Hospital-Acquired Anemia in Patients Hospitalized in the Intensive Care Unit: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Patients Data
2.3. IBL Data
2.4. Statistical Analysis
2.5. Ethics
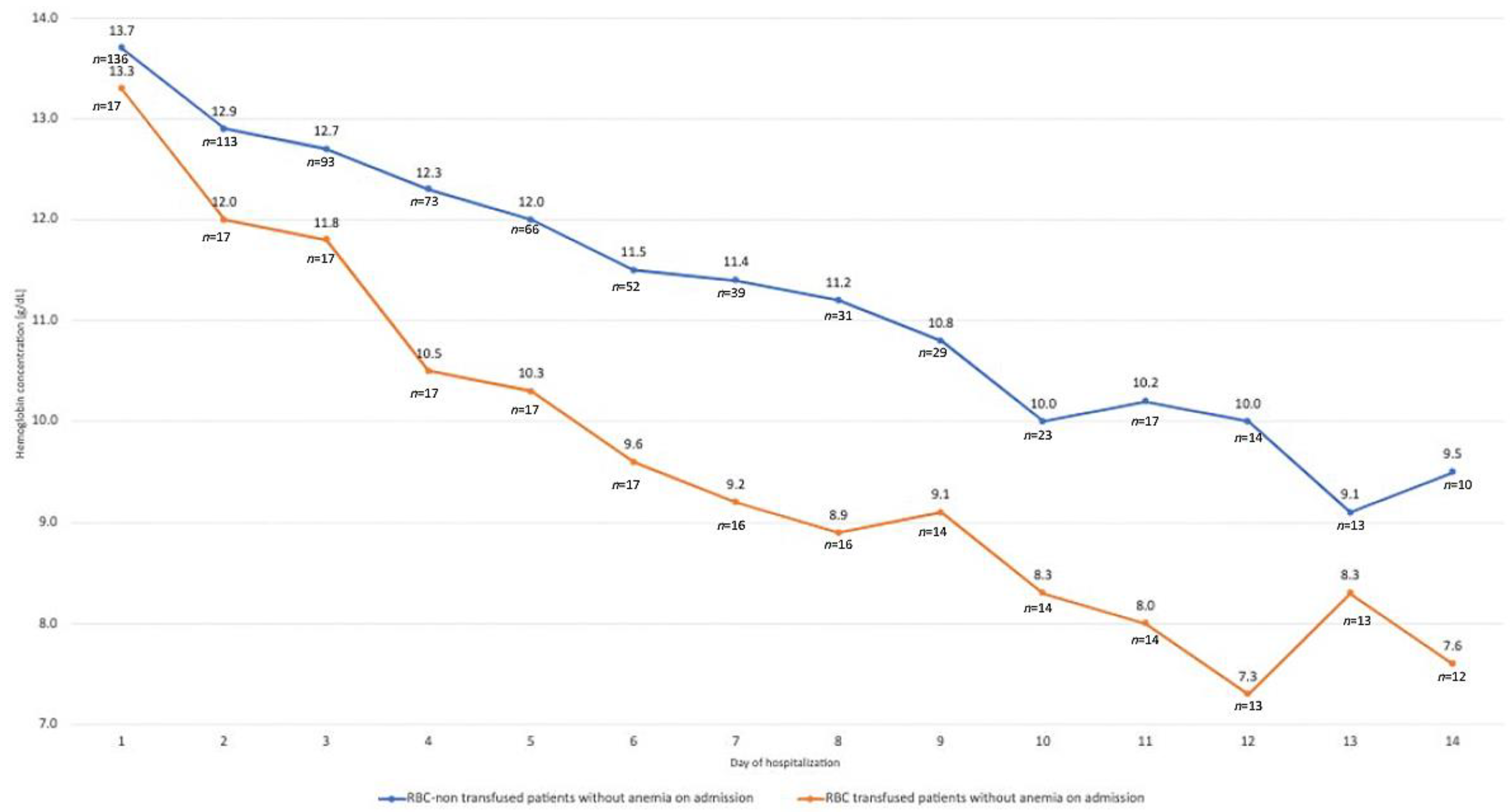
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, J.L.; Baron, J.F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D. Anemia and blood transfusion in critically ill patients. JAMA 2002, 288, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Corwin, H.L.; Gettinger, A.; Pearl, R.G.; Fink, M.P.; Levy, M.M.; Abraham, E.; MacIntyre, N.R.; Shabot, M.M.; Duh, M.-S.; Shapiro, M.J. The CRIT Study: Anemia and blood transfusion in the critically ill—Current clinical practice in the United States. Crit. Care Med. 2004, 32, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Jensen, L.; Nahirniak, S.; Gibney, R.T. Anemia and blood transfusion practices in the critically ill: A prospective cohort review. Heart Lung 2010, 39, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.G.; Li, L.; Sun, Z.; Hixson, E.D.; Tang, A.; Phillips, S.C.; Blackstone, E.H.; Henderson, J.M. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J. Hosp. Med. 2013, 8, 506–512. [Google Scholar] [CrossRef]
- Holland, J.; Peralta, R.M.; Moss, R.L.; Feane, K.; Uprichard, J. A single-centre review of iatrogenic anaemia in adult intensive care. Transfus. Med. 2020, 30, 196–200. [Google Scholar] [CrossRef]
- Witosz, K.; Wojnarowicz, O.; Krzych, Ł.J. Iatrogenic blood loss due to daily laboratory testing and the risk of subsequent anaemia in intensive care unit patients: Case series. Acta Biochim. Pol. 2021, 68, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Donahoe, L.; Clarke, F.J.; Tkaczyk, A.J.; Heels-Ansdell, D.; Zytaruk, N.; Cook, R.; Webert, K.E.; McDonald, E.; Cook, D.J. Bleeding during critical illness: A prospective cohort study using a new measurement tool. Clin. Investig. Med. 2007, 30, E93–E102. [Google Scholar] [CrossRef]
- Joannidis, M.; Oudemans-van Straaten, H.M. Clinical review: Patency of the circuit in continuous renal replacement therapy. Crit. Care 2007, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Angerås, U.; Bergqvist, D.; Eriksson, B.; Lassen, M.R.; Fisher, W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010, 8, 202–204. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Klum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef][Green Version]
- Lauzier, F.; Arnold, D.M.; Rabbat, C.; Heels-Ansdell, D.; Zarychanski, R.; Dodek, P.; Ashley, B.J.; Albert, M.; Khwaja, K.; Ostermann, M.; et al. Risk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxis. Intensive Care Med. 2013, 39, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Cable, C.A.; Razavi, S.A.; Roback, J.D.; Murphy, D.J. RBC Transfusion Strategies in the ICU: A Concise Review. Crit. Care Med. 2019, 47, 1637–1644. [Google Scholar] [CrossRef]
- Zouk, A.N.; Batra, H. Managing complications of percutaneous tracheostomy and gastrostomy. J. Thorac. Dis. 2021, 13, 5314–5330. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Bedert, L.L.; Delcroix, M.; Depuydt, P.; Lauwers, G.G.; Sokolov, Y.Y.; Van Meerhaeghe, A.; Van Schil, P. Tracheotomy: Clinical review and guidelines. Eur. J. Cardio-Thorac. Surg. 2007, 32, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Brass, P.; Hellmich, M.; Ladra, A.; Ladra, J.; Wrzosek, A. Percutaneous techniques versus surgical techniques for tracheostomy. Cochrane Database Syst. Rev. 2016, 7, CD008045. [Google Scholar] [CrossRef] [PubMed]
- Tosiri, P.; Kanitsap, N.; Kanitsap, A. Approximate iatrogenic blood loss in medical intensive care patients and the causes of anemia. J. Med. Assoc. Thail. 2010, 93, S271–S276. [Google Scholar]
- Cook, D.J.; E Griffith, L.; Walter, S.D.; Guyatt, G.H.; O Meade, M.; Heyland, D.K.; Kirby, A.; Tryba, M. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit. Care 2001, 5, 368–375. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Value |
|---|---|
| Sex (female/male) [n, %] | 168 (45.3)/203 (54.7) |
| Age (female/male), median, IQR 1 [years] | 65 (53–72)/65 (55–82) |
| Sepsis/septic shock [n, %] | 175 (47.2%) |
| Patients with acute injuries: | |
| -acute kidney injury [n, %] | 153 (41.2) |
| -acute liver injury [n, %] | 62 (16.7) |
| Severity of disease: | |
| -SAPS II 2, median, IQR [points] | 44 (31–59) |
| -APACHE II 3, median, IQR [points] | 18 (12–24) |
| -SOFA 4, median, IQR [points] | 9.0 (5–12) |
| ICU 5 length of stay, median, IQR [days] | 6.0 (3.0–14.0) |
| ICU mortality [n, %] | 150 (40.2%) |
| Parameter | All Patients (Median, IQR 1) | Anemia on Admission (n = 218) (Median, IQR) | No Anemia on Admission (n = 153) (Median, IQR) | p |
|---|---|---|---|---|
| Hb 2 [g/dL] | 11.6 (9.8–13.3) | 10.2 (9.1–11.2) | 13.6 (12.7–15.2) | <0.01 |
| Hct 3 [%] | 34.9 (29.9–40.1) | 30.6 (27.8–34.1) | 41.0 (37.4–45.0) | <0.01 |
| RBC 4 [x 106/µL] | 3.9 (3.3–4.4) | 3.4 (3.04–3.83) | 4.5 (4.2–5.0) | <0.01 |
| MCV 5 [fL] | 90.2 (86.4–94.6) | 89.5 (85.7–94.4) | 91.0 (87.6–94.7) | 0.20 |
| MCH 6 [pg] | 30.1 (28.8–31.6) | 29.9 (28.4–31.1) | 30.5 (29.1–31.8) | 0.01 |
| MCHC 7 [g/dL] | 33.3 (32.1–34.2) | 33.1 (31.7–34.0) | 33.8 (32.7–34.3) | <0.01 |
| RDW 8 [%] | 14.5 (13.4–16.1) | 15.3 (13.7–17.2) | 13.7 (12.8–14.8) | <0.01 |
| RDW-SD 9 [fL] | 48.4 (43.8–53.0) | 49.9 (46.0–55.8) | 45.9 (42.2–49.8) | <0.01 |
| Ferritin (n = 71) [ng/mL] | 773.1 (300.1–1599.5) | 795.6 (428.5–1760.9) | 646.9 (179.4–1391.0) | 0.30 |
| Iron (n = 69) [µg/dL] | 32.0 (16.7–69.5) | 32.0 (14.3–62.9) | 36.7 (18.1–102.0) | 0.21 |
| Transferrin (n = 69) [mg/dL] | 136.2 (96.4–182.1) | 119.8 (90.8–158.6) | 182.8 (151.7–205.9) | <0.01 |
| TS 10 (n = 69) [%] | 16.7 (7.9–33.5) | 17.6 (8.1–31.7) | 16.6 (7.5–55.7) | 0.99 |
| Fibrinogen [mg/dL] | 447.0 (300.0–619.7) | 481.5 (333.0–650.0) | 403.0 (271.3–561.5) | 0.01 |
| D-dimers [ng/mL] | 4139.5 (1818.0–7473.5) | 5107 (2265.5–7617.5) | 2430.0 (1327.0–7341.0) | <0.01 |
| Thrombin time [s] | 17.7 (15.8–20.8) | 17.8 (15.9–20.9) | 17.7 (15.7–21.2) | 0.90 |
| aPTT [s] | 35.1 (29.6–41.4) | 36.7 (30.1–43.1) | 32.3 (27.8–38.8) | <0.01 |
| Prothrombin time [s] | 14.1 (12.7–16.7) | 15.0 (13.0–17.8) | 13.2 (12.5–15.2) | <0.01 |
| INR | 1.2 (1.1–1.5) | 1.3 (1.1–1.6) | 1.2 (1.1–1.3) | <0.01 |
| Platelets [× 103/µL] | 228.0 (165.0–306.2) | 212.5 (141.0–318.0) | 242.0 (183.8–296.3) | 0.03 |
| Severity of Disease | Correlation Coefficient | p |
|---|---|---|
| All patients: | ||
| SAPS II 1 | −0.10 | 0.20 |
| APACHE II 2 | −0.04 | 0.65 |
| SOFA 3 | −0.13 | 0.09 |
| Non-anemic patients: | ||
| SAPS II | −0.11 | 0.43 |
| APACHE II | −0.07 | 0.58 |
| SOFA | −0.16 | 0.23 |
| Anemic patients: | ||
| SAPS II | −0.04 | 0.66 |
| APACHE II | −0.05 | 0.58 |
| SOFA | −0.09 | 0.38 |
| Procedure | Value |
|---|---|
| Continuous renal replacement therapy | |
| -number of patients [n, %] | 113 (30.5) |
| -duration, median, IQR [days] | 4 (2–8) |
| -circuit clotting [n, %] | 20 (17.7) |
| -bleeding at catheter insertion site [n, %] | 15 (13.3) |
| Therapeutic plasma exchange | |
| -number of patients [n, %] | 21 (5.7) |
| -circuit clotting [n, %] | 2 (9.5) |
| -bleeding at catheter insertion site [n, %] | 5 (23.8) |
| Central venous catheter | |
| -number of patients [n, %] | 257 (69.3) |
| -bleeding at insertion site [n, %] | 20 (7.8) |
| Tracheostomy | |
| -number of patients [n] | 60 (16.2) |
| -episodes of bleeding at insertion site [n, %] | 28 (46.7) |
| Percutaneous endoscopic gastrostomy | |
| -number of patients [n] | 42 (11.3) |
| -bleeding at insertion site [n, %] | 4 (9.5) |
| Factor | Value |
|---|---|
| Number of test tubes per patient daily [n, IQR 1]: | |
| -arterial blood gas (volume 1.0 mL) | 2.0 (1.9−2.4) |
| -biochemistry (2.5 mL) | 1.0 (0.9−1.2) |
| -complete blood count (2.0 mL) | 1.0 (0.7−1.1) |
| -blood culture (10 mL) | 0.6 (0.2−1.0) |
| -coagulation (2.7 mL) | 0.3(0.2−0.7) |
| Pts 2 with bleeding from tracheobronchial tree [n, %] | 66 (17.8) |
| Pts 2 with bloody postoperative drainage [n, %] | 65 (17.5) |
| Pts 2 with gastrointestinal bleeding [n, %] | 32 (8.6) |
| Pts 2 with bleeding from surgical wound [n, %] | 29 (7.8) |
| Pts 2 with genitourinary bleeding [n, %] | 20 (5.4) |
| Pts 2 with pleural bleeding [n, %] | 11 (3.0) |
| Characteristic | Patients with IBL Value | Patients without IBL Value | p |
|---|---|---|---|
| Sex (female/male) [n, %] | 72 (42.6)/97 (57.4) | 96 (47.5)/106 (53.5) | 0.34 |
| Age (female/male), median, IQR 1 [years] | 67 (55–71.5)/65 (54.8−73.3) | 64.5 (49.5−72)/65 (55.0−71.0) | 0.41/0.71 |
| Sepsis/septic shock [n, %] | 92 (52.6) | 83 (47.4) | 0.01 |
| Patients with acute injuries: | |||
| -acute kidney injury [n, %] | 74 (36.6) | 128 (63.4) | <0.05 |
| -acute liver injury [n, %] | 32 (51.6) | 30 (48.4) | 0.05 |
| Severity of disease: | |||
| -SAPS II 2, median, IQR 1 [points] | 45.0 (32.0−59.0) | 43.0 (29.0−58.0) | 0.35 |
| -APACHE II 3, median, IQR 1 [points] | 18.0 (13.0−24.3) | 18.0 (12.0−23.0) | 0.32 |
| -SOFA 4, median, IQR 1 [points] | 9.0 (6.0−12.0) | 8.0 (5.0−12.0) | 0.11 |
| ICU 5 length of stay, median, IQR 1 [days] | 12.0 (5.0–22.5) | 4 (2.0–8.0) | <0.01 |
| ICU mortality [n, %] | 73 (48.7) | 77 (51.3) | 0.05 |
| Hb drop 1–7, median, IQR 1 [g/dL] | 1.8 (0.6–3.1) | 1.3 (0.3–2.1) | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czempik, P.F.; Wilczek, D.; Herzyk, J.; Krzych, Ł.J. Hospital-Acquired Anemia in Patients Hospitalized in the Intensive Care Unit: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3939. https://doi.org/10.3390/jcm11143939
Czempik PF, Wilczek D, Herzyk J, Krzych ŁJ. Hospital-Acquired Anemia in Patients Hospitalized in the Intensive Care Unit: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(14):3939. https://doi.org/10.3390/jcm11143939
Chicago/Turabian StyleCzempik, Piotr F., Dawid Wilczek, Jan Herzyk, and Łukasz J. Krzych. 2022. "Hospital-Acquired Anemia in Patients Hospitalized in the Intensive Care Unit: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 14: 3939. https://doi.org/10.3390/jcm11143939
APA StyleCzempik, P. F., Wilczek, D., Herzyk, J., & Krzych, Ł. J. (2022). Hospital-Acquired Anemia in Patients Hospitalized in the Intensive Care Unit: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(14), 3939. https://doi.org/10.3390/jcm11143939







