Proximal Median Nerve Compression in the Differential Diagnosis of Carpal Tunnel Syndrome
Abstract
1. Introduction
2. Anatomy and Sites of Median Nerve Compression
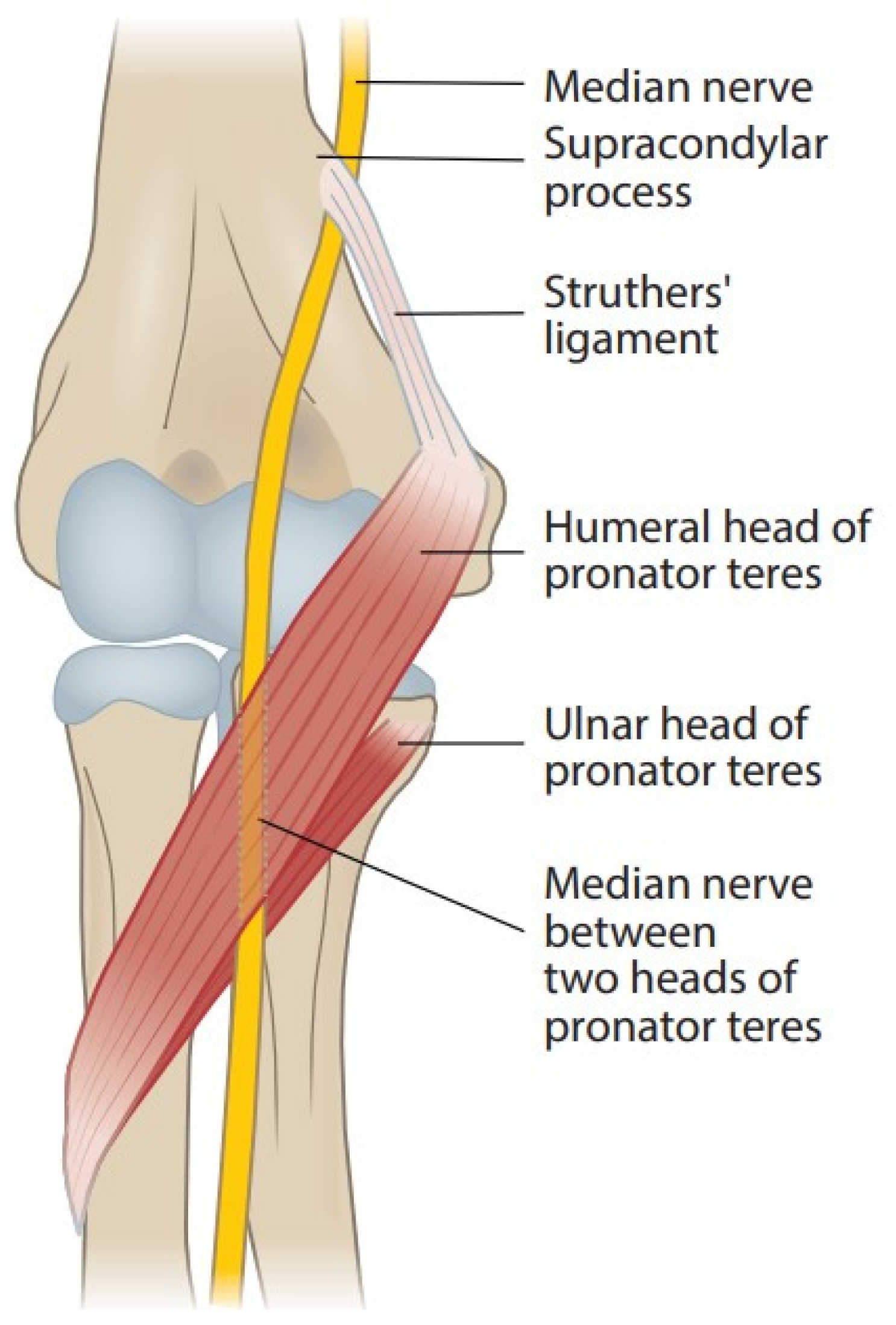
2.1. Supracondylar Process, Ligament of Struthers, and Supracondylar Process Syndrome
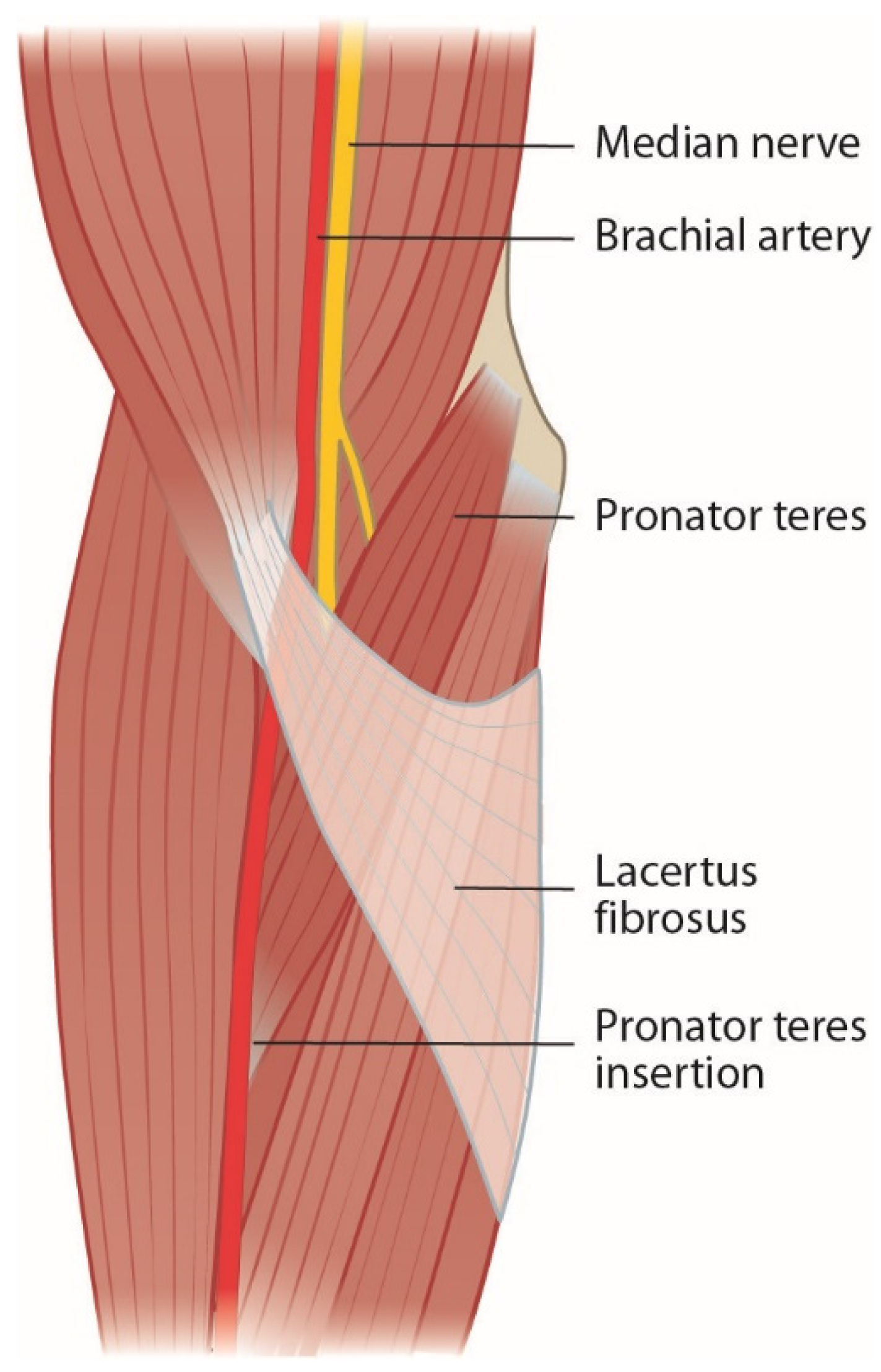
2.2. Lacertus Fibrosus and Lacertus Syndrome
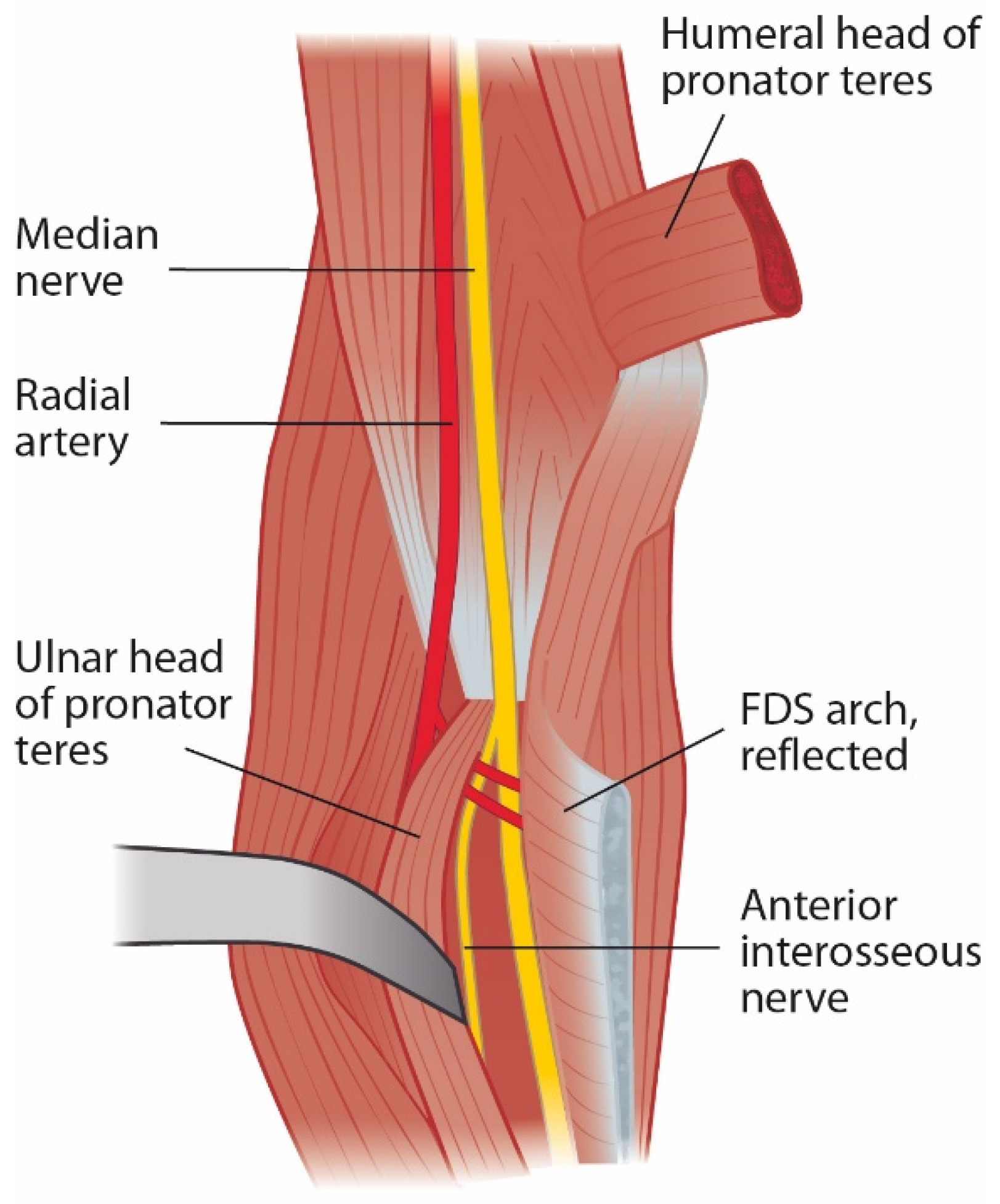
2.3. Pronator Teres Muscle and Pronator Syndrome
2.4. Flexor Digitorum Superficialis Arch and Superficialis/Pronator Syndrome
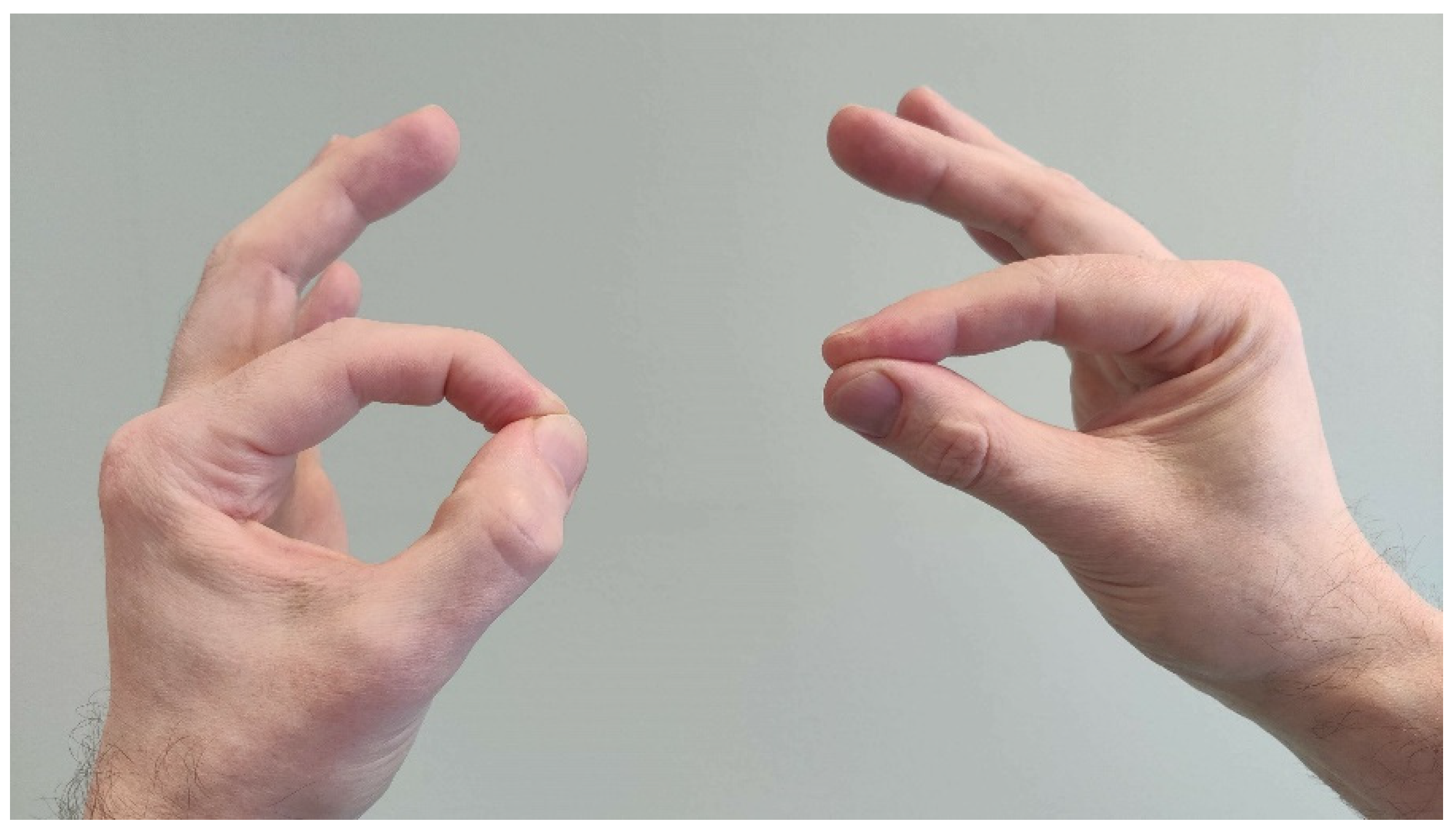
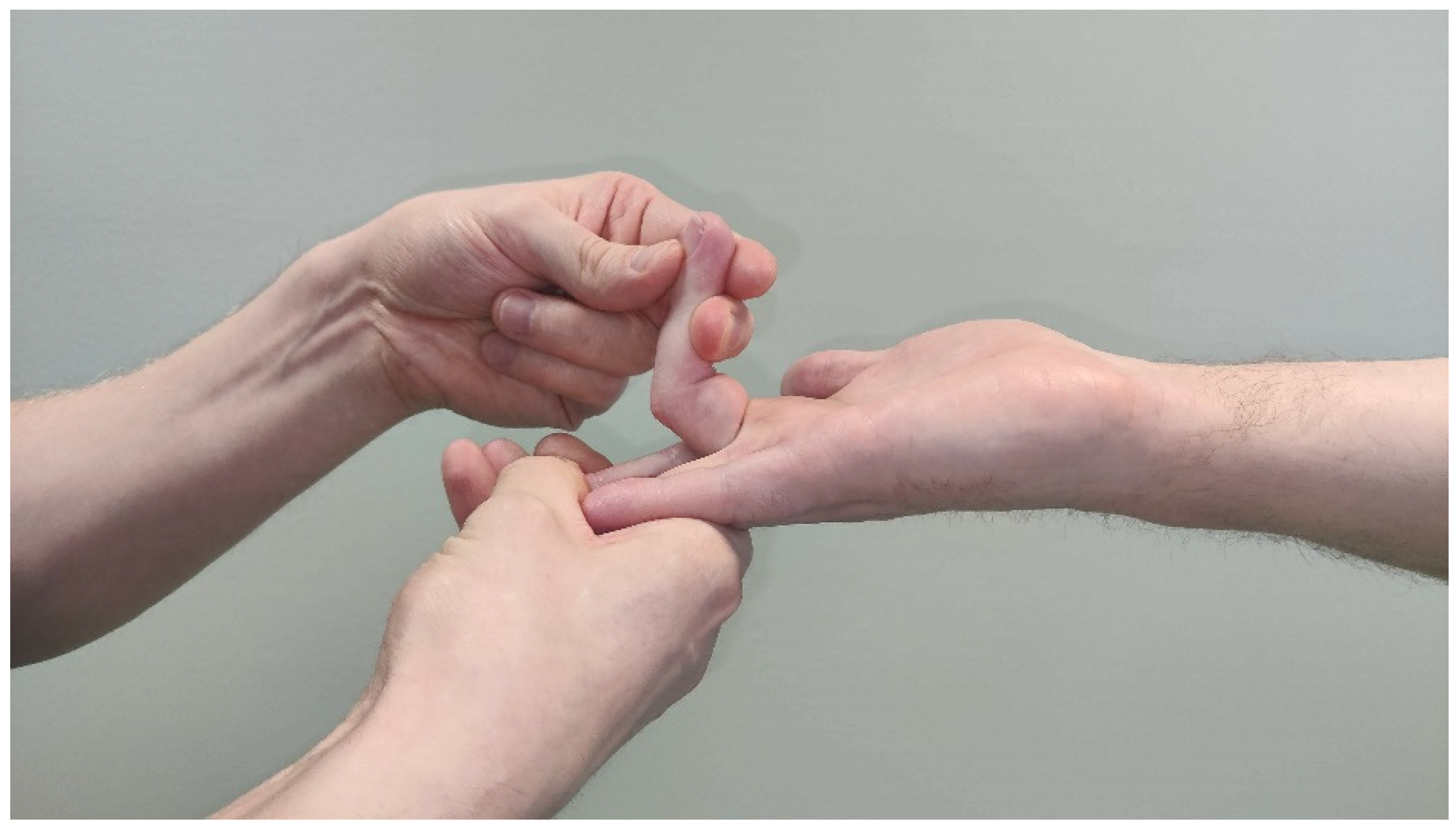
2.5. Anterior Interosseus Nerve and AIN Syndrome
2.6. Other Compressive Structures
2.7. Distal Course of the Median Nerve
2.8. Other Conflicting Factors
2.8.1. Martin–Gruber Anastomosis
2.8.2. Nerve Lesions and Renervation
2.8.3. Double Crush Syndrome
2.8.4. Neuralgic Amyotrophy
3. Clinical Presentation
3.1. Symptoms
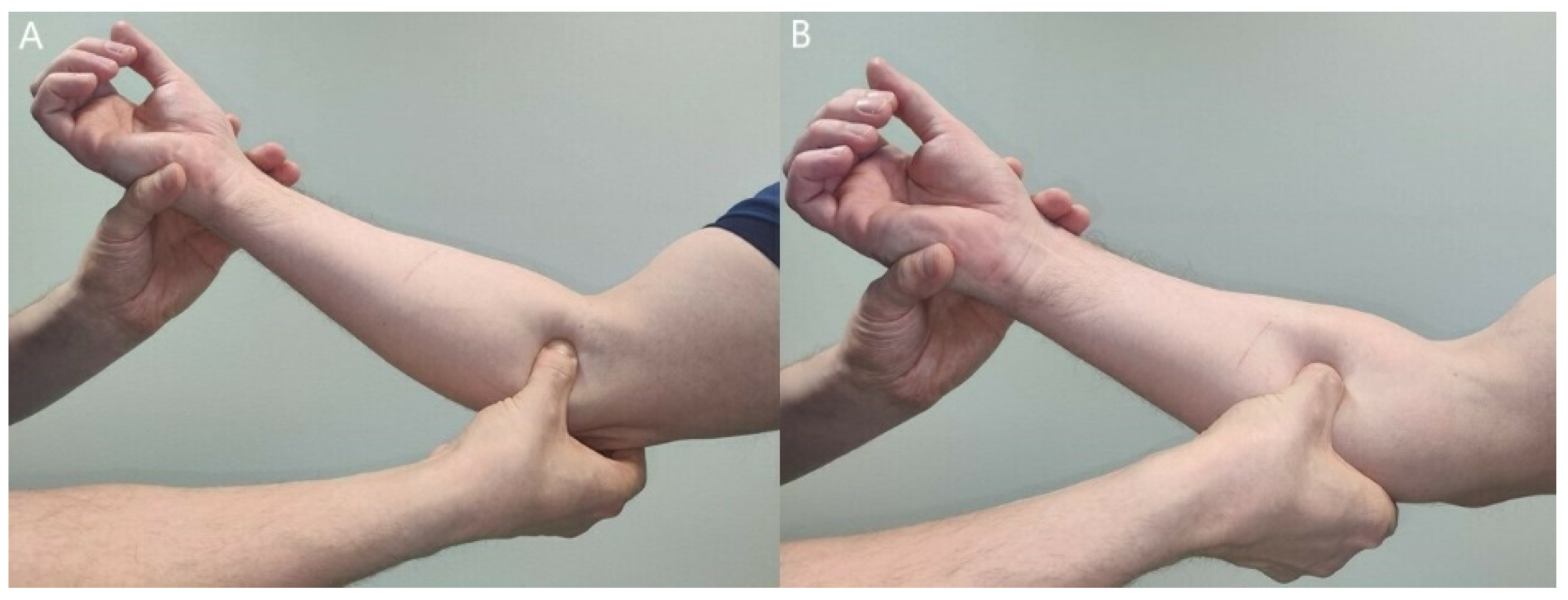
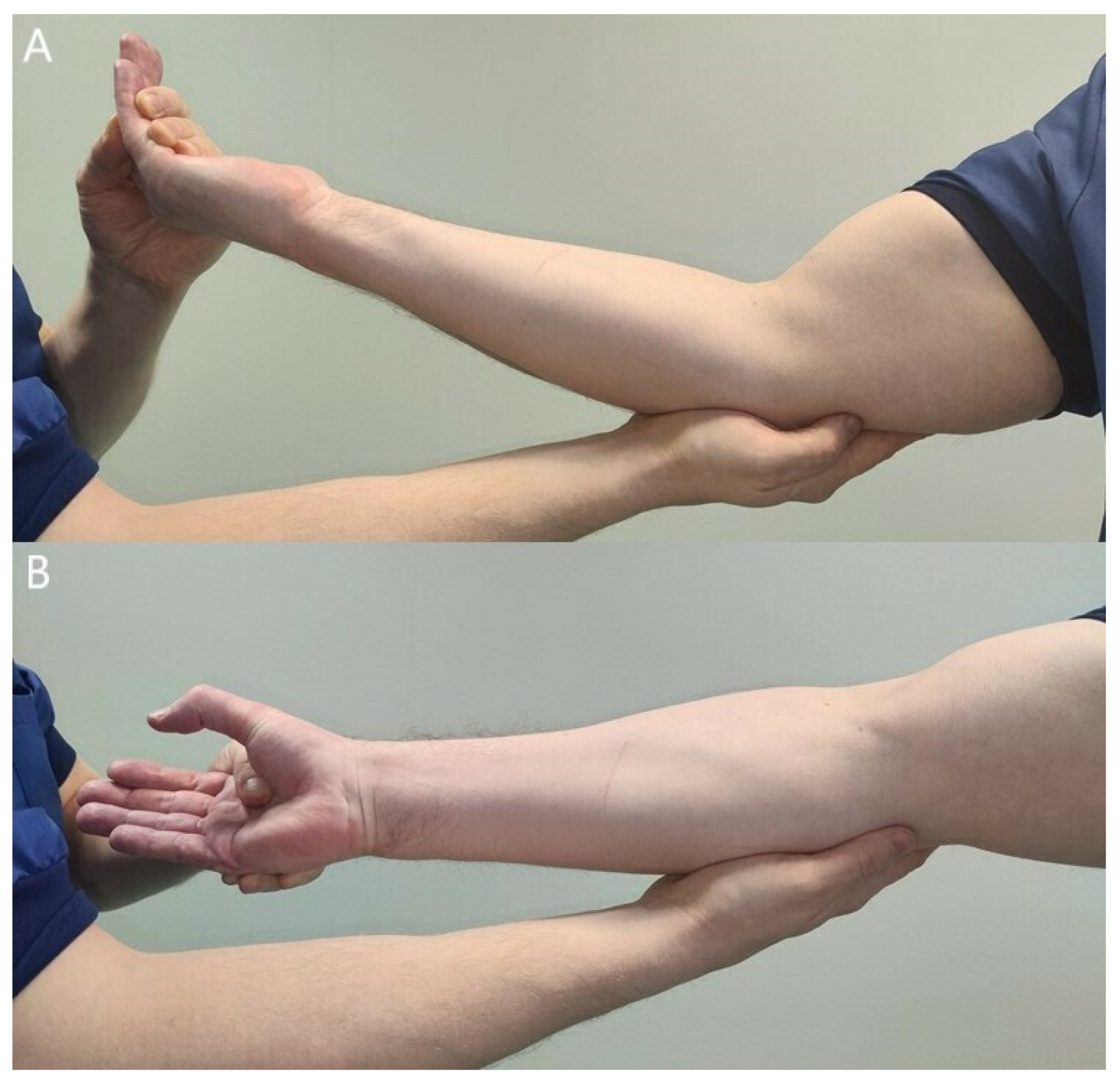
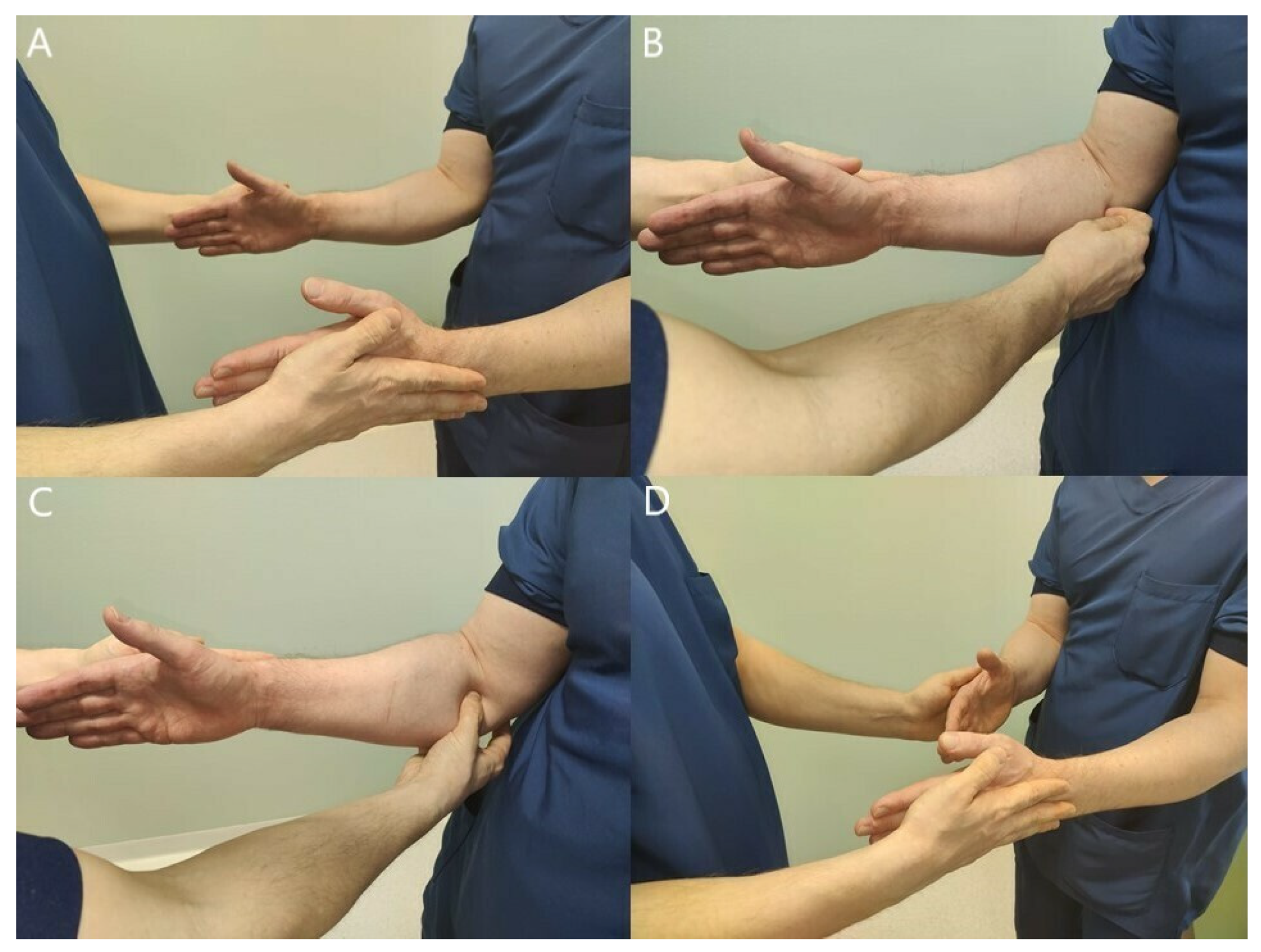
3.2. Clinical Findings
3.3. Electroneuromyography (ENMG)
3.4. Imaging Studies
4. Treatment
4.1. Non-Operative Treament
4.2. Operative Treatment
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asheghan, M.; Hollisaz, M.T.; Aghdam, A.S.; Khatibiaghda, A. The Prevalence of Pronator Teres among Patients with Carpal Tunnel Syndrome: Cross-Sectional Study. Int. J. Biomed. Sci. 2016, 12, 89–94. [Google Scholar] [PubMed]
- Hsiao, C.-W.; Shih, J.-T.; Hung, S.-T. Concurrent Carpal Tunnel Syndrome and Pronator Syndrome: A Retrospective Study of 21 Cases. Orthop. Traumatol. Surg. Res. 2017, 103, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Mujadzic, M.; Papanicolaou, G.; Young, H.; Tsai, T.-M. Simultaneous Surgical Release of Ipsilateral Pronator Teres and Carpal Tunnel Syndromes. Plast. Reconstr. Surg. 2007, 119, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Olehnik, W.K.; Manske, P.R.; Szerzinski, J. Median Nerve Compression in the Proximal Forearm. J. Hand Surg. Am. 1994, 19, 121–126. [Google Scholar] [CrossRef]
- Luangjarmekorn, P.; Tsai, T.M.; Honsawek, S.; Kitidumrongsook, P. Role of Pronator Release in Revision Carpal Tunnel Surgery. SICOT J. 2016, 2, 9. [Google Scholar] [CrossRef]
- Gainor, B.J. The Pronator Compression Test Revisited. A Forgotten Physical Sign. Orthop. Rev. 1990, 19, 888–892. [Google Scholar]
- Struthers, J. On a Peculiarity of the Humerus and Humeral Artery. Mon. J. Med. Sci. 1848, 28, 264–267. [Google Scholar] [CrossRef]
- Ivins, G.K. Supracondylar Process Syndrome: A Case Report. J. Hand Surg. Am. 1996, 21, 279–281. [Google Scholar] [CrossRef]
- Kessel, L.; Rang, M. Supracondylar Spur of the Humerus. J. Bone Jt. Surg. Br. 1966, 48, 765–769. [Google Scholar] [CrossRef]
- Barnard, L.B.; McCoy, S.M. The Supra Condyloid Process of the Humerus. J. Bone Jt. Surg. Am. 1946, 28, 845–850. [Google Scholar]
- Bilecenoglu, B.; Uz, A.; Karalezli, N. Possible Anatomic Structures Causing Entrapment Neuropathies of the Median Nerve: An Anatomic Study. Acta Orthop. Belg. 2005, 71, 169–176. [Google Scholar] [PubMed]
- Shon, H.-C.; Park, J.-K.; Kim, D.-S.; Kang, S.-W.; Kim, K.-J.; Hong, S.-H. Supracondylar Process Syndrome: Two Cases of Median Nerve Neuropathy Due to Compression by the Ligament of Struthers. J. Pain Res. 2018, 11, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Opanova, M.I.; Atkinson, R.E. Supracondylar Process Syndrome: Case Report and Literature Review. J. Hand Surg. Am. 2014, 39, 1130–1135. [Google Scholar] [CrossRef]
- Gessini, L.; Jandolo, B.; Pietrangeli, A. Entrapment Neuropathies of the Median Nerve at and above the Elbow. Surg. Neurol. 1983, 19, 112–116. [Google Scholar] [CrossRef]
- Sos, C.; Roulet, S.; Lafon, L.; Corcia, P.; Laulan, J.; Bacle, G. Median Nerve Entrapment Syndrome in the Elbow and Proximal Forearm. Anatomic Causes and Results for a 55-Case Surgical Series at a Mean 7years’ Follow-Up. Orthop. Traumatol. Surg. Res. 2021, 107, 102825. [Google Scholar] [CrossRef]
- Dellon, A.L.; Mackinnon, S.E. Musculoaponeurotic Variations along the Course of the Median Nerve in the Proximal Forearm. J. Hand Surg. Br. 1987, 12, 359–363. [Google Scholar] [CrossRef]
- Hartz, C.R.; Linscheid, R.L.; Gramse, R.R.; Daube, J.R. The Pronator Teres Syndrome: Compressive Neuropathy of the Median Nerve. J. Bone Jt. Surg. Am. 1981, 63, 885–890. [Google Scholar] [CrossRef]
- Hill, N.A.; Howard, F.M.; Huffer, B.R. The Incomplete Anterior Interosseous Nerve Syndrome. J. Hand Surg. Am. 1985, 10, 4–16. [Google Scholar] [CrossRef]
- Seitz, W.H.; Matsuoka, H.; McAdoo, J.; Sherman, G.; Stickney, D.P. Acute Compression of the Median Nerve at the Elbow by the Lacertus Fibrosus. J. Shoulder Elb. Surg. 2007, 16, 91–94. [Google Scholar] [CrossRef]
- Matsuzaki, A. Operative Treatment of Pronator Syndrome. Orthop. Traumatol. 1999, 7, 34–43. [Google Scholar] [CrossRef]
- Bennett, G.E. Injuries Characteristic of Particular Sports Elbow and Shoulder Lesions of Baseball Players. Am. J. Surg. 1959, 98, 484–492. [Google Scholar] [CrossRef]
- Hagert, E.; Lalonde, D.H. Lacertus Syndrome: Median Nerve Release at the Elbow. In Wide Awake Hand Surgery; Lalonde, D.H., Ed.; Thieme: New York, NY, USA, 2016; pp. 141–145. [Google Scholar]
- Hagert, E. Clinical Diagnosis and Wide-Awake Surgical Treatment of Proximal Median Nerve Entrapment at the Elbow: A Prospective Study. HAND 2013, 8, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Fuss, F.K.; Wurzl, G.H. Median Nerve Entrapment. Pronator Teres Syndrome. Surgical Anatomy and Correlation with Symptom Patterns. Surg. Radiol. Anat. 1990, 12, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Beaton, L.E.; Anson, B.J. The Relation of the Median Nerve to the Pronator Teres Muscle. Contribution no. 289 from the Anatomical Laboratories of Northwestern University Medical School. Anat. Rec. 1939, 75, 23–26. [Google Scholar] [CrossRef]
- Werner, C.O.; Rosén, I.; Thorngren, K.G. Clinical and Neurophysiologic Characteristics of the Pronator Syndrome. Clin. Orthop. Relat. Res. 1985, 197, 231–236. [Google Scholar] [CrossRef]
- Zancolli, E.R.; Zancolli, E.P.; Perrotto, C.J. New Mini-Invasive Decompression for Pronator Teres Syndrome. J. Hand Surg. Am. 2012, 37, 1706–1710. [Google Scholar] [CrossRef]
- Seyffarth, H. Primary Myoses in the M. Pronator Teres as Cause of Lesion of the N. Medianus (the Pronator Syndrome). Acta Psychiatr. Neurol. Scand. Suppl. 1951, 74, 251–254. [Google Scholar]
- Tubbs, R.S.; Marshall, T.; Loukas, M.; Shoja, M.M.; Cohen-Gadol, A.A. The Sublime Bridge: Anatomy and Implications in Median Nerve Entrapment. J. Neurosurg. 2010, 113, 110–112. [Google Scholar] [CrossRef]
- Tang, J.B. Median Nerve Compression: Lacertus Syndrome versus Superficialis-Pronator Syndrome. J. Hand. Surg. Eur. Vol. 2021, 46, 1017–1022. [Google Scholar] [CrossRef]
- Spinner, M.; Spencer, P.S. Nerve Compression Lesions of the Upper Extremity. A Clinical and Experimental Review. Clin. Orthop. Relat. Res. 1974, 104, 46–67. [Google Scholar] [CrossRef]
- Jabaley, M.E.; Wallace, W.H.; Heckler, F.R. Internal Topography of Major Nerves of the Forearm and Hand: A Current View. J. Hand Surg. Am. 1980, 5, 1–18. [Google Scholar] [CrossRef]
- Collins, D.N.; Weber, E.R. Anterior Interosseous Nerve Avulsion. Clin. Orthop. Relat. Res. 1983, 181, 175–178. [Google Scholar] [CrossRef]
- Howard, F.M. Compression Neuropathies in the Anterior Forearm. Hand Clin. 1986, 2, 737–745. [Google Scholar] [CrossRef]
- Vincelet, Y.; Journeau, P.; Popkov, D.; Haumont, T.; Lascombes, P. The Anatomical Basis for Anterior Interosseous Nerve Palsy Secondary to Supracondylar Humerus Fractures in Children. Orthop. Traumatol. Surg. Res. 2013, 99, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Duchenne de Boulogne, G.-B.-A. De L’électrisation Localisée et de Son Application À La Pathologie et À La Thérapeutique, 3rd ed.; Baillière, J.B., Ed.; J.-B. Baillière et Fils: Paris, France, 1872. [Google Scholar]
- Kiloh, L.G.; Nevin, S. Isolated Neuritis of the Anterior Interosseous Nerve. Br. Med. J. 1952, 1, 850–851. [Google Scholar] [CrossRef]
- Al-Qattan, M.M. Gantzer’s Muscle. An Anatomical Study of the Accessory Head of the Flexor Pollicis Longus Muscle. J. Hand Surg. Br. 1996, 21, 269–270. [Google Scholar] [CrossRef]
- Caetano, E.B.; Sabongi, J.J.; Vieira, L.Â.; Caetano, M.F.; Moraes, D.V. Gantzer Muscle. An Anatomical Study. Acta Ortop. Bras. 2015, 23, 72–75. [Google Scholar] [CrossRef]
- Roy, J.; Henry, B.M.; PĘkala, P.A.; Vikse, J.; Saganiak, K.; Walocha, J.A.; Tomaszewski, K.A. Median and Ulnar Nerve Anastomoses in the Upper Limb: A Meta-Analysis. Muscle Nerve 2016, 54, 36–47. [Google Scholar] [CrossRef]
- Haussmann, P.; Patel, M.R. Intraepineurial Constriction of Nerve Fascicles in Pronator Syndrome and Anterior Interosseous Nerve Syndrome. Orthop. Clin. N. Am. 1996, 27, 339–344. [Google Scholar] [CrossRef]
- Seddon, H.J. A Classification of Nerve Injuries. Br. Med. J. 1942, 2, 237–239. [Google Scholar] [CrossRef]
- Sunderland, S. A Classification of Peripheral Nerve Injuries Producing Loss of Function. Brain 1951, 74, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Upton, A.R.; McComas, A.J. The Double Crush in Nerve Entrapment Syndromes. Lancet 1973, 2, 359–362. [Google Scholar] [CrossRef]
- Mackinnon, S.E. Double and Multiple “Crush” Syndromes. Double and Multiple Entrapment Neuropathies. Hand Clin. 1992, 8, 369–390. [Google Scholar] [CrossRef]
- Lundborg, G.; Dahlin, L.B. Anatomy, Function, and Pathophysiology of Peripheral Nerves and Nerve Compression. Hand Clin. 1996, 12, 185–193. [Google Scholar] [CrossRef]
- Parsonege, M.J.; Turner, J.W.A. Neuralgic Amyotrophy; the Shoulder-Girdle Syndrome. Lancet 1948, 1, 973–978. [Google Scholar] [CrossRef]
- van Eijk, J.J.J.; Groothuis, J.T.; van Alfen, N. Neuralgic Amyotrophy: An Update on Diagnosis, Pathophysiology, and Treatment. Muscle Nerve 2016, 53, 337–350. [Google Scholar] [CrossRef]
- Lee, M.J.; LaStayo, P.C. Pronator Syndrome and Other Nerve Compressions That Mimic Carpal Tunnel Syndrome. J. Orthop. Sports Phys. Ther. 2004, 34, 601–609. [Google Scholar] [CrossRef][Green Version]
- Morris, H.H.; Peters, B.H. Pronator Syndrome: Clinical and Electrophysiological Features in Seven Cases. J. Neurol. Neurosurg. Psychiatry 1976, 39, 461–464. [Google Scholar] [CrossRef]
- El-Haj, M.; Ding, W.; Sharma, K.; Novak, C.; Mackinnon, S.E.; Patterson, J.M.M. Median Nerve Compression in the Forearm: A Clinical Diagnosis. Hand 2021, 16, 586–591. [Google Scholar] [CrossRef]
- Johnson, R.K.; Spinner, M.; Shrewsbury, M.M. Median Nerve Entrapment Syndrome in the Proximal Forearm. J. Hand Surg. Am. 1979, 4, 48–51. [Google Scholar] [CrossRef]
- Lalonde, D. Minimally Invasive Anesthesia in Wide Awake Hand Surgery. Hand Clin. 2014, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stål, M.; Hagert, C.G.; Moritz, U. Upper Extremity Nerve Involvement in Swedish Female Machine Milkers. Am. J. Ind. Med. 1998, 33, 551–559. [Google Scholar] [CrossRef]
- Mccue, F.C.I.; Alexander, E.J.; Baumgarten, T.E. Median Nerve Entrapment at the Elbow in Athletes. Oper. Tech. Sports Med. 1996, 4, 21–27. [Google Scholar] [CrossRef]
- Gross, P.T.; Tolomeo, E.A. Proximal Median Neuropathies. Neurol. Clin. 1999, 17, 425–445. [Google Scholar] [CrossRef]
- Özdemir, A.; Acar, M.A.; Güleç, A.; Durgut, F.; Cebeci, H. Clinical, Radiological, and Electrodiagnostic Diagnosis of Pronator Syndrome Concurrent With Carpal Tunnel Syndrome. J. Hand Surg. Am. 2020, 45, 1141–1147. [Google Scholar] [CrossRef]
- Sneag, D.B.; Arányi, Z.; Zusstone, E.M.; Feinberg, J.H.; Queler, S.C.; Nwawka, O.K.; Lee, S.K.; Wolfe, S.W. Fascicular Constrictions above Elbow Typify Anterior Interosseous Nerve Syndrome. Muscle Nerve 2020, 61, 301–310. [Google Scholar] [CrossRef]
- Schantz, K.; Riegels-Nielsen, P. The Anterior Interosseous Nerve Syndrome. J. Hand Surg. Br. 1992, 17, 510–512. [Google Scholar] [CrossRef]
- Park, I.-J.; Roh, Y.-T.; Jeong, C.; Kim, H.-M. Spontaneous Anterior Interosseous Nerve Syndrome: Clinical Analysis of Eleven Surgical Cases. J. Plast. Surg. Hand Surg. 2013, 47, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Na, K.-T.; Jang, D.-H.; Lee, Y.-M.; Park, I.-J.; Lee, H.-W.; Lee, S.-U. Anterior Interosseous Nerve Syndrome: Is It a Compressive Neuropathy? Indian J. Orthop. 2020, 54, 193–198. [Google Scholar] [CrossRef]
- Ulrich, D.; Piatkowski, A.; Pallua, N. Anterior Interosseous Nerve Syndrome: Retrospective Analysis of 14 Patients. Arch. Orthop. Trauma. Surg. 2011, 131, 1561–1565. [Google Scholar] [CrossRef]
- Nammour, M.; Desai, B.; Warren, M.; Sisco-Wise, L. Anterior Interosseous Nerve Palsy After Shoulder Arthroscopy Treated With Surgical Decompression: A Case Series and Systematic Review of the Literature. Hand 2021, 16, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.R.; Sneag, D.B.; Feinberg, J.H.; Wolfe, S.W. Anterior Interosseous Nerve Syndrome Reconsidered: A Critical Analysis Review. JBJS Rev. 2020, 8, e2000011. [Google Scholar] [CrossRef] [PubMed]
- Joist, A.; Joosten, U.; Wetterkamp, D.; Neuber, M.; Probst, A.; Rieger, H. Anterior Interosseous Nerve Compression after Supracondylar Fracture of the Humerus: A Metaanalysis. J. Neurosurg. 1999, 90, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Skouteris, D.; Thoma, S.; Andritsos, G.; Tasios, N.; Praxitelous, P.; Psychoyios, V. Simultaneous Compression of the Median and Ulnar Nerve at the Elbow: A Retrospective Study. J. Hand Surg. Asian Pac. Vol. 2018, 23, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Tapadia, M.; Mozaffar, T.; Gupta, R. Compressive Neuropathies of the Upper Extremity: Update on Pathophysiology, Classification, and Electrodiagnostic Findings. J. Hand Surg. Am. 2010, 35, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.T.; Jones, H.R. Proximal Median Neuropathies: Electromyographic and Clinical Correlation. Muscle Nerve 1992, 15, 390–395. [Google Scholar] [CrossRef]
- Lee, A.K.; Khorsandi, M.; Nurbhai, N.; Dang, J.; Fitzmaurice, M.; Herron, K.A. Endoscopically Assisted Decompression for Pronator Syndrome. J. Hand Surg. Am. 2012, 37, 1173–1179. [Google Scholar] [CrossRef]
- Pham, M.; Bäumer, P.; Meinck, H.-M.; Schiefer, J.; Weiler, M.; Bendszus, M.; Kele, H. Anterior Interosseous Nerve Syndrome: Fascicular Motor Lesions of Median Nerve Trunk. Neurology 2014, 82, 598–606. [Google Scholar] [CrossRef]
- Hide, I.G.; Grainger, A.J.; Naisby, G.P.; Campbell, R.S. Sonographic Findings in the Anterior Interosseous Nerve Syndrome. J. Clin. Ultrasound 1999, 27, 459–464. [Google Scholar] [CrossRef]
- Delzell, P.B.; Patel, M. Ultrasound-Guided Perineural Injection for Pronator Syndrome Caused by Median Nerve Entrapment. J. Ultrasound Med. 2020, 39, 1023–1029. [Google Scholar] [CrossRef]
- Youngner, J.M.; Matsuo, K.; Grant, T.; Garg, A.; Samet, J.; Omar, I.M. Sonographic Evaluation of Uncommonly Assessed Upper Extremity Peripheral Nerves: Anatomy, Technique, and Clinical Syndromes. Skelet. Radiol 2019, 48, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Akman, Y.E.; Yalcinkaya, M.; Arikan, Y.; Kabukcuoglu, Y. Atypically Localized Glomus Tumor Causing Anterior Interosseous Nerve Syndrome: A Case Report. Acta Orthop. Traumatol. Turc. 2017, 51, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A. Pronator Syndrome Due to Schwannoma. J. Hand Microsurg. 2015, 7, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.E.; Novak, C.B. Clinical Commentary: Pathogenesis of Cumulative Trauma Disorder. J. Hand Surg. Am. 1994, 19, 873–883. [Google Scholar] [CrossRef]
- Tsai, T.M.; Syed, S.A. A Transverse Skin Incision Approach for Decompression of Pronator Teres Syndrome. J. Hand Surg. Br. 1994, 19, 40–42. [Google Scholar] [CrossRef]
- Spinner, M. The Anterior Interosseous-Nerve Syndrome, with Special Attention to Its Variations. J. Bone Jt. Surg. Am. 1970, 52, 84–94. [Google Scholar] [CrossRef]
- Hagert, E.; Curtis, C. Failed Carpal Tunnel Release: Recognizing the Lacertus Syndrome. In Problems in Hand Surgery: Solutions to Recover Function; Neumeister, M.W., Ed.; Thieme: New York, NY, USA, 2020; ISBN 9781626237094. [Google Scholar]
- Jordan, S.E. The Lacertus Syndrome of the Elbow in Throwing Athletes. Clin. Sports Med. 2020, 39, 589–596. [Google Scholar] [CrossRef]
- Willick, S.E.; Deluigi, A.J.; Taskaynatan, M.; Petron, D.J.; Coleman, D. Bilateral Chronic Exertional Compartment Syndrome of the Forearm: A Case Report and Review of the Literature. Curr. Sports Med. Rep. 2013, 12, 170–174. [Google Scholar] [CrossRef]
- Smeraglia, F.; Tamborini, F.; Garutti, L.; Minini, A.; Basso, M.A.; Cherubino, M. Chronic Exertional Compartment Syndrome of the Forearm: A Systematic Review. EFORT Open Rev. 2021, 6, 101–106. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löppönen, P.; Hulkkonen, S.; Ryhänen, J. Proximal Median Nerve Compression in the Differential Diagnosis of Carpal Tunnel Syndrome. J. Clin. Med. 2022, 11, 3988. https://doi.org/10.3390/jcm11143988
Löppönen P, Hulkkonen S, Ryhänen J. Proximal Median Nerve Compression in the Differential Diagnosis of Carpal Tunnel Syndrome. Journal of Clinical Medicine. 2022; 11(14):3988. https://doi.org/10.3390/jcm11143988
Chicago/Turabian StyleLöppönen, Pekka, Sina Hulkkonen, and Jorma Ryhänen. 2022. "Proximal Median Nerve Compression in the Differential Diagnosis of Carpal Tunnel Syndrome" Journal of Clinical Medicine 11, no. 14: 3988. https://doi.org/10.3390/jcm11143988
APA StyleLöppönen, P., Hulkkonen, S., & Ryhänen, J. (2022). Proximal Median Nerve Compression in the Differential Diagnosis of Carpal Tunnel Syndrome. Journal of Clinical Medicine, 11(14), 3988. https://doi.org/10.3390/jcm11143988





