Abstract
Preoperative transfusion (PT) reduces acute postoperative vaso-occlusive events (VOE) in sickle cell disease (SCD), but exposes patients to alloimmunization, encouraging a recent trend towards transfusion sparing. The aim of this study was to investigate the benefit–risk ratio of PT before cholecystectomy on the occurrence of postoperative VOE. Adult SCD patients who underwent cholecystectomy between 2008 and 2019 in our center were included. Patients’ characteristics, collected retrospectively, were compared according to PT. A total of 79 patients were included, 66% of whom received PT. Gallbladder histopathology found chronic cholecystitis (97%) and gallstones (66%). Transfused patients underwent more urgent surgeries and had experienced more painful vaso-occlusive crises (VOC) in the month before surgery (p = 0.05). Four (8.5%) post-transfusion alloimmunizations occurred, and two of them caused a delayed hemolytic transfusion reaction (DHTR) (4.3%). The occurrence of postoperative VOE was similar between the groups (19.2% vs. 29.6%, p = 0.45). Though not statistically significant, a history of hospitalized VOC within 6 months prior to surgery seemed to be associated to postoperative VOE among non-transfused patients (75% vs. 31.6%, p = 0.10). PT before cholecystectomy exposes to risks of alloimmunization and DHTR that could be avoided in some patients. Recent VOCs appear to be associated with a higher risk of postoperative VOE and prompt the preemptive transfusion of these patients.
1. Introduction
Sickle cell disease (SCD) leads to chronic hemolysis, which is responsible for the accumulation of unconjugated bilirubin, which may result in cholelithiasis. The prevalence of pigmentary gallstones increases with age, reaching 50% in adulthood. They cause acute biliary complications in 10 to 20% of cases [1,2,3,4,5]. These complications, in addition to their own severity, also increase the incidence of vaso-occlusive events (VOE): painful vaso-occlusive crises (VOC) and acute chest syndromes (ACS) [6]. In the United Kingdom and the United States, cholecystectomy is recommended for SCD adults in case of symptomatic cholelithiasis [7,8]. In France, a cholecystectomy is recommended for all SCD patients (adults and children) with a demonstrated ultrasound cholelithiasis, even if asymptomatic, before the occurrence of any complication [9,10,11]. However, this surgery is at risk in this specific population since global stress, lack of oxygenation, and acidosis related to the surgical procedure may trigger VOC or ACS. The latter is a serious complication, with mortality ranging from 1.6 to 4.6% in hospitalized patients, according to previous studies [9,12,13,14]. Abdominal surgery is particularly associated with an increased risk of postoperative ACS, reaching up to 30% in non-transfused patients [13,14,15]. Peri-operative management therefore aims at avoiding these VOE as much as possible. Prophylactic preoperative transfusion (PT), which can be either a simple transfusion or exchange transfusion depending on the hemoglobin (Hb) level, reduces these postoperative VOE in adult SCD patients [13,16,17,18]. However, it exposes patients to alloimmunization and delayed hemolytic transfusion reactions (DHTR), the latter being a potentially lethal complication which explains a recent trend towards transfusion sparing [7,19,20]. However, whether PT can be avoided without risk for a simple surgery such as cholecystectomy is uncertain [21].
The primary objective of our study was to investigate the benefit–risk ratio of PT before a cholecystectomy on the occurrence of postoperative complications in a cohort of adult SCD patients, and to determine the factors associated with an increased risk of postoperative vaso-occlusive complications. The secondary objective was to describe patient management during a cholecystectomy.
2. Materials and Methods
We conducted a single-center, retrospective, and observational study in the adult Referral Center for SCD of the European Georges Pompidou University Hospital in Paris (France). Our hospital’s computerized data warehouse allowed for an exhaustive search of all consecutive adult SCD patients who underwent a cholecystectomy in our hospital between 1 January 2008 and 31 December 2019 (defined by codes D57.0, D57.01, D57.02, D57.09 for SCD and K82.9, Z90.4, Y83.6 for cholecystectomy, Table A1).
For all patients followed in the referral center, socio-demographic data, background treatments, acute and chronic complications of the SCD, comorbidities, and routine laboratory tests at steady state were prospectively collected. Nephropathy was defined as chronic renal failure (glomerular filtration rate < 60 mL/min/1.73 m2) or a urine albumin to creatinine ratio (ACR) > 3 mg/mmol on at least two measures at steady state, more than 6 months apart. Retinopathy was defined as any retinal change secondary to retinal ischemia and included proliferative and non-proliferative forms. Surgical and pathological data were collected from operative and pathological reports. Preoperative VOE were defined as all painful bone VOC and/or ACS requiring hospitalization within 6 months before surgery. Patients who underwent surgery in an emergency setting were differentiated from those who underwent elective planned surgery. VOE in the immediate postoperative period and up to one year after the surgery, the need for admission in the intensive care unit (ICU) and orotracheal intubation, as well as deaths and the need for re-hospitalization in the month following the surgery were retrospectively collected.
Concerning the transfusion data, the French Blood Establishment database was used to confirm the dates of red blood cells (RBC) transfusion, the number of RBC units received, the emergence of post-transfusion allo-antibodies, and their type. Any new allo-antibody that had an impact on the choice of the type of RBC during subsequent transfusions, or triggered a delayed hemolytic transfusion reaction (DHTR) (defined as the appearance, at least 3 days after a RBC transfusion, of biological markers of hemolysis associated with a deeper anemia than before transfusion (minimum decrease 30%), and a significant drop in hemoglobin A percentage) was considered significant.
The primary endpoint was the occurrence of significant VOE (painful VOC and/or ACS requiring an extension of hospital stay or readmission) or death in the month following surgery. Secondary endpoints included transfusion-related complications (alloimmunization, DHTR), postoperative complications other than VOE, need for readmission, and need for postoperative transfusion. The parameters studied were compared between two groups of patients (preoperative transfusion or not, postoperative complication or not) by Student or Wilcoxon test for quantitative variables according to their distribution, and by Chi-2 or Fischer test for qualitative variables, according to the numbers. The non-transfused patients who did and did not present postoperative complications were then compared. We also performed the same analyses in the sub-population of patients with the S/C genotype. A multivariate analysis by logistic regression was performed to search for factors associated with the occurrence of a postoperative complication in the whole sample. Given the small number of events, the models tested included only two explanatory variables: preoperative transfusion and another variable (Hb phenotype (S/S and S/β0 vs. other), VOC in the previous month, ACS in the previous 6 months or lifetime, and acute cholecystitis or not). p values less than 0.05 were considered significant. All analyses were performed with the R software (Ross Ihaka, Robert Gentleman, Auckland, New Zealand) (version 4.0.3, 2020).
The local institutional board approved this study on 26 March 2020 (IRB registration number #00011928, Comité d’Ethique de la Recherche Assistance Publique Hôpitaux de Paris (CERAPHP.5)) as non-interventional research using data collected for routine clinical practice. In line with the French legislation on retrospective studies of routine clinical practice, participants were not required to give their written informed consent. Patients included in this study were all informed that their medical data could be used for research purposes, in accordance with General Data Protection Regulation 2016/679.
3. Results
3.1. Patients’ Characteristics and Transfusion Rate
A total of 79 SCD adult patients (43% male) were included. The median age was 27.6 years (IQR 23.6–32.5) and 64 (81%) were of the S/S or S/β0-thalassemia genotype. The main characteristics of the patients are reported in Table 1. All patients underwent a laparoscopic cholecystectomy, with a median total length of hospital stay of 4 days (IQR 3–9.5). The pathological analysis of the gallbladder found lesions of chronic cholecystitis (n = 73, 97%) and the presence of gallstones (n = 50, 66%). Lesions of acute or subacute cholecystitis were observed in 15 patients (20%).

Table 1.
Patients’ characteristics and comparison according to prophylactic transfusion.
The trend over the study period of the percentage of patients with SCD who received pre-cholecystectomy PT is reported in Figure 1. Although the decrease is statistically not significant (p = 0.20), we observed that PT in our center is no longer systematic since 2010. There was no other major management practice change during the study period.
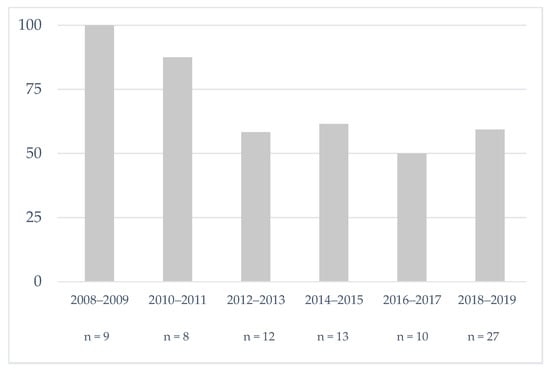
Figure 1.
Evolution of the percentage of sickle cell patients who received pre-cholecystectomy prophylactic transfusion in our center.
3.2. Comparison According to Prophylactic Transfusion
We compared the 52 patients (66%) who received PT before a cholecystectomy with those who did not (Table 1). Transfused patients were more likely to have a S/S or S/β0 genotype (88.5% vs. 66.7%, p = 0.04) and to receive hydroxyurea therapy (42.6% vs. 23.8%, p = 0.13). There was no other difference in demographics, chronic complications, or history of ACS before surgery between transfused and non-transfused patients. The pre-procedure hemoglobin levels (pre-operative or pre-PT) were not statistically different between the two groups (9.2 vs. 9.5 g/dL, p = 0.59). However, transfused patients had more preoperative vaso-occlusive complications, including more VOC in the month before surgery (39.2% vs. 14.8%, p = 0.05), with the same trend in the 6 preoperative months (62.7% vs. 44.4%, p = 0.19). They were more often operated in an emergency setting (38.5% vs. 14.8%, p = 0.06). The median length of stay was comparable between the two groups: 4 (3.8–10) days in the transfused group versus 3 (3–6.5) days in the non-transfused group (p = 0.11).
The prevalence of VOE (VOC or ACS or death) in the postoperative month between transfused and non-transfused patients were not statistically different (19.2% vs. 29.6%, p = 0.45) (Table 2). One non-transfused S/C patient died of an unexplained coma one day after surgery. Four patients in the transfused group (7.7%) required readmission within one month of discharge: one for VOC, one for VOC and DHTR, one for DHTR, and one for a postoperative collection requiring revision surgery. In the non-transfused group, five patients (18.5%) were readmitted (p = 0.26 compared to transfused patients): four for ACS and one for VOC. Five patients required postoperative transfusion, four (7.7%) in the transfused group and one (3.7%) in the non-transfused group (p = 0.84).

Table 2.
Postoperative complications according to prophylactic transfusion.
Four patients acquired new allo-antibodies with implications for subsequent transfusions (8.5%) in the transfused group: anti RH4, RH6, MNS1, MNS3 (n = 1), anti JKB (n = 1), anti RH10 private (n = 1), and anti RH2 KEL3 and MNS3 (n = 1). Two of these patients (4.3% of transfused patients), 34- and 23-year-old S/S patients, developed DHTR at 11 and 19 days of prophylactic transfusion, respectively, and both required readmission. The lowest hemoglobin levels for these two patients were 5 g/dL and 6.7 g/dL, respectively. Considering all severe complications (hospitalized VOC or ACS or death or alloimmunization) in the postoperative month, the incidence was not statistically different between the two groups (26.9% in transfused vs. 29.6% in non-transfused).
3.3. Comparison of Non-Transfused Patients
We then focused on the 27 patients who did not receive PT. As already shown, 8 of them (29.6%) developed VOC (n = 1), ACS (n = 6), or died (n = 1). Only one of these patients was transfused postoperatively, without secondary alloimmunization, for an ACS that occurred at day 8 after surgery, without secondary alloimmunization. We compared the characteristics of these 8 patients with the 19 non-transfused patients who did not develop any postoperative complications. The sociodemographic characteristics, proportion of S/S or S/β0-thalassemia patients, prevalence of chronic complications, and history of ACS were comparable between the 2 groups (Table 3). Non-transfused patients with a history of VOC hospitalized within 6 months before surgery were more frequent among the non-transfused patients who developed post-operative complications: 6/8 (75%) versus 6/19 (31.6%) among those who did not (p = 0.10). The median time from last VOE to surgery was 105 (52.3–204) days in patients with postoperative complications, versus 270 (120–366) days in other non-transfused patients (p = 0.14). Patients operated on in an emergency setting also seemed to undergo more VOE (25% vs. 10.5%), although this was not statistically significant (p = 0.71).

Table 3.
Characteristics of patients without prophylactic transfusion according to the occurrence of postoperative complications.
A total of 34 patients of our cohort had neither hospitalized VOC within 6 months preoperatively nor emergency surgery. These patients had fewer postoperative VOE, regardless of their preoperative transfusion status: 13.3% (2/15) in non-transfused patients and 15.8% (3/19) in transfused patients. Among these 19 patients with PT, there were 2 (10.5%) transfusion-related complications: 1 alloimmunization and 1 DHTR.
3.4. Characteristics of Patients Experiencing VOE
Regardless of PT status, 18 patients (22.8%) had experienced a postoperative VOE within one month after surgery (n = 17) or death (n = 1). The characteristics of these patients are shown in Table A2. Overall, 13 (72.2%) had a history of VOCs within 6 months before the surgery versus 30/59 (50.8%) in those without post-operative VOE (p = 0.18). Moreover, 8 (44.4%) were operated in an emergency setting versus 15 (25%) in patients without post operative VOE, (p = 0.20). A gallbladder histopathological analysis revealed more frequent lesions of acute or subacute cholecystitis in patients with post-operative VOE (7/18, 38.9% vs. 8/57, 14%, p = 0.05).
3.5. Outcome in S/C Population
Our population included 11 patients with the S/C genotype (63% male) and a median age of 35.8 years (IQR 30.2–39.9). None was receiving hydroxyurea therapy at the time of the surgery. They had a comparable history of ACS as patients with S/S and S/β0 genotypes (8/11, 72.7%, vs. 44/64, 68.8%, p = 1). PT was performed in 5 (45.5%) of them versus 46 (71.9%) in patients with S/S and S/β0 genotypes (p = 0.15). Two patients with the S/C genotype (18%), who had not been transfused, experienced severe postoperative VOE: an ACS at D3 postoperative that required readmission (without the need of a transfusion), and a death at day 1 postoperative because of an unexplained coma, without associated VOE.
4. Discussion
This study shows an evolution of practice in our referral center towards transfusion sparing since 2010. We did not find any statistically significant difference in terms of prevalence of occurrence of VOC or ACS or death in the postoperative month between patients who did or did not receive PT for a cholecystectomy. In the randomized trial published in 2013 by Howard et al. [16] comparing prophylactic transfusion before various low and medium-risk surgeries (including 44% abdominal surgeries) in 70 patients, there were 39% postoperative complications in non-transfused patients compared with 15% complications in transfused patients postoperatively. The complication rate in non-transfused patients was higher than in our study (39% vs. 29.6%): this can be explained by several factors. First, the retrospective nature of our study induces a bias of indication of PT, with an enrichment of less severe SCD phenotypes in the group of patients without PT even when considering patients with S/S and S/β0-genotype, as in the study by Howard et al. (27.7% of complication rate). Second, in our study, the proportion of patients treated by hydroxyurea therapy was higher (36.8% vs. 11%).
In our study, the most frequent VOE that occurred after surgery were ACS (44.4% of all VOE). This rate is comparable to that found in the study by Howard et al. and is consistent with numerous studies finding an increased risk of ACS perioperatively in abdominal surgery, although almost all of the surgeries were performed by laparoscopy [13,14,21]. One possible pathophysiological explanation is the use of carbon dioxide to perform the pneumoperitoneum, which may cause postoperative hypercapnia [15].
Our study also shows that the practice of transfusion sparing has prevented transfusion complications. The rates of alloimmunization and DHTR in our series (8.5% and 4.3%, respectively) among the transfused patients are consistent with the results of the literature (7.5% of alloimmunization in the study by Howard et al., but no DHTR). If VOC or ACS can be managed (including with postoperative transfusion, if necessary), alloimmunization, in addition to the immediate lethal risk of DHTR (5 to 10% depending on the series) [22,23,24], often may condemn the possibilities of subsequent transfusions in these young patients during severe VOE.
The analysis of the 8/27 non-transfused patients with postoperative complications (29.6%) revealed that these patients did not have more preoperative history of ACS than patients without postoperative complications (75% vs. 73.7%) but had more frequently been hospitalized for a VOC in the past 6 months (75% vs. 31.6%). The latter result was not statistically significant, possibly because of a lack of power due to the small sample size. However, it is reasonable to assume that a recently hospitalized crisis may be a ground for postoperative vaso-occlusive complications.
The occurrence of a postoperative VOE in our series was more frequent in patients operated in an urgent setting (44%). In a large American pediatric study evaluating the risk factors associated with the occurrence of postoperative vaso-occlusive complications in abdominal surgery, the urgent nature of the surgery was the only significant predictive factor of complications (OR 1.83, 95% CI 1.02–3.29, p = 0.04), whether the patients were transfused or not [25]. Likewise, in the French series conducted at Tenon Hospital by Muroni et al. (103 patients), postoperative vaso-occlusive complications were less frequent in the case of a prophylactic laparoscopic cholecystectomy in asymptomatic patients (11.5%) than in patients with an acute vesicular complication (25.5%) [26]. Significant differences were also found by Currò et al. (study that included 30 patients) in terms of operative time, morbidity rate, postoperative length of stay, and total length of hospitalization between children operated before the onset of abdominal symptoms and children operated after the onset of symptoms [27]. These patients are therefore at greater risk of postoperative complications and could clearly benefit from PT.
The modalities of perioperative management have rarely been evaluated in patients with the S/C genotype: two trials specifically excluded these patients [16,28], and one trial included only nine of them [20]. However, the risk of vaso-occlusive complications seems to be increased in case of abdominal surgery in these patients [29]. Our study, though it included a limited number of S/C patients (n = 11), shows that the rate of postoperative complications is significant (18% of patients, including one unexplained death). This indicates that S/C patients are also at risk of complications in the peri-operative period and could also benefit from prophylactic transfusion in case of recent VOE.
As mentioned above, the main limitation of our study is related to the indication bias for PT that artificially reduces the difference of post-operative complications between transfused and non-transfused patients. In addition, the data were recorded retrospectively, but the existence of a prospective database in our center systematically collecting SCD data at each appointment in our center allowed us to minimize the risk of missing data. Nevertheless, the reasons for indication (or non-indication) of transfusion were unfortunately not specified in the files. Furthermore, the small size of the study induces a lack of statistical power, although the sample size is comparable to the main studies studying the benefit of preoperative prophylactic transfusion in patients with SCD [16,30].
5. Conclusions
Transfusion sparing before a planned cholecystectomy seems to be a safe option in properly selected stable SCD patients, with no history of hospitalization for VOC within the 6 previous months, and in the context of planned surgery. This targeted strategy could allow avoiding potentially dangerous alloimmunizations for the long-term management of sickle cell patients. It should therefore be tested in a randomized controlled study, taking both the short-term risk of VOE and the long-term risk of alloimmunization into account.
Author Contributions
Conceptualization, E.R. and J.-B.A.; formal analysis, B.R.; investigation, E.R., S.T., L.J., N.B., R.D., A.F., J.P. and J.-B.A.; methodology, E.R. and J.-B.A.; validation, B.R., J.P. and J.-B.A.; writing—original draft, E.R. and J.-B.A.; writing—review & editing, B.R., L.J., N.B., R.D., A.F. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of European Georges Pompidou University Hospital (Comité d’Ethique de la Recherche Assistance Publique Hôpitaux de Paris (CERAPHP.5)) (IRB registration number #00011928, 26 March 2020).
Informed Consent Statement
Patient written informed consent was not required in line with the French legislation on retrospective studies of routine clinical practice. Patients included in this study were all informed that their medical data could be used for research purpose, in accordance with General Data Protection Regulation 2016/679.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Djamal Khimoud and all participants in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Disease coding used (International Classification of Diseases).
Table A1.
Disease coding used (International Classification of Diseases).
| D57.0 | Hb-SS disease with crisis |
| D57.01 | Hb-SS disease with acute chest syndrome |
| D57.02 | Hb-SS disease with splenic sequestration |
| D57.09 | Hb-SS disease with other specified complication |
| K82.9 | Disease of gallbladder, unspecified |
| Z90.4 | Acquired absence of other specified parts of digestive tract |
| Y83.6 | Removal of other organ (partial) (total) as the cause of abnormal reaction of the patient, or of later complication, without mention of misadventure at the time of the procedure |
Hb: hemoglobin.

Table A2.
Comparison of patients according to postoperative vaso-occlusive events (VOC and/or ACS) and/or death.
Table A2.
Comparison of patients according to postoperative vaso-occlusive events (VOC and/or ACS) and/or death.
| No Postoperative VOE or Death n = 60 | Postoperative VOE (VOC and/or ACS) and/or Death n = 18 | p | |
|---|---|---|---|
| Age | 27.1 (23.4–30.8) | 30.1 (23.9–35.2) | 0.14 |
| Sex (M) | 27/60 (45) | 6/18 (33.3) | 0.54 |
| BMI (kg/m2) | 21.9 (19.9–24.5) | 20.7 (18.2–23.7) | 0.1 |
| Sickle cell genotype | |||
| S/S + S/β0-thalassemia | 48/60 (80) | 15/18 (83.3) | 1 |
| S/β+-thalassemia | 3/60 (5) | 1/18 (5.6) | 1 |
| S/C | 9/60 (15) | 2/18 (11.1) | 1 |
| Current hydroxyurea treatment among S/S and S/β0 patients 1 | 18/48 (37.5) | 6/15 (40) | 1 |
| Dose (mg) (mean ± SD) | 1125 ± 385.9 | 1000 ± 418.3 | 0.5 |
| Preoperative alloimmunization | 2/57 (3.5) | 4/16 (25) | 0.02 |
| Preoperative transfusion, lifetime | 41/60 (68.3) | 10/18 (55.6) | 0.47 |
| Number of transfused RBC units | 2 (2–2) | 2 (2–2) | 0.26 |
| Urgent surgery | 15/60 (25) | 8/18 (44.4) | 0.2 |
| Preoperative acute complications | |||
| VOC in the past 1 month | 15/59 (25.4) | 8/18 (44.4) | 0.21 |
| VOC in the past 6 months | 30/59 (50.8) | 13/18 (72.2) | 0.18 |
| ACS in the past 6 months | 15/59 (25.4) | 3/18 (16.7) | 0.65 |
| VOC and/or ACS in the past 6 months | 31/59 (52.5) | 13/18 (72.2) | 0.23 |
| Time between the last VOE and surgery (days) | 170.0 (30–366) | 45 (30–312) | 0.18 |
| History of ACS, lifetime | 41/60 (68.3) | 13/18 (72.2) | 0.98 |
| Number of previous ACS | 1 (0–2) | 1 (0.25–2.75) | 0.91 |
| Chronic complications with percentage > 20% | |||
| Nephropathy | 14/60 (23.3) | 2/18 (11.1) | 0.34 |
| Retinopathy | 18/60 (30) | 3/18 (16.7) | 0.37 |
| Osteonecrosis | 14/60 (23.3) | 5/18 (27.8) | 0.76 |
| Priapism (in men only) | 7/27 (25.9) | 1/6 (16.7) | 1 |
| Length of hospital stay (days) | 4 (3–6) | 10 (5.5–12) | 0.001 |
| Anatomical pathology | |||
| Presence of gallstones | 38/57 (66.7) | 12/18 (66.7) | 1 |
| Chronic cholecystitis | 55/56 (98.2) | 17/18 (94.4) | 0.43 |
| Acute or subacute cholecystitis | 8/57 (14) | 7/18 (38.9) | 0.05 |
| Postoperative complications | |||
| Death (%) | 0 | 1/18 (5.6) | 0.23 |
| VOC and/or ACS (%) | - | 17/18 (94.4) | - |
| ICU admission (%) | 2/60 (3.3) | 3/18 (16.7) | 0.08 |
| Postoperative infection (except ACS) | 3/60 (5) | 3/18 (16.7) | 0.13 |
| Readmission < 1 month after surgery | 2/60 (3.3%) 2 | 7/18 (38.9) | 0.001 |
| Time between surgery and readmission (days) | 4 (2.5–5.5) | 13.0 (6.8–24.8) | 0.32 |
| Postoperative transfusion less than 1 month after surgery | 2/60 (3.3) | 3/18 (16.7) | 0.08 |
| Number of RBC units transfused | 2 (1.5–2.5) | 2 (2–3) | 0.6 |
Values are expressed as median (IQR) or n/N (%) unless specified. ACS: acute chest syndrome, BMI: body mass index, DHTR: delayed haemolytic transfusion reaction, ICU: intensive care unit, RBC: red blood cells, VOC: vaso-occlusive bone crisis, VOE: vaso-occlusive event. 1 No current hydroxyurea treatment among patients with S/C genotype. 2 Reason for readmission: 1 DHTR, 1 postoperative collection.
References
- Alhawsawi, Z.M.; Alshenqeti, A.M.; Alqarafi, A.M.; Alhussayen, L.K.; Turkistani, W.A. Cholelithiasis in patients with paediatric sickle cell anaemia in a Saudi hospital. J. Taibah Univ. Med. Sci. 2019, 14, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sarnaik, S.; Slovis, T.L.; Corbett, D.P.; Emami, A.; Whitten, C.F. Incidence of cholelithiasis in sickle cell anemia using the ultrasonic gray-scale technique. J. Pediatr. 1980, 96, 1005–1008. [Google Scholar] [CrossRef]
- Martins, R.A.; Soares, R.S.; De Vito, F.B.; Barbosa, V.D.F.; Silva, S.S.; Moraes-Souza, H.; Martins, P.R.J. Cholelithiasis and its complications in sickle cell disease in a university hospital. Rev. Bras. Hematol. Hemoter. 2017, 39, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Issa, H.; Al-Salem, A.H. Hepatobiliary Manifestations of Sickle Cell Anemia. Gastroenterol. Res. 2010, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Ferguson, V.; Nelson, M.A. Treatment of Cholelithiasis in Children with Sickle Cell Disease. AORN J. 2003, 77, 1169–1182. [Google Scholar] [CrossRef]
- Arlet, J.-B.; de Luna, G.; Khimoud, D.; Odièvre, M.-H.; de Montalembert, M.; Joseph, L.; Chantalat-Auger, C.; Flamarion, E.; Bartolucci, P.; Lionnet, F.; et al. Prognosis of patients with sickle cell disease and COVID-19: A French experience. Lancet Haematol. 2020, 7, e632–e634. [Google Scholar] [CrossRef]
- Yawn, B.P.; Buchanan, G.R.; Afenyi-Annan, A.N.; Ballas, S.K.; Hassell, K.L.; James, A.H.; Jordan, L.; Lanzkron, S.; Lottenberg, R.; Savage, W.J.; et al. Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA 2014, 312, 1033–1048. [Google Scholar] [CrossRef]
- Sickle Cell Society. Standards for the Clinical Care of Adults with Sickle Cell Disease in the UK. 2018. Available online: https://www.sicklecellsociety.org/wp-content/uploads/2018/05/Standards-for-the-Clinical-Care-of-Adults-with-Sickle-Cell-in-the-UK-2018.pdf (accessed on 11 December 2020).
- Habibi, A.; Arlet, J.-B.; Stankovic, K.; Gellen-Dautremer, J.; Ribeil, J.-A.; Bartolucci, P.; Lionnet, F. French guidelines for the management of adult sickle cell disease: 2015 update. Rev. Med. Internet 2015, 36, S3–S84. [Google Scholar]
- Haute Autorité de Santé (HAS). Guide Drépanocytose Adulte. 2010. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2010-04/ald_10_lap_drepano_adulte_web.pdf (accessed on 11 December 2020).
- Haute Autorité de Santé (HAS). Prise en Charge de la Drépanocytose chez L’enfant et L’adolescent—Recommandations Pour la Pratique Clinique. 2005. Available online: https://www.has-sante.fr/jcms/c_240664/fr/prise-en-charge-de-la-drepanocytose-chez-l-enfant-et-l-adolescent (accessed on 12 December 2020).
- Vichinsky, E.P.; Neumayr, L.D.; Earles, A.N.; Williams, R.; Lennette, E.T.; Dean, D.; Nickerson, B.; Orringer, E.; McKie, V.; Bellevue, R.; et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N. Engl. J. Med. 2000, 342, 1855–1865. [Google Scholar] [CrossRef] [Green Version]
- Haberkern, C.M.; Neumayr, L.D.; Orringer, E.P.; Earles, A.N.; Robertson, S.M.; Black, D.; Abboud, M.R.; Koshy, M.; Idowu, O.; Vichinsky, E.P. Cholecystectomy in sickle cell anemia patients: Perioperative outcome of 364 cases from the National Preoperative Transfusion Study. Preoperative Transfusion in Sickle Cell Disease Study Group. Blood 1997, 89, 1533–1542. [Google Scholar]
- Wales, P.W.; Carver, E.; Crawford, M.W.; Kim, P.C. Acute chest syndrome after abdominal surgery in children with sickle cell disease: Is a laparoscopic approach better? J. Pediatr. Surg. 2001, 36, 718–721. [Google Scholar] [CrossRef] [PubMed]
- de’Angelis, N.; Abdalla, S.; Carra, M.C.; Lizzi, V.; Martínez-Pérez, A.; Habibi, A.; Bartolucci, P.; Galactéros, F.; Laurent, A.; Brunetti, F. Low-impact laparoscopic cholecystectomy is associated with decreased postoperative morbidity in patients with sickle cell disease. Surg. Endosc. 2018, 32, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Malfroy, M.; Llewelyn, C.; Choo, L.; Hodge, R.; Johnson, T.; Purohit, S.; Rees, D.C.; Tillyer, L.; Walker, I.; et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: A randomised, controlled, multicentre clinical trial. Lancet 2013, 381, 930–938. [Google Scholar] [CrossRef]
- Adams, D.M.; Ware, R.E.; Schultz, W.H.; Ross, A.K.; Oldham, K.T.; Kinney, T.R. Successful surgical outcome in children with sickle hemoglobinopathies: The Duke University experience. J. Pediatr. Surg. 1998, 33, 428–432. [Google Scholar] [CrossRef]
- Goodwin, E.F.; Partain, P.I.; Lebensburger, J.D.; Fineberg, N.S.; Howard, T.H. Elective cholecystectomy reduces morbidity of cholelithiasis in pediatric sickle cell disease. Pediatr. Blood Cancer 2017, 64, 113–120. [Google Scholar] [CrossRef]
- Griffin, T.C.; Buchanan, G.R. Elective surgery in children with sickle cell disease without preoperative blood transfusion. J. Pediatric Surg. 1993, 28, 681–685. [Google Scholar] [CrossRef]
- Al Jaouni, S.; Al-Muhayawi, S.; Qari, M.; Nawas, M.; Al-Mazrooa, A. Randomized Clinical Trial to Evaluate the Safety of Avoiding Pre-operative Transfusion in Sickle Cell Anemia. Bahrain Med. Bull. 2006, 28, 164–167. [Google Scholar]
- Estcourt, L.J.; Kimber, C.; Trivella, M.; Doree, C.; Hopewell, S. Preoperative blood transfusions for sickle cell disease. Cochrane Database Syst. Rev. 2020, 7, CD003149. [Google Scholar] [CrossRef] [Green Version]
- Rieux, C.; Brittenham, G.; Bachir, D.; De Meyer, E.; Boudjedir, K. Delayed hemolytic transfusion reaction in the French hemovigilance system. Transfus. Clin. Biol. 2019, 26, 109–111. [Google Scholar] [CrossRef]
- Habibi, A.; Mekontso-Dessap, A.; Guillaud, C.; Michel, M.; Razazi, K.; Khellaf, M.; Chami, B.; Bachir, D.; Rieux, C.; Melica, G.; et al. Delayed hemolytic transfusion reaction in adult sickle-cell disease: Presentations, outcomes, and treatments of 99 referral center episodes. Am. J. Hematol. 2016, 91, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Narbey, D.; Habibi, A.; Chadebech, P.; Mekontso-Dessap, A.; Khellaf, M.; Lelièvre, J.-D.; Godeau, B.; Michel, M.; Galactéros, F.; Djoudi, R.; et al. Incidence and predictive score for delayed hemolytic transfusion reaction in adult patients with sickle cell disease. Am. J. Hematol. 2017, 92, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, C.W.; Bludevich, B.M.; Gonzalez, R.; Danielson, P.D.; Chandler, N.M. Risk factors for complications after abdominal surgery in children with sickle cell disease. J. Pediatr. Surg. 2021, 56, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Muroni, M.; Loi, V.; Lionnet, F.; Girot, R.; Houry, S. Prophylactic laparoscopic cholecystectomy in adult sickle cell disease patients with cholelithiasis: A prospective cohort study. Int. J. Surg. 2015, 22, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Currò, G.; Meo, A.; Ippolito, D.; Pusiol, A.; Cucinotta, E. Asymptomatic Cholelithiasis in Children with Sickle Cell Disease. Ann. Surg. 2007, 245, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.P.; Haberkern, C.M.; Neumayr, L.; Earles, A.N.; Black, D.; Koshy, M.; Pegelow, C.; Abboud, M.; Ohene-Frempong, K.; Iyer, R.V. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N. Engl. J. Med. 1995, 333, 206–213. [Google Scholar] [CrossRef]
- Neumayr, L.; Koshy, M.; Haberkern, C.; Earles, A.N.; Bellevue, R.; Hassell, K.; Miller, S.; Black, D.; Vichinsky, E. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. Am. J. Hematol. 1998, 57, 101–108. [Google Scholar] [CrossRef]
- Fu, T.; Corrigan, N.J.; Quinn, C.T.; Rogers, Z.R.; Buchanan, G.R. Minor elective surgical procedures using general anesthesia in children with sickle cell anemia without pre-operative blood transfusion. Pediatr. Blood Cancer 2005, 45, 43–47. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).