Time to Total Knee Arthroplasty after Intra-Articular Hyaluronic Acid or Platelet-Rich Plasma Injections: A Systematic Literature Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
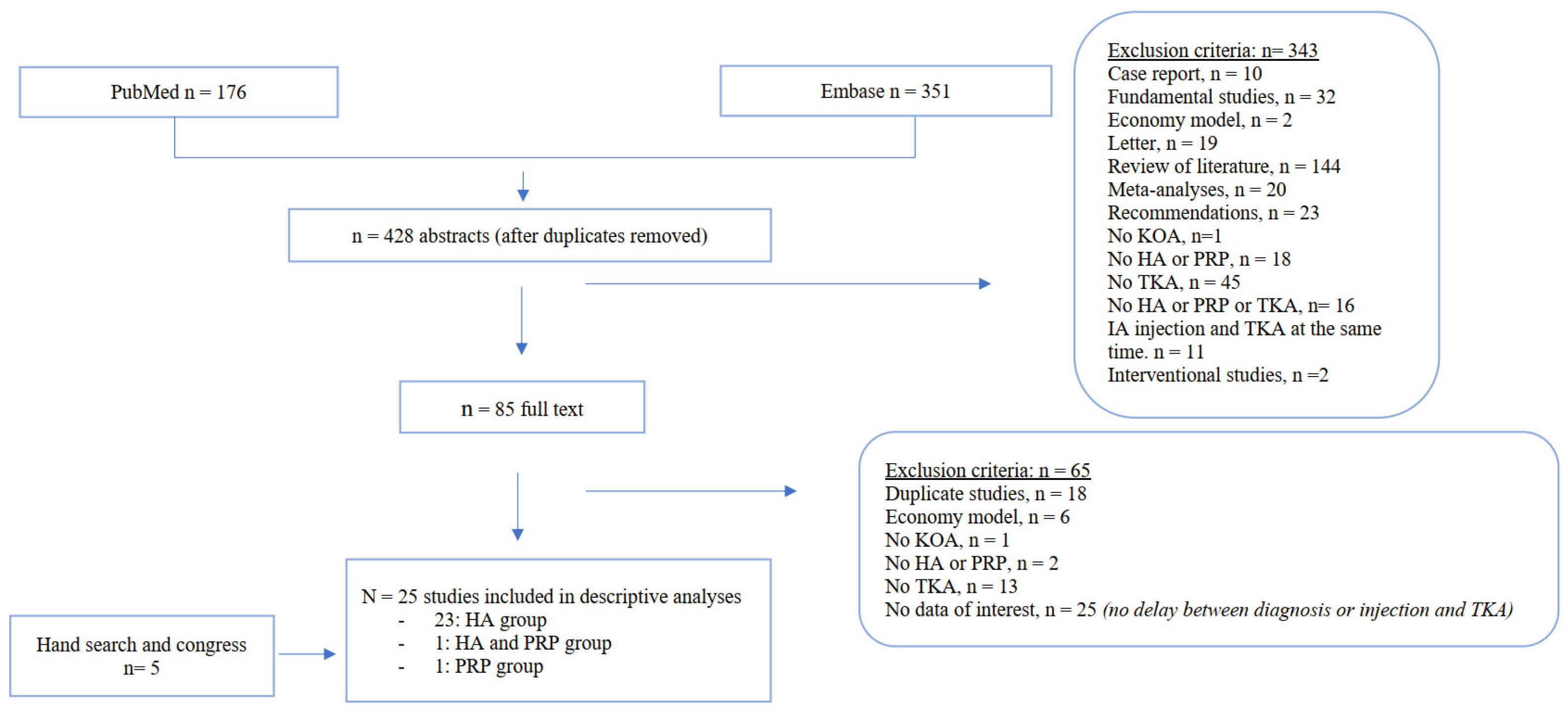
3.1. Literature Search and Characteristics of Included Studies
3.2. Patient and Product Characteristics
3.3. Primary Outcome: Time from KOA Declared Diagnosis in the Database to TKA (HA Injection)
3.3.1. Descriptive Delay from Each Study
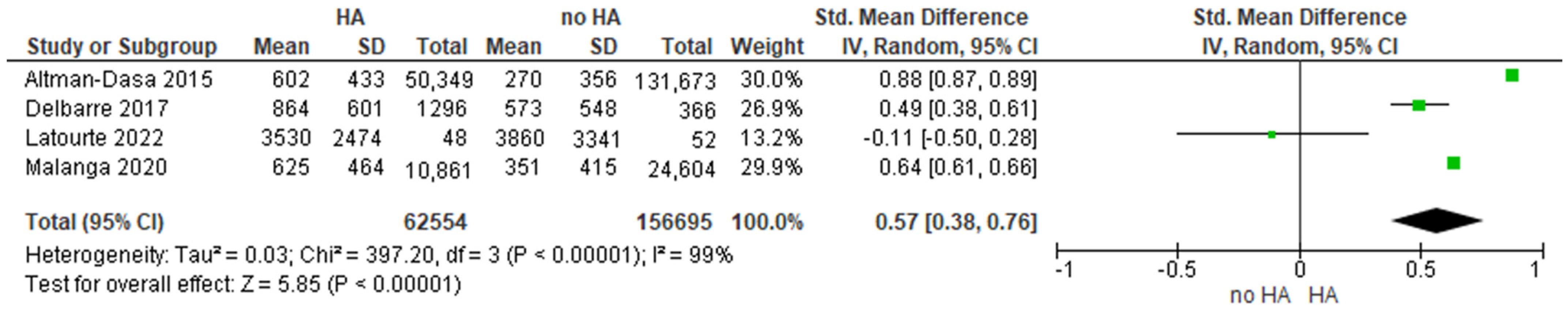
3.3.2. Pooled Mean Delay and ES
3.3.3. Multiple Courses of HA Injections
3.4. Secondary Outcomes: Time from IA Injection to TKA (HA Injection)
3.5. Secondary Outcomes: Prevalence of TKA at 2 Years after a Declared Diagnosis of KOA
3.6. Secondary Outcomes: Prevalence of TKA at Different Times after IA HA Injection
3.7. PRP Injections
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swain, S.; Sarmanova, A.; Coupland, C.; Doherty, M.; Zhang, W. Comorbidities in Osteoarthritis: A Systematic Review and Meta-Analysis of Observational Studies. Arthritis Care Res. 2020, 72, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Louati, K.; Vidal, C.; Berenbaum, F.; Sellam, J. Association between diabetes mellitus and osteoarthritis: Systematic literature review and meta-analysis. RMD Open 2015, 1, e000077. [Google Scholar] [CrossRef] [PubMed]
- Baudart, P.; Louati, K.; Marcelli, C.; Berenbaum, F.; Sellam, J. Association between osteoarthritis and dyslipidaemia: A systematic literature review and meta-analysis. RMD Open 2017, 3, e000442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nüesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef] [Green Version]
- The Burden of Musculoskeletal Diseases in the United States. Available online: https://www.boneandjointburden.org/ (accessed on 19 July 2020).
- Ornetti, P.; Fortunet, C.; Morisset, C.; Gremeaux, V.; Maillefert, J.; Casillas, J.; Laroche, D. Clinical effectiveness and safety of a distraction-rotation knee brace for medial knee osteoarthritis. Ann. Phys. Rehabil. Med. 2015, 58, 126–131. [Google Scholar] [CrossRef]
- Zeng, C.-Y.; Zhang, Z.-R.; Tang, Z.-M.; Hua, F.-Z. Benefits and Mechanisms of Exercise Training for Knee Osteoarthritis. Front. Physiol. 2021, 12, 794062. [Google Scholar] [CrossRef]
- Sellam, J.; Courties, A.; Eymard, F.; Ferrero, S.; Latourte, A.; Ornetti, P.; Bannwarth, B.; Baumann, L.; Berenbaum, F.; Chevalier, X.; et al. Recommandations de la Société française de rhumatologie sur la prise en charge pharmacologique de la gonarthrose. Rev. Rhum. 2020, 87, 439–446. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Kolasinski, S.L.; Neogi, T.; Neogi, T.; Hochberg, M.C.; Hochberg, M.C.; Oatis, C.; Oatis, C.; Guyatt, G.; Guyatt, G.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Richette, P. Hyaluronic acid: Still useful in knee osteoarthritis? Jt. Bone Spine 2017, 84, 655–656. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [Green Version]
- Brophy, R.H.; Fillingham, Y.A. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. J. Am. Acad. Orthop. Surg. 2022, 30, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.A.; Balazs, E.A.; Belmonte, C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp. Brain Res. 1997, 116, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Ghosh, P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol. Int. 1987, 7, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, T.; Mochizuki, S.; Takizawa, M.; Chijiiwa, M.; Okada, A.; Kimura, T.; Fujita, Y.; Matsumoto, H.; Toyama, Y.; Okada, Y. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann. Rheum. Dis. 2009, 68, 1051–1058. [Google Scholar] [CrossRef]
- Listrat, V.; Ayral, X.; Patarnello, F.; Bonvarlet, J.-P.; Simonnet, J.; Amor, B.; Dougados, M. Arthroscopic evaluation of potential structure modifying activity of hyaluronan (Hyalgan®) in osteoarthritis of the knee. Osteoarthr. Cartil. 1997, 5, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.; Le Henanff, A.; Ravaud, P.; Dieppe, P.; Paolozzi, L.; Dougados, M. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomised controlled study in symptomatic knee osteoarthritis. Ann. Rheum. Dis. 2004, 63, 1611–1617. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hall, S.; Hanna, F.; Wluka, E.A.; Grant, G.; Marks, P.; Feletar, M.; Cicuttini, F.M. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: A two-year single-blind clinical trial. BMC Musculoskelet. Disord. 2011, 12, 195. [Google Scholar] [CrossRef] [Green Version]
- Eymard, F.; Ornetti, P.; Maillet, J.; Noel, É.; Adam, P.; Legré-Boyer, V.; Boyer, T.; Allali, F.; Gremeaux, V.; Kaux, J.-F.; et al. Intra-articular injections of platelet-rich plasma in symptomatic knee osteoarthritis: A consensus statement from French-speaking experts. Knee Surg. Sports Traumatol. Arthrosc. 2020, 29, 3195–3210. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- Patel, S.; Dhillon, M.S.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment with Platelet-Rich Plasma Is More Effective than Placebo for Knee Osteoarthritis. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef]
- Smith, P.A. Intra-articular Autologous Conditioned Plasma Injections Provide Safe and Efficacious Treatment for Knee Osteoarthritis. Am. J. Sports Med. 2016, 44, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients with Knee Osteoarthritis. JAMA J. Am. Med. Assoc. 2021, 326, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Fice, M.P.; Miller, J.; Christian, R.; Hannon, C.P.; Smyth, N.; Murawski, C.D.; Cole, B.J.; Kennedy, J.G. The Role of Platelet-Rich Plasma in Cartilage Pathology: An Updated Systematic Review of the Basic Science Evidence. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 961–976.e3. [Google Scholar] [CrossRef]
- Ornetti, P.; Nourissat, G.; Berenbaum, F.; Sellam, J.; Richette, P.; Chevalier, X. Does platelet-rich plasma have a role in the treatment of osteoarthritis? Jt. Bone Spine 2016, 83, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging 2020, 32, 547–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Levin, G.; Nikolov, N.P.; Abugov, R.; Rothwell, R. Concept End Points Informing Design Considerations for Confirmatory Clinical Trials in Osteoarthritis. Arthritis Care Res. 2020, 74, 1154–1162. [Google Scholar] [CrossRef]
- McAlindon, T.; Driban, J.; Henrotin, Y.; Hunter, D.; Jiang, G.-L.; Skou, S.; Wang, S.; Schnitzer, T. OARSI Clinical Trials Recommendations: Design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, 747–760. [Google Scholar] [CrossRef] [Green Version]
- Dougados, M.; Nguyen, M.; Listrat, V.; Amor, B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: A 1 year placebo-controlled trial. Osteoarthr. Cartil. 1993, 1, 97–103. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook/current (accessed on 1 June 2020).
- PRISMA. Available online: http://prisma-statement.org/PRISMAStatement/Checklist (accessed on 3 February 2022).
- Guillemin, F.; Rat, A.-C.; Roux, C.H.; Fautrel, B.; Mazieres, B.; Chevalier, X.; Euller-Ziegler, L.; Fardellone, P.; Verrouil, E.; Morvan, J.; et al. The KHOALA cohort of knee and hip osteoarthritis in France. Jt. Bone Spine 2012, 79, 597–603. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Altman, R.; Fredericson, M.; Bhattacharyya, S.K.; Bisson, B.; Abbott, T.; Yadalam, S.; Kim, M. Association between Hyaluronic Acid Injections and Time-to-Total Knee Replacement Surgery. J. Knee Surg. 2016, 29, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Lim, S.; Steen, R.G.; Dasa, V. Hyaluronic Acid Injections Are Associated with Delay of Total Knee Replacement Surgery in Patients with Knee Osteoarthritis: Evidence from a Large U.S. Health Claims Database. PLoS ONE 2015, 10, e0145776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.L.; Anderson, A.F.; Niazi, F.; Fierlinger, A.L.; Kurtz, S.M.; Altman, R.D. Hyaluronic Acid Injections in Medicare Knee Osteoarthritis Patients Are Associated with Longer Time to Knee Arthroplasty. J. Arthroplast. 2016, 31, 1667–1673. [Google Scholar] [CrossRef]
- Ong, K.L.; Runa, M.; Lau, E.; Altman, R. Is Intra-Articular Injection of Synvisc Associated with a Delay to Knee Arthroplasty in Patients with Knee Osteoarthritis? Cartilage 2018, 10, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Runa, M.; Lau, E.; Altman, R.D. Cost-of-illness of knee osteoarthritis: Potential cost savings by not undergoing arthroplasty within the first 2 years. Clin. Outcomes Res. 2019, 11, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Etter, K.; Chitnis, A.S.; Holy, E.C.; Gray, F.S.; Manalac, F.J.; Bisson, B.; Bhattacharyya, S.K. High-concentration nonavian high-molecular weight hyaluronan injections and time-to-total knee replacement surgery. J. Comp. Eff. Res. 2020, 9, 795–805. [Google Scholar] [CrossRef]
- Dasa, V.; Lim, S.; Heeckt, P. Real-World Evidence for Safety and Effectiveness of Repeated Courses of Hyaluronic Acid Injections on the Time to Knee Replacement Surgery. Am. J. Orthop. 2018, 47, 58. [Google Scholar] [CrossRef]
- Joseph, B.; Waddell, D.D. Delayed Total Knee Replacement with Hylan G-F 20. J. Knee Surg. 2014, 29, 159–168. [Google Scholar] [CrossRef]
- Bowman, E.N.; Hallock, J.D.; Throckmorton, T.W.; Azar, F.M. Hyaluronic acid injections for osteoarthritis of the knee: Predictors of successful treatment. Int. Orthop. 2018, 42, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, Z.T.; Sytsma, T.T.; Greenlund, L.S. Rethinking Viscosupplementation: Ultrasound- Versus Landmark-Guided Injection for Knee Osteoarthritis. J. Ultrasound Med. 2020, 39, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.L.; Sloniewsky, M.J.; Gibbons, E.T.; Johnston, J.G.; Vosler, K.D.; Nasir, S. Long-term clinical benefit and cost-effectiveness of an 8-week multimodal knee osteoarthritis management program incorporating intra-articular sodium hyaluronate (Hyalgan®) injections. J. Pain Res. 2017, 10, 1045–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, A.; Balduini, F.; Rogers, K. Hyaluronic acid in management of advanced osteoarthritis of the knee: Retrospective analysis. Eur. J. Orthop. Surg. Traumatol. 2010, 20, 645–649. [Google Scholar] [CrossRef]
- Barrett, J.P.; Siviero, P.; Barrett, J.P. Retrospective Study of Outcomes in Hyalgan®-Treated Patients with Osteoarthritis of the Knee. Clin. Drug Investig. 2002, 22, 87–97. [Google Scholar] [CrossRef]
- Malanga, G.; Niazi, F.; Kidd, V.D.; Lau, E.; Kurtz, S.M.; Ong, K.L.; Concoff, A.L. Knee Osteoarthritis Treatment Costs in the Medicare Patient Population. Am. Health Drug Benefits 2020, 13, 144–153. [Google Scholar]
- Abbott, T.; Altman, R.D.; Dimeff, R.; Fredericson, M.; Vad, V.; Vitanzo, P.; Yadalam, S.; Levine, R.; Bisson, B.; Bhattacharyya, S. Do Hyaluronic Acid Injections Delay Total Knee Replacement Surgery? In Arthritis and Rheumatism; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Volume 65, pp. S910–S911. [Google Scholar]
- Evanich, J.D.; Evanich, C.J.; Wright, M.B.; Rydlewicz, J.A. Efficacy of Intraarticular Hyaluronic Acid Injections in Knee Osteoarthritis. Clin. Orthop. Relat. Res. 2001, 390, 173–181. [Google Scholar] [CrossRef]
- Jurado, M.R.; Fidalgo, A.E.; Villar, V.R.; Medina, J.M.; López, B.S. Factores que influyen sobre el tiempo hasta la necesidad de intervenir un paciente en la lista de espera para prótesis de rodilla. Reumatol. Clín. 2013, 9, 148–155. [Google Scholar] [CrossRef]
- Sánchez, M.; Jorquera, C.; Sánchez, P.; Beitia, M.; García-Cano, B.; Guadilla, J.; Delgado, D. Platelet-rich plasma injections delay the need for knee arthroplasty: A retrospective study and survival analysis. Int. Orthop. 2020, 45, 401–410. [Google Scholar] [CrossRef]
- Delbarre, A.; Amor, B.; Bardoulat, I.; Tetafort, A.; Pelletier-Fleury, N. Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis—A Cox model analysis. PLoS ONE 2017, 12, e0187227. [Google Scholar] [CrossRef] [Green Version]
- Mazières, B.; Bard, H.; Ligier, M.; Bru, I.; D’Orsay, G.G.; Le Pen, C. Medicoeconomic evaluation of hyaluronic acid for knee osteoarthritis in everyday practice: The MESSAGE study. Jt. Bone Spine 2007, 74, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Rat, A.; Omorou, A.; Ngueyon-Sime, W.; Eymard, F.; Sellam, J.; Roux, C.; Ea, H.; Cohen-Solal, M.; Bardin, T.; et al. Do Glucocorticoid Injections Increase the Risk of Knee Osteoarthritis Progression Over 5 Years? Arthritis Rheumatol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Korkmaz, M.; Erdogan, Y.; Okur, A.; Göçmen, A.Y.; Günaydin, I. Comparison of the Effects of Intraarticular Hyaluronic Acid and Antiinflammatory Drug Treatments on the Surgical Intervention Rates in Patients with Gonarthrosis. Turk. J. Med. Sci. 2013, 43, 222–226. [Google Scholar] [CrossRef]
- Turajane, T.; Amphansap, T.; Labpiboonpong, V.; Maungsiri, S. Total knee replacement following repeated cycles of intra-articular sodium hyaluronate (500-730 Kda) in failed conservative treatment of knee osteoarthritis: A 54-month follow-up. J. Med. Assoc. Thail. 2009, 92, S63–S68. [Google Scholar]
- Annaniemi, J.A.; Pere, J.; Giordano, S. Platelet-rich plasma versus hyaluronic acid injections for knee osteoarthritis: A propensity-score analysis. Scand. J. Surg. 2018, 108, 329–337. [Google Scholar] [CrossRef]
- Campbell, D.; Angel, K.R.; Dobson, P.J.; Lewis, P.; Tandon, S. Experiences of viscosupplementation for knee osteoarthritis. Aust. Fam. Physician 2004, 33, 863–864. [Google Scholar]
- Whitman, C.S.; Allen, D.; Comadoll, J.L.; Thomason, H.C.; Oweida, S.D. A Retrospective Study of SUPARTZ® and Repeat Treatment for Osteoarthritis Pain in the Knee. J. Manag. Care Med. 2010, 13, 43–47. [Google Scholar]
- McHugh, G.A.; Luker, K.A. Influences on individuals with osteoarthritis in deciding to undergo a hip or knee joint replacement: A qualitative study. Disabil. Rehabil. 2009, 31, 1257–1266. [Google Scholar] [CrossRef]
- Tang, A.; Almetwali, O.; Zak, S.G.; Bernstein, J.A.; Schwarzkopf, R.; Aggarwal, V.K. Do preoperative intra-articular corticosteroid and hyaluronic acid injections affect time to total joint arthroplasty? J. Clin. Orthop. Trauma 2020, 16, 49–57. [Google Scholar] [CrossRef]
- Ong, K.L.; Niazi, F.; Lau, E.; Mont, M.A.; Concoff, A.; Shaw, P.; Kurtz, S.M. Knee OA cost comparison for hyaluronic acid and knee arthroplasty. J. Orthop. Surg. Res. 2020, 15, 305. [Google Scholar] [CrossRef]
- Electricwala, A.J.; Jethanandani, R.G.; Narkbunnam, R.; Huddleston, J.I.; Maloney, W.J.; Goodman, S.B.; Amanatullah, D.F. Elevated Body Mass Index Is Associated with Early Total Knee Revision for Infection. J. Arthroplast. 2017, 32, 252–255. [Google Scholar] [CrossRef]
- Ward, D.T.; Metz, L.N.; Horst, P.K.; Kim, H.T.; Kuo, A.C. Complications of Morbid Obesity in Total Joint Arthroplasty: Risk Stratification Based on BMI. J. Arthroplast. 2015, 30, 42–46. [Google Scholar] [CrossRef] [PubMed]
| Author and Year of Publication | Quality Score STROBE (%) | Patients with IA Injection (n) (Population) | Injection Products (Intervention) | Patient without IA Injection (n) (Comparator) | Data of Interest Available (Outcome) | |||
|---|---|---|---|---|---|---|---|---|
| Time from KOA Diagnosis to TKA | Prevalence of TKA from Diagnosis | Time from IA Injection to TKA | Prevalence of TKA from IA Injection | |||||
| Altman-dasa, 2015 [38] | 65% | 50,349 | All HA | 131,673 | Yes | Yes | No | No |
| Delbarre, 2017 [55] | 81% | 1296 | All HA | 366 | Yes | Yes | No | No |
| Ong, 2016 [39] | 76% | 9586 | All HA | 25,560 | Yes | No | No | No |
| Altman-kim, 2015 [37] | 76% | 8423 | All HA | 14,132 | Yes | No | No | No |
| Ong, 2019a [41] * | 66% | 88,501 | All HA | 1,941,996 | No | Yes | No | No |
| Ong, 2019b [40] * | 76% | 37,160 | All HA | 104,145 | Yes | Yes | No | No |
| Etter, 2020 [42] | 77% | 4376 | HMW HA | 90,316 | Yes | No | No | No |
| Abbott, 2013 [51] | Abstract | 6981 | All HA | 19,627 | Yes | No | No | No |
| Malanga, 2020 [50] | 68% | 45,801 | HA | 229,455 | Yes | No | Yes | No |
| Latourte, 2022 [57] | Cohort | 191 | All HA | 465 | Yes | No | No | No |
| Korzmaz, 2013 [58] | 44% | 197 | LMW HA | 487 | No | No | No | Yes |
| Jurado, 2012 [53] | 61% | 202 | HMW HA | 22 | No | No | Yes | No |
| Dasa, 2018 [39] | 68% | 50,389 | HA | 0 | No | No | Yes | No |
| Waddell, 2014 [44] | 67% | 1342 | HMW HA | 0 | No | No | Yes | Yes |
| Bowman, 2018 [45] | 67% | 120 | HMW HA | 0 | No | No | Yes | Yes |
| Miler, 2017 [47] | 69% | 218 | HMW HA | 0 | No | No | No | Yes |
| Turajane, 2008 [59] | 44% | 183 | LMW HA | 0 | No | No | Yes | Yes |
| Lundstrom, 2019 [46] | 53% | 1147 | LMW HA | 0 | No | No | No | Yes |
| Anand, 2018 [48] | 55% | 130 | HMW HA | 0 | No | No | No | Yes |
| Barrett, 2002 [49] | 67% | 176 | LMW HA | 0 | No | No | No | Yes |
| Whitman, 2010 [62] | 27% | 220 | HMW HA | 0 | No | No | No | Yes |
| Campbell, 2004 [61] | 29% | 61 | HMW HA | 0 | No | No | No | Yes |
| Evanich, 2001 [52] | 67% | 70 | HMW HA | 0 | No | No | Yes | Yes |
| Mazieres, 2007 [56] | 67% | 296 | HMW HA | 0 | No | No | No | Yes |
| Annaniemi, 2019 [60] | 59% | 8694 | All HA PRP | 0 | No | No | No | Yes |
| Sanchez, 2020 [54] | 67% | 186 | PRP | 0 | No | No | Yes | Yes |
| Author and Year of Publication | Patients (n) | Database/Years of Inclusion | OA Diagnostic Criteria | With TKA | Without TKA | Age (years), Mean ± SD | Female (%) | Mean BMI (kg/m²) |
|---|---|---|---|---|---|---|---|---|
| Altman-dasa, 2015 [38] | 182,022 | IMS Health Database/ 2007–2013 | Codes | Yes | No | 61 ± 8.9 | 58.7% | - |
| Delbarre, 2017 [55] | 14,782 | French medical insurance/ 2006–3013 | X-ray images of the knee followed by an IA injection, prescribed by an OA specialist | Yes | Yes | 67.67 ± 10.41 | 66% | - |
| Ong, 2016 [39] | 35,142 | 5% Medicare/ 2005–2012 | OA knee or osteoarthritis with pain leg codes | Yes | No | - | 61.2% | - |
| Altman-kim, 2015 [37] | 22,555 | Truven market scan commercial/ 2006–2011 | Codes | Yes | No | - | 61.7% | - |
| Ong, 2019a [41] | 2,030,497 | Optum informatics/ 2006–2016 | Codes | Yes | Yes | - | - | - |
| Ong, 2019b [40] | 141,305 | Codes | Yes | No | - | - | - | |
| Etter, 2020 [42] | 30,028 | Truven market scan commercial/ 2008–2017 | Codes by an orthopedics | Yes | No | - | 58.2% | - |
| Abbott, 2013 [51] | - | Truven market scan commercial/ 2007–2011 | First visit to an OA specialist | Yes | No | - | - | - |
| Malanga, 2020 [50] | 275,256 | 5% Medicare/2010–2015 | Codes | Yes | Yes | - | - | - |
| Latourte, 2022 [57] | 656 | No/2013 | ACR | Yes | Yes | 62.21 ± 8.45 | 70.3% | 30.3 ± 6.2 |
| Korzmaz, 2013 [58] | 684 | No/2007–2009 | NA | Yes | Yes | 55.1 ± 12.8 | 83.6% | - |
| Jurado, 2012 [53] | 224 | No/2006–2009 | Spanish recommendations | Yes | Yes | - | 67.9% | - |
| Dasa, 2018 [43] | 50,389 | IMS Health Database/2007–2010 | First injection of HA | Yes | No | 57.5 ± 10.5 | 59.9% | - |
| Waddell, 2014 [44] | 1342 | No/1997–2010 | - | Yes | Yes | 67.5 ± 10.1 | 60.2% | 31.5 ± 7 |
| Bowman, 2018 [45] | 102 | No/2013–2016 | - | Yes | Yes | 60.1 ± - | 71.6% | 33 ± - |
| Miller, 2017 [47] | 218 | No/NA | ACR | Yes | Yes | 70.5 ± 9.2 | 46.3% | 30.5 ± 6.9 |
| Turajane, 2008 [59] | 183 | No/2001–2004 | ACR | Yes | Yes | 68.7 ± - | 74.9% | 25.1 ± - |
| Lundstrom, 2019 [46] | 1147 | No/2008–2014 | - | Yes | Yes | 62.2 ± 14 | 65.7% | 25.21 ± - |
| Anand, 2018 [48] | 130 | No/1999–2003 | - | Yes | Yes | - | 57.7% | - |
| Barrett, 2002 [49] | 376 | No/- | ACR | Yes | Yes | 72 ± 11 | - | - |
| Whitman, 2010 [62] | 220 | No/- | - | Yes | Yes | - | 74.5% | - |
| Campbell, 2004 [61] | 61 | No/- | X-ray images or arthroscopy | Yes | Yes | 62.2 ± - | 44.3% | 55.7% |
| Evanich, 2001 [52] | 70 | No/1997 | X-ray images | Yes | Yes | 66 ± 14 | 61% | - |
| Annaniemi, 2019 [60] | 180 | No/2014–2017 | Radiography | Yes | Yes | 61.3 ± 8.8 | 60.3% | 29.8 ± 4.8 |
| Mazieres, 2007 [56] | 296 | No/2003–2004 | ACR | Yes | Yes | 69 ± 10 | 65% | 28 ± 5 |
| Sanchez, 2020 [54] | 481 | No/2014–2019 | Radiography | Yes | Yes | 63.9 ± - | 49.3% | - |
| 186 | 63.9 ± 7.4 | - | - |
| After IA HA Injection | After PRP Injection | ||
|---|---|---|---|
| 6 months | Barett, 2002 [49] Mazieres, 2007 [56] | 20.3% 0.7% | - - |
| 8 months | Campbell, 2004 [61] | 11.4% * | |
| 10 months | Evanich, 2001 [52] | 28.6% * | - |
| 1 year | Whitman, 2010 [62] Korkmaz, 2013 [58] Miller, 2017 [47] | 1% 3.2% 10.4% | - - - |
| 17 months | Annaniemi, 2019 [60] | 36% * | 3.3% * |
| 2 years | Miller, 2017 [47] | 18% | - |
| 27 months | Bowman, 2018 [45] | 19.6% | - |
| 3 years | Dasa, 2018 [43] | 25.7% | - |
| 3.5 years | Turajane, 2008 [59] Miller, 2017 [47] | 25% 37.2% * | - - |
| 5 years | Latourte, 2022 [57] | 25.7% | - |
| 6 years | Sanchez, 2020 [54] | - | 9.4% |
| 6.5/7 years | Lundstrom, 2019 [46] | 50% | - |
| 7.3 years | Sanchez, 2020 [54] | - | 31.6% |
| 8 years | Waddell, 2014 [44] | 25% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berkani, S.; Courties, A.; Eymard, F.; Latourte, A.; Richette, P.; Berenbaum, F.; Sellam, J.; Louati, K. Time to Total Knee Arthroplasty after Intra-Articular Hyaluronic Acid or Platelet-Rich Plasma Injections: A Systematic Literature Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3985. https://doi.org/10.3390/jcm11143985
Berkani S, Courties A, Eymard F, Latourte A, Richette P, Berenbaum F, Sellam J, Louati K. Time to Total Knee Arthroplasty after Intra-Articular Hyaluronic Acid or Platelet-Rich Plasma Injections: A Systematic Literature Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(14):3985. https://doi.org/10.3390/jcm11143985
Chicago/Turabian StyleBerkani, Sabryne, Alice Courties, Florent Eymard, Augustin Latourte, Pascal Richette, Francis Berenbaum, Jérémie Sellam, and Karine Louati. 2022. "Time to Total Knee Arthroplasty after Intra-Articular Hyaluronic Acid or Platelet-Rich Plasma Injections: A Systematic Literature Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 14: 3985. https://doi.org/10.3390/jcm11143985
APA StyleBerkani, S., Courties, A., Eymard, F., Latourte, A., Richette, P., Berenbaum, F., Sellam, J., & Louati, K. (2022). Time to Total Knee Arthroplasty after Intra-Articular Hyaluronic Acid or Platelet-Rich Plasma Injections: A Systematic Literature Review and Meta-Analysis. Journal of Clinical Medicine, 11(14), 3985. https://doi.org/10.3390/jcm11143985






