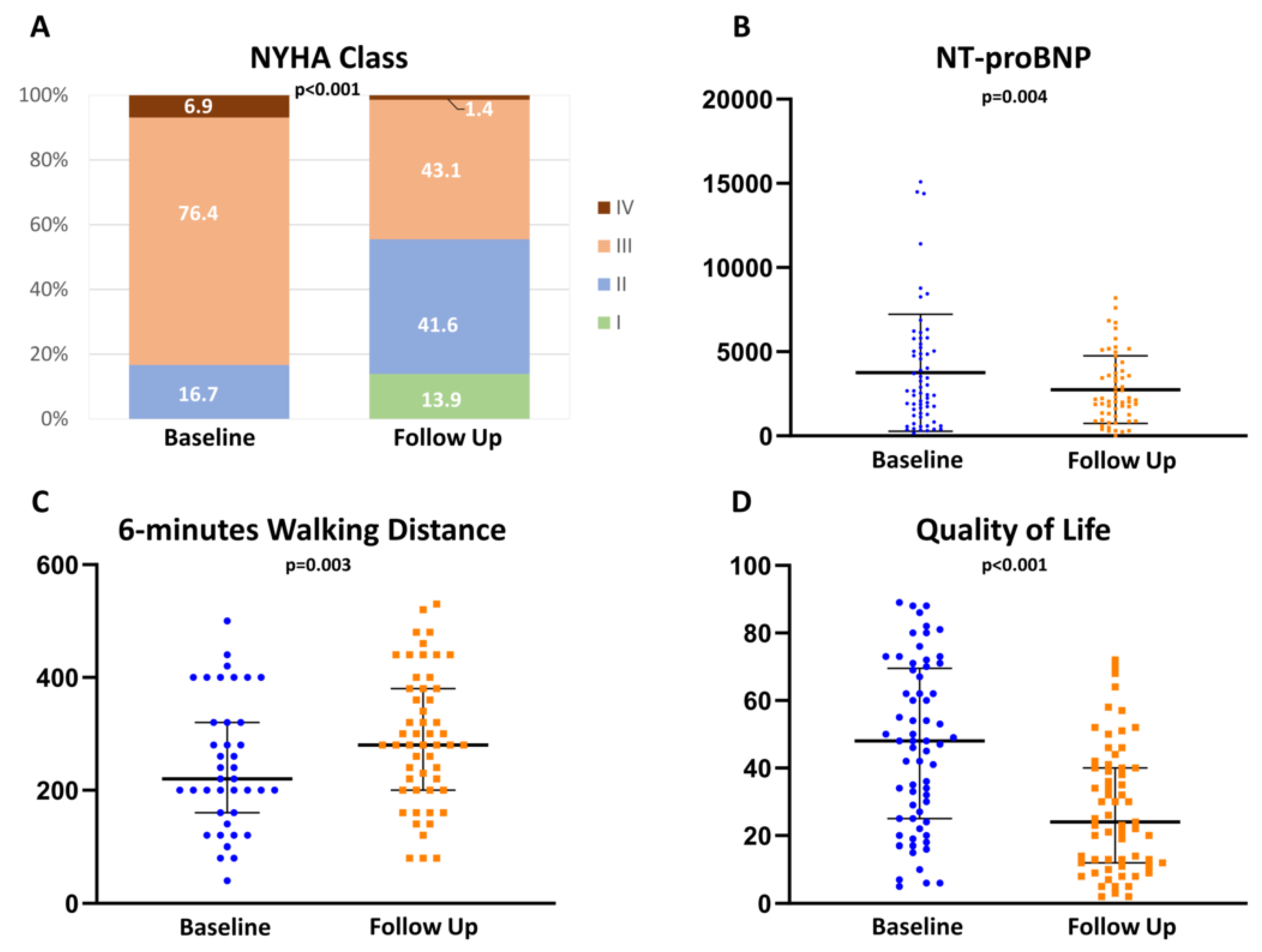
Cognitive Function and Clinical Parameters Improved after TMVR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chehab, O.; Roberts-Thomson, R.; Ng Yin Ling, C.; Marber, M.; Prendergast, B.D.; Rajani, R.; Redwood, S.R. Secondary mitral regurgitation: Pathophysiology, proportionality and prognosis. Heart 2020, 106, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Kar, S.; Mack, M.J.; Lindenfeld, J.; Abraham, W.T.; Asch, F.M.; Weissman, N.J.; Enriquez-Sarano, M.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; et al. Relationship Between Residual Mitral Regurgitation and Clinical and Quality-of-Life Outcomes After Transcatheter and Medical Treatments in Heart Failure: COAPT Trial. Circulation 2021, 144, 426–437. [Google Scholar] [CrossRef]
- Mauri, V.; Besler, C.; Riebisch, M.; Al-Hammadi, O.; Ruf, T.; Gerçek, M.; Horn, P.; Grothusen, C.; Mehr, M.; Becher, M.U.; et al. German Multicenter Experience With a New Leaflet-Based Transcatheter Mitral Valve Repair System for Mitral Regurgitation. JACC Cardiovasc. Interv. 2020, 13, 2769–2778. [Google Scholar] [CrossRef]
- Gerçek, M.; Roder, F.; Rudolph, T.K.; Fortmeier, V.; Zittermann, A.; Rudolph, V.; Friedrichs, K.P. PASCAL mitral valve repair system versus MitraClip: Comparison of transcatheter edge-to-edge strategies in complex primary mitral regurgitation. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 1890–1899. [Google Scholar] [CrossRef]
- Van der Velpen, I.F.; Yancy, C.W.; Sorond, F.A.; Sabayan, B. Impaired Cardiac Function and Cognitive Brain Aging. Can. J. Cardiol. 2017, 33, 1587–1596. [Google Scholar] [CrossRef]
- De La Torre, J.C. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc. Psychiatry Neurol. 2012, 2012, 367516. [Google Scholar] [CrossRef]
- Erkelens, C.D.; van der Wal, H.H.; de Jong, B.M.; Elting, J.W.; Renken, R.; Gerritsen, M.; van Laar, P.J.; van Deursen, V.M.; van der Meer, P.; van Veldhuisen, D.J. Dynamics of cerebral blood flow in patients with mild non-ischaemic heart failure. Eur. J. Heart Fail. 2017, 19, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; O’Gara, P.T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.A.; Satpathy, R.; et al. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 75, 2236–2270. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.H.; Creavin, S.T.; Yip, J.L.; Noel-Storr, A.H.; Brayne, C.; Cullum, S. Montreal Cognitive Assessment for the detection of dementia. Cochrane Database Syst. Rev. 2021, 7, Cd010775. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM 5 Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; p. 947. [Google Scholar]
- Sanford, A.M. Mild Cognitive Impairment. Clin. Geriatr. Med. 2017, 33, 325–337. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Beiser, A.S.; Himali, J.J.; Seshadri, S.; O’Donnell, C.J.; Manning, W.J.; Wolf, P.A.; Au, R.; Benjamin, E.J. Low cardiac index is associated with incident dementia and Alzheimer disease: The Framingham Heart Study. Circulation 2015, 131, 1333–1339. [Google Scholar] [CrossRef] [Green Version]
- Cacciamani, F.; Tandetnik, C.; Gagliardi, G.; Bertin, H.; Habert, M.O.; Hampel, H.; Boukadida, L.; Révillon, M.; Epelbaum, S.; Dubois, B. Low Cognitive Awareness, but Not Complaint, is a Good Marker of Preclinical Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 59, 753–762. [Google Scholar] [CrossRef]
- Toledo, C.; Lucero, C.; Andrade, D.C.; Díaz, H.S.; Schwarz, K.G.; Pereyra, K.V.; Arce-Álvarez, A.; López, N.A.; Martinez, M.; Inestrosa, N.C.; et al. Cognitive impairment in heart failure is associated with altered Wnt signaling in the hippocampus. Aging 2019, 11, 5924–5942. [Google Scholar] [CrossRef]
- Kelley, R.E.; Kelley, B.P. Heart-Brain Relationship in Stroke. Biomedicines 2021, 9, 1835. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsumoto, Y.; Ota, H.; Sugimura, K.; Takahashi, J.; Ito, K.; Miyata, S.; Furukawa, K.; Arai, H.; Fukumoto, Y. Hippocampal blood flow abnormality associated with depressive symptoms and cognitive impairment in patients with chronic heart failure. Circ. J. 2016, 80, 1773–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browndyke, J.N.; Berger, M.; Harshbarger, T.B.; Smith, P.J.; White, W.; Bisanar, T.L.; Alexander, J.H.; Gaca, J.G.; Welsh-Bohmer, K.; Newman, M.F.; et al. Resting-State Functional Connectivity and Cognition After Major Cardiac Surgery in Older Adults without Preoperative Cognitive Impairment: Preliminary Findings. J. Am. Geriatr. Soc. 2017, 65, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Bown, C.W.; Do, R.; Khan, O.A.; Liu, D.; Cambronero, F.E.; Moore, E.E.; Osborn, K.E.; Gupta, D.K.; Pechman, K.R.; Mendes, L.A.; et al. Lower Cardiac Output Relates to Longitudinal Cognitive Decline in Aging Adults. Front. Psychol. 2020, 11, 569355. [Google Scholar] [CrossRef] [PubMed]
- Nikendei, C.; Schäfer, H.; Weisbrod, M.; Huber, J.; Geis, N.; Katus, H.A.; Bekeredjian, R.; Herzog, W.; Pleger, S.T.; Schultz, J.H. The Effects of Mitral Valve Repair on Memory Performance, Executive Function, and Psychological Measures in Patients With Heart Failure. Psychosom. Med. 2016, 78, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Terhoeven, V.; Nikendei, C.; Cranz, A.; Weisbrod, M.; Geis, N.; Raake, P.W.; Katus, H.A.; Herzog, W.; Friederich, H.C.; Schultz, J.H.; et al. Effects of MitraClip on cognitive and psychological function in heart failure patients: The sicker the better. Eur. J. Med. Res. 2019, 24, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, S.A.; Heaps, J.M.; Bolzenius, J.D.; Salminen, L.E.; Baker, L.M.; Scott, S.E.; Paul, R.H. Longitudinal Change in Performance on the Montreal Cognitive Assessment in Older Adults. Clin. Neuropsychol. 2015, 29, 824–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | |
|---|---|
| Age | 81.0 [76.0; 84.5] |
| Female | 39.7% (29) |
| Body mass index kg/m2 | 26.2 [23.0; 29.2] |
| EuroScore II (%) | 4.4 [2.9; 7.7] |
| STS Score for mitral valve repair | 2.5 [1.5; 3.9] |
| Atrial fibrillation | 63.0% (46) |
| Diabetes mellitus | 12.3% (9) |
| Chronic obstructive pulmonary disease | 16.7% (12) |
| Coronary artery disease | 46.6% (34) |
| History of myocardial infarction | 13.7% (10) |
| History of cardiac surgery | 24.7% (18) |
| Stroke | 27.4% (20) |
| Dialysis | 2.7% (2) |
| NTpro-BNP [pg/mL] * | 2680.0 [1520.0; 5292.5] |
| Imaging Parameters | Baseline | Follow Up | p-Value |
|---|---|---|---|
| LV ejection fraction [%] | 51.9 ± 15.2 | 49.4 ± 14.0 | 0.51 |
| LV end-diastolic diameter [mm] | 57.0 [50.0; 61.0] | 53.0 [46.5; 60.0] | 0.009 * |
| LV end-diastolic volume [mL] | 104.0 [72.3; 148.8] | 88.0 [66.5; 124.0] | 0.016 * |
| LV end-systolic diameter [mm] | 39.0 [34.0; 50.0] | 37.0 [32.0; 48.8] | 0.82 |
| LV end-systolic volume [mL] | 46.0 [32.0; 79.8] | 39.0 [28.5; 69.0] | 0.40 |
| LA volume [mL] | 121.0 [92.5; 153.0] | 108.0 [78.8; 140.3] | 0.20 |
| LA volume index [mL/m2] | 66.0 [51.5; 82.5] | 59.0 [45.0; 77.0] | 0.12 |
| Mitral regurgitation etiology | |||
| Primary Secondary Mixed | 431% (31) 54.2% (39) 2.8% (2) | ∅ | ∅ |
| Mitral regurgitation grade | |||
| Trace Mild Mild-to-Moderate Moderate-to-Severe Severe | ∅ ∅ ∅ 38.9% (28) 61.1% (44) | 8.3% (6) 68.1% (49) 16.6% (12) 5.6% (4) 1.4% (1) | <0.001 + |
| MR vena contracta width [mm] | 8.0 [6.0; 11.0] | 3.0 [2.0; 4.0] | <0.001 * |
| MR EROA [cm2] | 0.4 [0.2; 0.7] | 0.1 [0.1; 0.1] | <0.001 * |
| MR regurgitant volume [mL] | 56.0 [35.5; 89.5] | 13.0 [7.0; 20.0] | <0.001 * |
| Mean transmitral gradient [mmHg] | 2.0 [1.0; 3.0] | 3.0 [2.0; 4.0] | <0.001 * |
| Degree of tricuspid regurgitation | |||
| Trace Mild Moderate Severe | 1.4% (1) 52.7% (38) 26.4% (19) 19.5% (14) | 4.2% (3) 58.3% (40) 23.6% (17) 13.9% (10) | 0.003 + |
| Systolic pulmonary artery pressure [mmHg] | 43.5 ± 16.9 | 36.6 ± 15.7 | 0.004 # |
| Parameter | Baseline | Follow Up | p-Value |
|---|---|---|---|
| MoCA result | 22.0 [19.0; 24.5] | 24.0 [22.0; 26.0] | <0.001 * |
| MoCA standard deviation results | −1.2 ± 1.0 | −0.4 ± 1.1 | <0.001 # |
| Executive Function | 3.0 [2.0; 4.0] | 4.0 [3.0; 4.5] | <0.001 * |
| Naming | 3.0 [3.0; 3.0] | 3.0 [3.0; 3.0] | 0.52 |
| Memory | 2.0 [1.0; 3.0] | 3.0 [2.0;4.0] | <0.001 * |
| Attention | 5.0 [4.0; 6.0] | 6.0 [5.0; 6.0] | <0.001 * |
| Language | 2.0 [1.0; 2.0] | 2.0 [1.0; 2.0] | 0.33 |
| Abstraction | 1.0 [1.0; 2.0] | 2 [2.0; 2.0] | <0.001 * |
| Orientation | 6.0 [6.0; 6.0] | 6.0 [6.0; 6.0] | 0.39 |
| 6-min-walking distance | 220.0 [160.0; 320.0] | 280.0 [200.0; 380.0] | 0.003 * |
| Quality of Life (MLHFQ) | 47.5 [25; 69.3] | 24.0 [12.0; 40.0] | <0.001 * |
| NTproBNP | 2680.0 [1520.0; 5292.5] | 2150.0 [1232.5; 3847.5] | 0.004 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerçek, M.; Irimie, A.A.; Gerçek, M.; Fox, H.; Fortmeier, V.; Rudolph, T.K.; Rudolph, V.; Friedrichs, K.P. Dynamics of Cognitive Function in Patients with Heart Failure Following Transcatheter Mitral Valve Repair. J. Clin. Med. 2022, 11, 3990. https://doi.org/10.3390/jcm11143990
Gerçek M, Irimie AA, Gerçek M, Fox H, Fortmeier V, Rudolph TK, Rudolph V, Friedrichs KP. Dynamics of Cognitive Function in Patients with Heart Failure Following Transcatheter Mitral Valve Repair. Journal of Clinical Medicine. 2022; 11(14):3990. https://doi.org/10.3390/jcm11143990
Chicago/Turabian StyleGerçek, Muhammed, Anca A. Irimie, Mustafa Gerçek, Henrik Fox, Vera Fortmeier, Tanja K. Rudolph, Volker Rudolph, and Kai P. Friedrichs. 2022. "Dynamics of Cognitive Function in Patients with Heart Failure Following Transcatheter Mitral Valve Repair" Journal of Clinical Medicine 11, no. 14: 3990. https://doi.org/10.3390/jcm11143990
APA StyleGerçek, M., Irimie, A. A., Gerçek, M., Fox, H., Fortmeier, V., Rudolph, T. K., Rudolph, V., & Friedrichs, K. P. (2022). Dynamics of Cognitive Function in Patients with Heart Failure Following Transcatheter Mitral Valve Repair. Journal of Clinical Medicine, 11(14), 3990. https://doi.org/10.3390/jcm11143990






