Early, Delayed and Late Cardiac Implantable Electronic Device Infections: Do the Timing of Onset and Pathogens Matter?
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Analysis of Infectious Complications in Specific Time Intervals
2.3. Definitions
2.4. Statistical Analysis
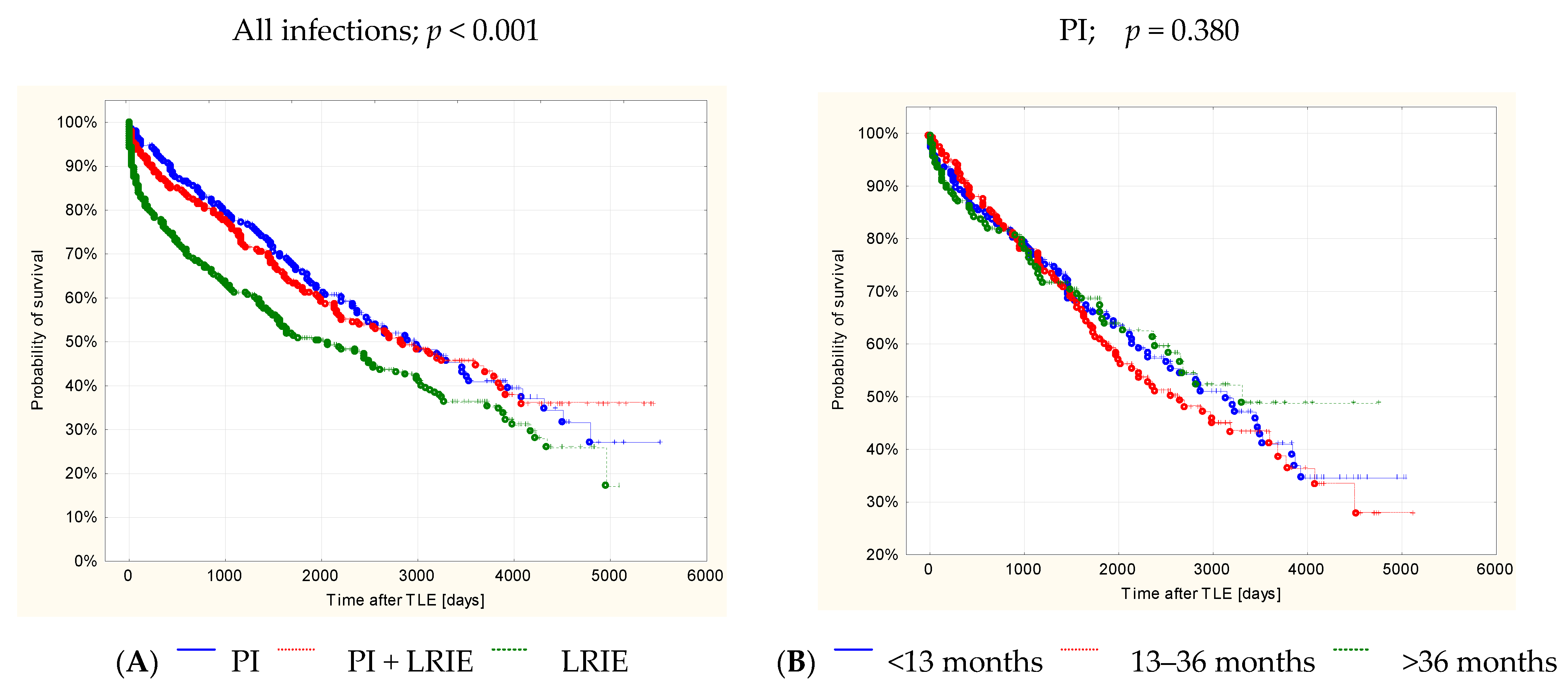
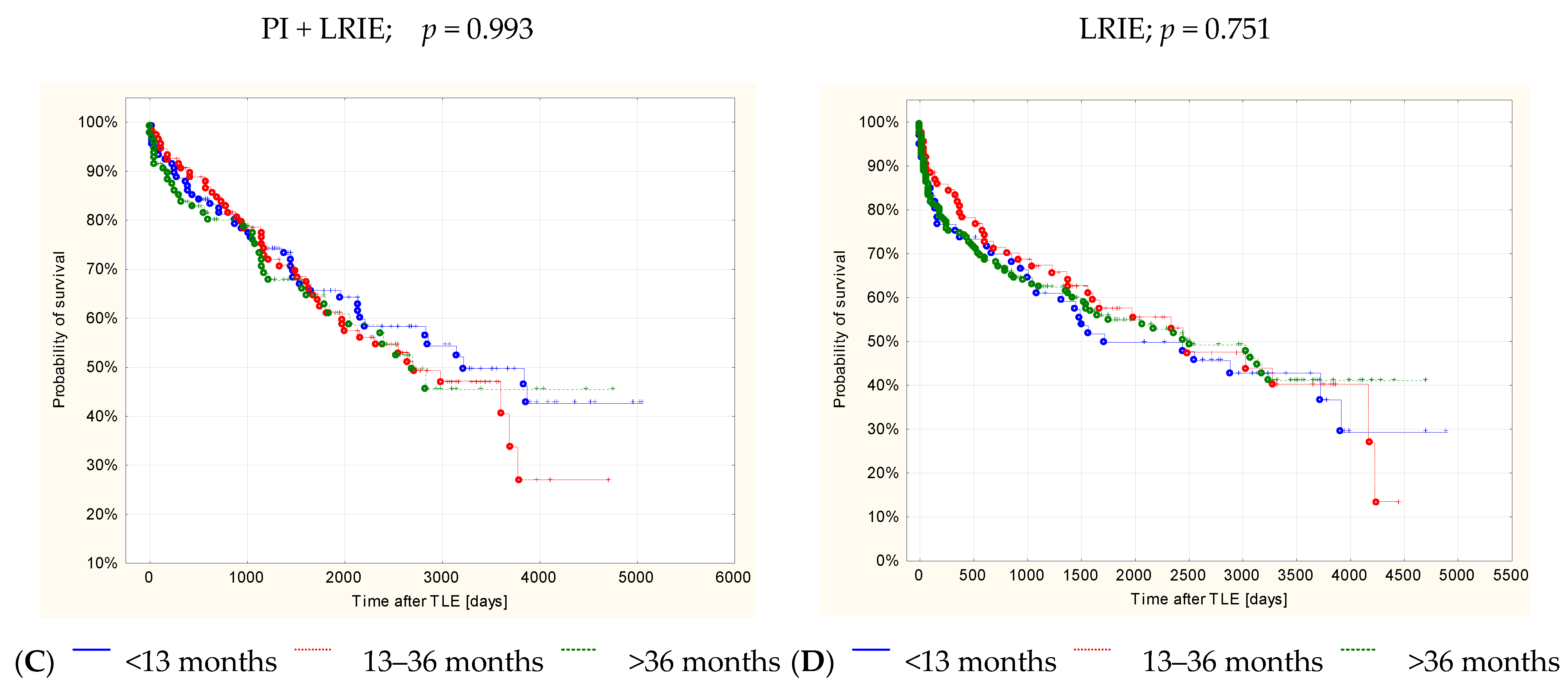
3. Results
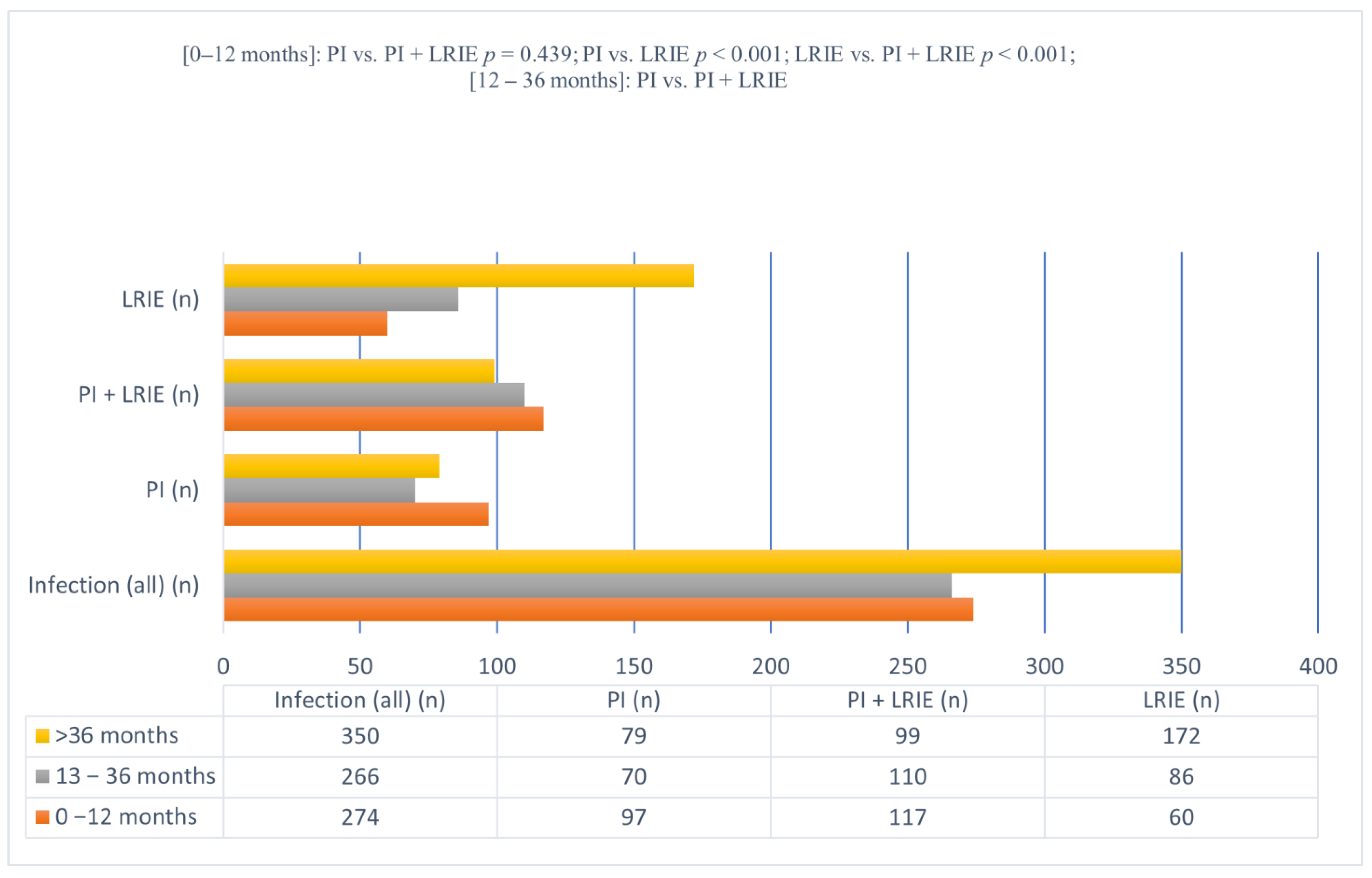
| Number of Cases | 0–12 Months | 13–36 Months | >36 Months | |
|---|---|---|---|---|
| Infection (all); n (%) | 890 (100.0%) | 274 (30.79%) | 266 (29.89%) | 350 (39.32%) |
| Isolated pocket infection (PI); n (%) | 246 (100.0%) | 97 (39.43%) | 70 (28.46%) | 79 (32.11%) |
| Pocket infection with concomitant lead-related infective endocarditis (PI + LRIE); n (%) | 326 (100.0%) | 117 (35.89%) (p = 0.439 vs. PI) | 110 (33.74%) (p = 0.209 vs. PI) | 99 (30.37%) (p = 0.722 vs. PI) |
| Isolated lead-related infective endocarditis (LRIE); n (%) | 318 (100.0%) | 60 (18.87%) (p < 0.001 vs. PI; p < 0.001 vs. PI + LRIE) | 86 (27.04%) (p = 0.782 vs. PI; p < 0.034 vs. PI + LRIE) | 172 54.09% (p < 0.001 vs. PI; p < 0.001 vs. PI + LRIE) |
| Early (E) | Delayed (D) | Late (L) | ANOVA Kruskal-Wallis 3–5 | Early (E) | Delayed (D) | Late (L) | ANOVA Kruskal-Wallis 7–9 | Early (E) | Delayed (D) | Late (L) | ANOVA Kruskall-Wallis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 11 12 13 | 2 6 10 | 3 7 11 | 4 8 12 | 5 9 13 | |||
| All | PI (All) | 0–12 month | 13–36 month | >36 month | p | PI +LRIE (All) | 0–12 month | 13–36 month | >36 month | p | LRIE (All) | 0–12 month | 13–36 month | >36 month | p | p | p | p | p | |
| Time to TLE since last CIED procedure [months], (mean ± SD) | 35.73 ±33.03 | 31.46 ±31.34 | 5.84 ±3.55 | 23.94 ±7.94 | 69.59 ±26.05 | 28.81 ±27.41 | 5.54 ±3.62 | 22.64 ±7.44 | 63.17 ±23.09 | <0.001 | 46.13 ±36.85 | 6.53 ±3.38 | 23.24 ±7.07 | 71.38 ±32.14 | <0.001 | <0.001 | 0.200 | 0.447 | 0.062 | |
| Patient characteristics | ||||||||||||||||||||
| Number of patients; n (%) | 890 (100) | 246 (100.0) | 97 (39.43) | 70 E (28.46) | 79 (32.11) | 326 (100.0) | 117 (35.89) | 110 (33.74) | 99 (30.37) | 318 (100.0) | 60 (18.87) | 86 E (27.04) | 172 DDD EEE (54.09) | |||||||
| Patient age during last procedure before TLE [years] | 65.20 ±14.31 | 67.19 ±13.39 | 68.88 ±13.83 | 66.64 ±13.79 | 65.61 ±12.36 | 0.079 | 66.66 ±13.79 | 66.81 ±13.77 | 66.59 ±15.40 | 66.57 ±11.94 | 0.665 | 62.18 222 666 ±14.98 | 63.76 3 ±15.41 | 64.53 ±13.56 | 60.46 D 5 99 ±15.38 | 0.052 | <0.001 | 0.061 | 0.278 | 0.004 |
| Female patient; n (%) | 275 (30.90) | 72 (29.27) | 37 (38.14) | 11 E (15.71) | 24 (30.38) | 0.007 | 99 (30.37) | 41 (35.04) | 32 (29.09) | 26 (26.26) | 0.354 | 104 (32.70) | 19 (31.67) | 29 4 (33.72) | 56 (32.56) | 0.965 | 0.426 | 0.786 | 0.044 | 0.518 |
| NYHA [class]; (median, IQR) | 2 [1–2] | 2 [1–2] | 2 [1–2] | 2 [1–2] | 2 [1–2] | 0.992 | 2 [1–2] | 2 [1–2] | 2 [1–2] | 2 [1–2] | 0.991 | 2 2 66 [1–3] | 2 [1–2] | 2 [1–2] | 2 5 99 [2–3] | 0.288 | 0.002 | 0.689 | 0.491 | 0.006 |
| LVEF [%]; (mean±SD) | 47.92 ±14.74 | 48.57 ±14.23 | 48.49 ±14.10 | 46.19 ±14.23 | 51.0 D ±14.19 | 0.079 | 47.72 ±14.50 | 48.34 ±14.99 | 46.09 ±14.55 | 48.79 ±13.95 | 0.355 | 47.64 ±15.36 | 47.72 ±14.77 | 45.18 ±15.96 | 48.68 ±15.21 | 0.270 | 0.626 | 0.939 | 0.958 | 0.543 |
| AF permanent; n (%) | 234 (26.29) | 77 (31.30) | 28 (28.87) | 23 (32.86) | 26 (32.91) | 0.803 | 77 (23.62) | 29 (24.79) | 22 (20.00) | 26 (26.26) | 0.531 | 80 (25.16) | 12 (20.00) | 20 (23.26) | 48 (27.91) | 0.428 | 0.107 | 0.461 | 0.114 | 0.502 |
| Diabetes (any) n (%) | 204 (22.92) | 43 (17.48) | 18 (18.56) | 9 (12.86) | 16 (20.25) | 0.465 | 74 (22.70) | 25 (21.37) | 28 (25.45) | 21 (21.21) | 0.699 | 87 22 (27.36) | 19 (31.67) | 25 4 (29.07) | 43 (25.00) | 0.559 | 0.013 | 0.161 | 0.039 | 0.561 |
| Creatinine level [mg / dl]; (mean±SD) | 1.38 ±1.09 | 1.24 ±0.86 | 1.26 ±0.75 | 1.33 ±1.29 | 1.13 ±0.35 | 0.371 | 1.29 ±1.00 | 1.28 ±0.76 | 1.34 ±1.29 | 1.25 ±0.89 | 0.166 | 1.59 222 ±1.29 | 1.70 33 7 ±1.37 | 1.51 8 ±1.13 | 1.59 555 999 ±1.34 | 0.416 | <0.001 | 0.020 | 0.151 | 0.001 |
| System and history of pacing | ||||||||||||||||||||
| HV lead presence before TLE n (%) | 278 31.24) | 74 (30.08) | 32 (32.99) | 28 (40.00) | 14 EEE DDD (17.72) | 0.009 | 98 (30.06) | 38 (32.48) | 34 (30.91) | 26 (26.26) | 0.595 | 106 (33.33) | 25 (41.67) | 35 E (40.70) | 46 D E (26.74) | 0.026 | 0.714 | 0.387 | 0.606 | 0.595 |
| CS lead presence before TLE n (%) | 176 (19.78) | 36 (14.63) | 17 (17.53) | 10 (14.29) | 9 (11.39) | 0.518 | 58 (17.79) | 31 (26.50) | 15 E (13.64) | 12 E (12.12) | 0.009 | 82 22 (25.79) | 20 3 (33.33) | 23 (26.74) | 39 (22.67) | 0.261 | 0.002 | 0.047 | 0.046 | 0.019 |
| Number of leads in the heart before TLE (mean ± SD) | 2.08 ±0.80 | 1.97 ±0.73 | 2.12 ±0.74 | 1.87 E ±0.76 | 1.86 E ±0.66 | 0.026 | 2.16 2 ±0.80 | 2.37 ±0.92 | 2.15 4 ±0.75 | 1.94 EEE L ±0.62 | <0.001 | 2.08 ±0.83 | 2.28 ±1.04 | 2.09 ±0.81 | 2.01 ±0.76 | 0.261 | 0.018 | 0.107 | 0.043 | 0.333 |
| Presence of abandoned leads before TLE n (%) | 123 (13.82) | 26 (10.57) | 15 (15.64) | 5 (7.14) | 6 (7.59) | 0.132 | 56 (17.18) | 32 (27.35) | 16 E (14.55) | 8 E (8.08) | <0.001 | 41 (12.98) | 9 (15.00) | 10 (11.63) | 22 (12.79) | 0.835 | 0.070 | 0.035 | 0.428 | 0.291 |
| Number of previous CIED-related procedures (median, IQR) | 2 [1–2] | 2 [1–2] | 2 [2–2] | 2 EE [1–2] | 2 EE [1–2] | 0.015 | 2 [1–2] | 2 [2–3] | 2 [1–2] | 1 EEE [1–2] | <0.001 | 1 2 66 [1–2] | 2 333 [1–2] | 2 [1–2] | 1 E [1–2] | 0.012 | 0.004 | 0.002 | 0.712 | 0.994 |
| Dwell time of the oldest lead per patient during TLE [months] (mean ±SD) | 91.64 ±69.65 | 92.39 ±68.86 | 89.59 ±58.71 | 72.23 ±70.32 | 113.7 DDD ±73.77 | <0.001 | 90.45 ±69.93 | 97.69 ±74.91 | 81.52 ±75.53 | 91.82 D ±55.44 | 0.034 | 92.29 ±70.19 | 67.33 3 77 ±64.63 | 85.62 ±84.79 | 104.3 EEE DDD ±61.07 | <0.001 | 0.967 | 0.016 | 0.758 | 0.238 |
| Dwell time of the oldest lead per patient during last procedure before TLE [months] (mean ±SD) | 55.40 ±69.46 | 57.84 ±64.75 | 79.97 ±58.45 | 48.84 EEE ±70.28 | 38.39 EEE ±59.39 | <0.001 | 62.28 ±73.46 | 91.47 ±76.66 | 57.83 ±75.34 | 33.00 EEE ±52.36 | <0.001 | 46.35 22 66 ±67.94 | 61.48 77 ±65.15 | 61.19 ±84.70 | 33.52 EEE DDD ±6.24 | <0.001 | 0.003 | 0.167 | 0.589 | 0.632 |
| History of early CIED intervention n (%) | 44 (4.94) | 14 (5.69) | 8 (8.25) | 4 (5.71) | 2 (2.53) | 0.267 | 17 (5.21) | 11 (9.40) | 3 (2.73) | 3 (3.09) | 0.040 | 13 (4.09) | 3 (5.00) | 7 (8.14) | 3 (1.74) | 0.047 | 0.682 | 0.593 | 0.210 | 0.800 |
| Large lead loop presence in X-Ray before TLE n (%) | 46 (5.17) | 8 (3.25) | 3 (3.09) | 1 (1.43) | 4 (5.06) | 0.457 | 14 (4.29) | 5 (4.27) | 6 (5.45) | 3 (3.03) | 0.690 | 24 2 (7.55) | 3 (5.00) | 5 (5.81) | 16 (9.30) | 0.431 | 0.010 | 0.568 | 0.314 | 0.006 |
| Leads on the both side of the chest n (%) | 28 (3.15) | 4 (1.63) | 2 (2.06) | 1 (1.43) | 1 (1.27) | 0.907 | 9 (2.76) | 4 (3.42) | 4 (3.64) | 1 (1.01) | 0.443 | 15 (4.72) | 4 (6.67) | 3 (3.49) | 8 (4.65) | 0.672 | 0.093 | 0.323 | 0.670 | 0.133 |
| Lead abrasion; n (%) | 225 (25.28) | 56 (22.76) | 22 (22.68) | 19 (27.14) | 15 (18.99) | 0.614 | 66 (20.25) | 31 (26.50) | 14 4 (12.73) | 21 (21.21) | 0.041 | 113 22 666 (35.53) | 21 (6.67) | 29 888 (33.72) | 63 55 9 (20.93) | 0.840 | <0.001 | 0.147 | 0.002 | 0.001 |
| The type of last procedure before TLE | ||||||||||||||||||||
| Primary system implantation; n (%) | 404 (45.39) | 100 (40.65) | 16 (16.49) | 36 EEE (51.43) | 48 EEE (60.76) | <0.001 | 133 (40.80) | 17 (14.53) | 55 EEE (50.00) | 61 EEE (61.62) | <0.001 | 171 22 66 (53.37) | 21 7 (35.00) | 38 (44.19) | 112 EEE DD (65.12) | <0.001 | <0.001 | 0.003 | 0.712 | 0.893 |
| Unit replacement; n (%) | 307 (34.49) | 102 (41.46) | 58 (59.79) | 21 EEE (30.00) | 23 EEE (29.11) | <0.001 | 124 (38.04) | 61 (52.14) | 38 EE (34.55) | 25 EE (25.25) | 0.002 | 81 222 666 (25.47) | 23 3 (38.33) | 25 (29.07) | 33 EE (19.19) | 0.009 | <0.001 | 0.030 | 0.645 | 0.381 |
| Up-grading of the system; n (%) | 57 (6.40) | 16 (6.50) | 7 (7.22) | 7 (10.00) | 2 (2.53) | 0.172 | 20 (6.13) | 8 (6.84) | 7 (6.36) | 5 (5.05) | 0.856 | 21 (6.60) | 4 (6.67) | 11 (12.79) | 6 D (3.49) | 0.018 | 0.952 | 0.990 | 0.281 | 0.686 |
| Any CIED-related procedure (excluding primary system implantation); n (%) | 486 (54.61) | 146 (59.34) | 81 (83.51) | 34 EEE (48.57) | 31 EEE (39.24) | <0.001 | 193 (59.20) | 100 (85.47) | 55 EEE (50.00) | 38 EEE (38.38) | <0.001 | 147 22 66 (46.27 | 39 3 7 (65.00) | 48 (55.81) | 60 EEE DD (34.88) | <0.001 | <0.001 | <0.001 | 0.809 | 0.893 |
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clementy, N.; Carion, P.L.; de Leotoing, L.; Lamarsalle Wilquin-Bequet, F.; Brown, B.; Verhees, K.J.P.; Fernandes, J.; Deharo, J.C. Infections and associated costs following cardiovascular implantable electronic device implantations: A nationwide cohort study. Europace 2018, 20, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Theis, C.; Brown, B.; Witthohn, A.; Lux, W.; Goette, A. Incidence and costs of cardiac device infections: Retrospective analysis using German health claims data. J. Comp. Eff. Res. 2018, 7, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoe, J.A.; Barlow, G.; Chambers, J.B.; Gammage, M.; Guleri, A.; Howard, P.; Olson, E.; Perry, J.D.; Prendergast, B.D.; Spry, M.J.; et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J. Antimicrob. Chemother. 2015, 70, 325–359. [Google Scholar]
- Uslan, D.Z.; Sohail, M.R.; St Sauver, J.L.; Friedman, P.A.; Hayes, D.L.; Stoner, S.M.; Wilson, W.R.; Steckelberg, J.M.; Baddour, L.M. Permanent pacemaker and implantable cardioverter defibrillator infection: A population-based study. Arch. Intern. Med. 2007, 167, 669–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baddour, L.M.; Epstein, A.E.; Erickson, C.C.; Knight, B.P.; Levison, M.E.; Lockhart, P.B.; Masoudi, F.A.; Okum, E.J.; Wilson, W.R.; Beerman, L.B.; et al. Update on cardiovascular implantable electronic device infections and their management: A scientific statement from the American Heart Association. Circulation 2010, 121, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Korkerdsup, T.; Ngarmukos, T.; Sungkanuparph, S.; Phuphuakrat, A. Cardiac implantable electronic device infection in the cardiac referral center in Thailand: Incidence, microbiology, risk factors, and outcomes. J. Arrhythm. 2018, 34, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.; Uslan, D.Z.; Greenspon, A.J.; Sohail, M.R.; Baddour, L.M.; Blank, E.; Carrillo, R.G.; Danik, S.B.; Del Rio, A.; Hellinger, W.; et al. Variability in Clinical Features of Early Versus Late Cardiovascular Implantable Electronic Device Pocket Infections. Pacing Clin. Electrophysiol. 2014, 37, 955–962. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Wang, Q.; Zhang, Y.; Wang, H. Microbiological Characteristics and Clinical Features of Cardiac Implantable Electronic Device Infections at a Tertiary Hospital in China. Front. Microbiol. 2017, 8, 360. [Google Scholar]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. ESC guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.C.; et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infec-tions-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 41, 2012–2032. [Google Scholar]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley GH 3rd Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; Schaerf, R.H.; et al. Transvenous Lead Extraction: Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management. Heart Rhythm 2009, 6, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.-M.; Clancy, J.; Deharo, J.-C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef] [PubMed]
- Kutarski, A.; Małecka, B.; Kołodzinska, A.; Grabowski, M. Mutual Abrasion of Endocardial Leads: Analysis of Explanted Leads. Pacing Clin. Electrophysiol. 2013, 36, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Catanchin, A.; Murdock, C.J.; Athan, E. Pacemaker Infections: A 10-Year Experience. Heart Lung Circ. 2007, 16, 434–439. [Google Scholar] [CrossRef]
- Nery, P.B.; Fernandes, R.; Nair, G.M.; Sumner, G.L.; Ribas, C.S.; Menon, S.M.D.; Wang, X.; Krahn, A.D.; Morillo, C.A.; Connolly, S.J.; et al. Device-Related Infection Among Patients with Pacemakers and Implantable Defibrillators: Incidence, Risk Factors, and Consequences. J. Cardiovasc. Electrophysiol. 2010, 21, 786–790. [Google Scholar] [CrossRef]
- Sohail, M.R.; Uslan, D.Z.; Khan, A.H.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Jenkins, S.M.; Baddour, L.M. Infective Endocarditis Complicating Permanent Pacemaker and Implantable Cardioverter-Defibrillator Infection. Mayo Clin. Proc. 2008, 83, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Dai, M.; Cai, C.; Vaibhav, V.; Sohail, M.R.; Hayes, D.L.; Hodge, D.O.; Tian, Y.; Asirvatham, R.; Cochuyt, J.J.; Huang, C.; et al. Trends of Cardiovascular Implantable Electronic Device Infection in 3 Decades: A Population-Based Study. JACC Clin. Electrophysiol. 2019, 5, 1071–1080. [Google Scholar] [CrossRef]
- Greenspon, A.J.; Prutkin, J.M.; Sohail, M.R.; Vikram, H.R.; Baddour, L.M.; Danik, S.B.; Peacock, J.; Falces, C.; Miro, J.M.; Blank, E.; et al. Timing of the Most Recent Device Procedure Influences the Clinical Outcome of Lead-Associated Endocarditis: Results of the MEDIC (Multicenter Electrophysiologic Device Infection Cohort). J. Am. Coll. Cardiol. 2012, 59, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Klug, D.; Lacroix, D.; Savoye, C.; Goullard, L.; Grandmougin, D.; Hennequin, J.L.; Kacet, S.; Lekieffre, J. Systemic Infection Related to Endocarditis on Pacemaker Leads. Circulation 1997, 95, 2098–2107. [Google Scholar] [CrossRef]
- Ursaru, A.M.; Haba, C.M.; Popescu, E.; Crișu, D.; Petriș, A.O.; Tesloianu, N.D. A Rare Entity–Percutaneous Lead Extraction in a Very Late Onset Pacemaker Endocarditis: Case Report and Review of Literature. Diagnostics 2021, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Machino, T.; Sekiguchi, Y. Positive Pocket Cultures From Cardiac Implantable Electrophysiological Devices Without Infection—Contamination or Colonization? Circ. J. 2015, 79, 1680–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichlmaier, M.; Marwitz, V.; Kühn, C.; Niehaus, M.; Klein, G.; Bara, C.; Haverich, A.; Abraham, W.R. High prevalence of asymptomatic bacterial colonization of rhythm management devices. Europace 2008, 10, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, T.; Becker, T.; Strauss, M.; Dyck, N.; Weisse, U.; Saggau, W.; Burkhardt, U.; Seidl, K. Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace 2010, 12, 58–63. [Google Scholar] [CrossRef]
- Rohacek, M.; Weisser, M.; Kobza, R.; Schoenenberger, A.W.; Pfyffer, G.E.; Frei, R.; Erne, P.; Trampuz, A. Bacterial Colonization and Infection of Electrophysiological Cardiac Devices Detected with Sonication and Swab Culture. Circulation 2010, 121, 1691–1697. [Google Scholar] [CrossRef] [Green Version]
- Mason, P.K.; Dimarco, J.P.; Ferguson, J.D.; Mahapatra, S.; Mangrum, J.M.; Bilchick, K.C.; Wiggins, D.; Mounsey, J.P.; Moorman, J.R. Sonication of explanted cardiac rhythm management devices for the diagnosis of pocket infections and asymptomatic bacterial colonization. Pacing Clin. Electrophysiol. 2011, 34, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Okada, M.; Kashiwase, K.; Hirata, A.; Nemoto, T.; Matsuo, K.; Murakami, A.; Ueda, Y. Bacterial Contamination During Pacemaker Implantation Is Common and Does Not Always Result in Infection. Circ. J. 2015, 79, 1712–1718. [Google Scholar] [CrossRef] [Green Version]
- Kołodzińska, A.; Kutarski, A.; Koperski, Ł.; Grabowski, M.; Małecka, B.; Opolski, G. Differences in encapsulating lead tissue in patients who underwent transvenous lead removal. Europace 2012, 14, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Kołodzińska, A.; Kutarski, A.; Kozłowska, M.; Grabowski, M.; Marchel, H.; Drela, N.; Opolski, G. Biodegradation of the outer silicone insulation of endocardial leads. Circ. Arrhythm. Electrophysiol. 2013, 6, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Polewczyk, A.; Jacheć, W.; Janion, M.; Podlaski, R.; Kutarski, A. Lead-Dependent Infective Endocarditis: The Role of Factors Predisposing to Its Development in an Analysis of 414 Clinical Cases. Pacing Clin. Electrophysiol. 2015, 38, 846–856. [Google Scholar] [CrossRef]
- Mitacchione, G.; Arabia, G.; Schiavone, M.; Cerini, M.; Gasperetti, A.; Salghetti, F.; Bontempi, L.; Viecca, M.; Curnis, A.; Forleo, G.B. Intraoperative sensing increase predicts long-term pacing threshold in leadless pacemakers. J. Interv. Card. Electrophysiol. 2022, 63, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Gasperetti, A.; Schiavone, M.; Ziacchi, M.; Vogler, J.; Breitenstein, A.; Laredo, M.; Palmisano, P.; Ricciardi, D.; Mitacchione, G.; Compagnucci, P.; et al. Long-term complications in patients implanted with subcutaneous implantable cardioverter-defibrillators: Real-world data from the extended ELISIR experience. Heart Rhythm 2021, 18, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
| Early (E) | Delayed (D) | Late (L) | ANOVA 3–5 | Early (E) | Delayed (D) | Late (L) | ANOVA 7–9 | Early (E) | Delayed (D) | Late (L) | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | (11 12 13) | (2 6 10) | (3 7 11) | (4 8 12) | (5 9 13) | |||
| All pts | PI (All) | 0–12 month. | 13–36 month | >36 month | p | PI + LRIE (All) | 0–12 month | 13–36 month | >36 month | p | LRIE (All) | 0–12 month | 13–36 month | >36 month | p | p | p | p | p | |
| Number of patients (n, %) | 890 | 246 (100.0) | 97 (39.43) | 70 (28.46) | 79 (32.1) | 326 (100.0) | 117 (35.89) | 110 (33.74) | 99 (30.37) | 318 (100.0) | 60 (18.87) | 86 (27.04) | 172 (54.09) | |||||||
| CoNS; n (%) | 40 (4.49) | 16 (6.50) | 6 (6.91) | 8 (11.43) | 2 (2.53) | 0.890 | 13 (3.99) | 5 (4.27) | 5 (4.55) | 3 (3.03) | 0.839 | 11 (3.46) | 4 (6.67) | 5 (5.81) | 2 (1.16) | 0.050 | 0.669 | 0.747 | 0.190 | 0.669 |
| Staph epidermidis; n (%) | 183 (20.56) | 39 (15.85) | 12 (12.37) | 14 (20.00) | 13 (16.46) | 0.407 | 82 (25.15) | 33 E 33 (28.21) | 35 (31.82) | 14 E DD (14.14) | 0.009 | 62 (19.50) | 12 (20.00) | 14 D 8 (16.28) | 36 (20.93) | 0.670 | 0.450 | 0.018 | 0.034 | 0.449 |
| Staph aureus; n (%) | 102 (11.46) | 20 (8.13) | 11 11.44) | 4 (5.71) | 5 (6.33) | 0.330 | 54 22 (16.56) | 20 (17.09) | 16 (14.55) | 18 E 5 (18.18) | 0.766 | 28 66 (8.81) | 8 (13.33) | 7 (8.14) | 13 L 99 (7.56) | 0.386 | 0.012 | 0.476 | 0.928 | 0.012 |
| Staph—other; n (%) | 11 (1.24) | 6 (2.44) | 4 (4.12) | 2 (2.86) | 0 (0.00) | 0.205 | 5 (1.53) | 2 (1.71) | 1 (0.91) | 2 (2.02) | 0.794 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 | 0.154 | 0.207 | 0.241 | 0.154 |
| STI n (%) | 60 (6.74) | 10 (4.07) | 2 (2.06) | 4 (5.71) | 4 (5.06) | 0.431 | 22 (6.75) | 4 (3.42) | 11 (10.00) | 7 (7.07) | 0.141 | 28 8.81) | 5 (8.33) | 9 (10.47) | 14 (8.14) | 0.817 | 0.669 | 0.138 | 0.512 | 0.669 |
| Streptococcus; n (%) | 3 (0.34) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1.000 | 1 (0.31) | 0 (0.00) | 1 (0.91) | 0 (0.00) | 0.375 | 2 (0.63) | 0 (0.00) | 0 (0.00) | 2 (1.16) | 0.427 | 0.514 | 1.00 | 0.497 | 0.514 |
| Other bacteria; n (%) | 64 (7.19) | 10 (4.07) | 6 (6.91) | 3 (4.29) | 1 (1.27) | 0.259 | 23 (7.06) | 10 (8.55) | 5 (4.55) | 8 (8.08) | 0.448 | 31 (9.75) | 6 (10.00) | 9 (10.47) | 16 (9.30) | 0.955 | 0.077 | 0.670 | 0.155 | 0.077 |
| Culture negative; n (%) | 243 (27.30) | 88 (35.77) | 37 (38.14) | 23 (32.86) | 28 (35.44) | 0.780 | 70 (21.47) | 24 EE 33 (20.51 | 18 E 4 (16.36) | 28 (28.28) | 0.108 | 85 (26.73) | 15 (25.00) | 28 D 88 (32.56) | 42 (24.42) | 0.358 | 0.137 | 0.014 | 0.010 | 0.137 |
| Lack of culture result; n (%) | 184 (20.67) | 57 (23.17) | 19 (19.59) | 12 (17.14) | 26 (32.91 | 0.043 | 56 (17.18) | 19 (16.24) | 18 (16.36 | 19 (19.19) | 0.817 | 71 (22.33) | 10 (16.67) | 14 (16.28) | 47 (27.33) | 0.068 | 0.340 | 0.799 | 0.962 | 0.340 |
| Multivariate Cox Regression Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | 12 Months Follow-Up | 36 Months Follow-Up | Over 36 Months Follow-Up | ||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Isolated PI | |||||||||
| n = 2929 | n = 2642 | n = 2307 | |||||||
| Patient’s age during last procedure before TLE (years) | 1.035 | 1.015–1.055 | 0.001 | 1.028 | 1.006–1.051 | 0.013 | 1.042 | 1.020–1.066 | <0.001 |
| Sex (% of female patients) (%) | 1.008 | 0.647–1.571 | 0.972 | 0.295 | 0.151–0.574 | 0.000 | 0.468 | 0.284–0.769 | 0.003 |
| AF permanent | 1.287 | 0.806–2.054 | 0.291 | 1.529 | 0.914–2.557 | 0.106 | 1.016 | 0.625–1.649 | 0.950 |
| Device type—CRT-D | 1.130 | 0.467–2.734 | 0.787 | 1.174 | 0.431–3.193 | 0.754 | 0.631 | 0.135–2.954 | 0.559 |
| Number of leads in the system before TLE | 1.054 | 0.716–1.552 | 0.788 | 0.922 | 0.599–1.417 | 0.710 | 0.948 | 0.618–1.453 | 0.806 |
| HV lead presence before TLE | 0.898 | 0.505–1.595 | 0.713 | 1.076 | 0.589–1.967 | 0.811 | 1.162 | 0.593–2.276 | 0.663 |
| Early CIED intervention in history | 1.210 | 0.570–2.567 | 0.619 | 1.129 | 0.343–3.721 | 0.841 | 1.069 | 0.260–4.396 | 0.926 |
| Dwell time of the oldest lead in the patient before TLE | 0.993 | 0.989–0.996 | 0.000 | 0.986 | 0.980–0.991 | 0.000 | 0.994 | 0.990–0.998 | 0.002 |
| Any CIED-related procedure | 7.735 | 5.507–10.865 | 0.000 | 4.294 | 2.837–6.501 | 0.000 | 3.863 | 2.513–5.936 | <0.001 |
| PI + LRIE | |||||||||
| n = 2503 | n = 2642 | n = 2307 | |||||||
| Patient’s age during last procedure before TLE (years) | 1.018 | 1.001–1.036 | 0.043 | 1.028 | 1.010–1.047 | 0.002 | 1.048 | 1.027–1.071 | <0.001 |
| Sex (% of female patients) (%) | 0.862 | 0.569–1.304 | 0.481 | 0.593 | 0.383–0.919 | 0.019 | 0.452 | 0.280–0.728 | 0.001 |
| LVEF (%) | 1.410 | 0.806–2.469 | 0.229 | 1.304 | 0.699–2.433 | 0.403 | 1.325 | 0.688–2.554 | 0.400 |
| Diabetes (any) | 1.007 | 0.632–1.605 | 0.977 | 1.369 | 0.874–2.144 | 0.170 | 1.024 | 0.622–1.686 | 0.926 |
| Creatinine level (mg%) | 1.262 | 1.026–1.552 | 0.028 | 1.233 | 1.035–1.468 | 0.019 | 1.124 | 0.881–1.434 | 0.348 |
| Long-term anticoagulation therapy | 0.696 | 0.421–1.152 | 0.159 | 0.484 | 0.280–0.835 | 0.009 | 0.514 | 0.285–0.929 | 0.027 |
| Number of leads in the system before TLE | 1.414 | 0.946–2.113 | 0.091 | 1.655 | 1.083–2.532 | 0.020 | 1.109 | 0.701–1.756 | 0.658 |
| Presence of abandoned lead before TLE | 3.216 | 1.886–5.486 | 0.000 | 2.294 | 1.164–4.519 | 0.016 | 3.183 | 1.342- 7.548 | 0.009 |
| HV lead presence before TLE | 0.812 | 0.509–1.295 | 0.381 | 0.916 | 0.577–1.453 | 0.709 | 1.099 | 0.644–1.876 | 0.729 |
| CS lead presence before TLE | 0.951 | 0.512–1.769 | 0.875 | 0.433 | 0.207–0.907 | 0.026 | 0.819 | 0.375–1.787 | 0.616 |
| Early CIED intervention in history | 1.447 | 0.732–2.859 | 0.288 | 0.653 | 0.202–2.107 | 0.476 | 0.936 | 0.281–3.123 | 0.915 |
| Upgrading or additional lead implantation | 1.346 | 0.806–2.245 | 0.256 | 1.701 | 0.930–3.110 | 0.084 | 0.265 | 0.091–0.765 | 0.014 |
| Dwell time of the oldest lead before TLE | 0.994 | 0.991–0.998 | 0.001 | 0.989 | 0.985–0.993 | 0.000 | 0.985 | 0.980–0.991 | <0.001 |
| Any CIED-related procedure | 8.388 | 6.070–11.591 | 0.000 | 4.619 | 3.271–6.523 | 0.000 | 4.276 | 2.851–6.414 | <0.001 |
| Isolated LRIE | |||||||||
| n = 2693 | n = 2462 | n = 2216 | |||||||
| Patient’s age during last procedure before TLE (years) | 1.014 | 0.988–1.041 | 0.289 | 1.029 | 1.008–1.051 | 0.007 | 1.004 | 0.989–1.018 | 0.622 |
| Female gender | 0.896 | 0.481–1.669 | 0.730 | 0.901 | 0.542–1.497 | 0.687 | 0.666 | 0.466–0.954 | 0.027 |
| NYHA I-IV | 1.050 | 0.652–1.693 | 0.840 | 0.857 | 0.565–1.300 | 0.467 | 1.490 | 1.123–1.978 | 0.006 |
| LVEF | 1.015 | 0.990–1.040 | 0.241 | 0.988 | 0.970–1.007 | 0.228 | 1.008 | 0.995–1.021 | 0.230 |
| Diabetes (any) | 1.806 | 0.986–3.307 | 0.056 | 1.675 | 1.016–2.761 | 0.043 | 1.296 | 0.886–1.896 | 0.181 |
| Creatinine level (mg%) | 1.244 | 1.065–1.452 | 0.006 | 1.164 | 0.986–1.374 | 0.073 | 1.270 | 1.137–1.418 | 0.000 |
| Long-term antiplatelet therapy | 0.746 | 0.412–1.351 | 0.334 | 0.856 | 0.528–1.387 | 0.527 | 0.966 | 0.680–1.370 | 0.844 |
| Number of leads in the system | 1.379 | 0.823–2.312 | 0.222 | 1.061 | 0.697–1.613 | 0.783 | 0.741 | 0.538–1.019 | 0.065 |
| HV lead presence before TLE | 1.187 | 0.588–2.397 | 0.633 | 1.474 | 0.839–2.589 | 0.177 | 1.405 | 0.911–2.167 | 0.124 |
| CS lead presence before TLE | 1.505 | 0.691–3.281 | 0.304 | 1.219 | 0.641–2.321 | 0.546 | 2.322 | 1.471–3.664 | <0.001 |
| Lead implanted before age of 20 | 3.466 | 0.635–18.923 | 0.151 | 6.105 | 1.563–23.837 | 0.009 | 0.658 | 0.199–2.170 | 0.491 |
| Presence of abandoned lead before TLE | 1.458 | 0.536–3.970 | 0.460 | 1.360 | 0.610–3.030 | 0.452 | 2.210 | 1.213–4.029 | 0.010 |
| Abrasion of the lead | 2.603 | 1.371–4.943 | 0.003 | 2.402 | 1.428–4.041 | 0.001 | 2.610 | 1.792–3.801 | <0.001 |
| Leads on both sides of the chest before TLE | 3.752 | 1.036–13.588 | 0.044 | 1.923 | 0.533–6.945 | 0.318 | 1.387 | 0.538–3.578 | 0.499 |
| Dwell time of the oldest lead before TLE | 0.982 | 0.976–0.988 | 0.000 | 0.987 | 0.982–0.992 | 0.000 | 0.978 | 0.973–0.983 | <0.001 |
| Any CIED-related procedure | 5.733 | 3.662–8.975 | 0.000 | 5.497 | 3.768–8.019 | 0.000 | 5.230 | 3.890–7.030 | <0.001 |
| Infectious Complications (All) | |||||||
|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | ||||||
| OR | 95% CI | p | OR | 95% CI | p | ||
| E | Patient age during the last procedure before TLE (one year) | 1.018 | 1.007–1.031 | 0.002 | 1.024 | 1.008–1.038 | 0.002 |
| L | NYHA (one class) | 1.269 | 1.012–1.591 | 0.039 | 1.654 | 1.213–2.256 | <0.001 |
| E | BMI (one unit) | 1.043 | 1.003–1.082 | 0.033 | 1.053 | 1.004–1.103 | 0.032 |
| E | CRT-D (yes/no) | 3.040 | 1.718–5.376 | <0.001 | 1.451 | 0.545–3.861 | 0.456 |
| E | Number of leads in the system before TLE (by one) | 1.650 | 1.282–2.123 | <0.001 | 2.387 | 1.188–4.785 | 0.014 |
| E | Number of leads in the heart before TLE (by one) | 1.618 | 1.312–1.992 | <0.001 | 0.741 | 0.398–1.377 | 0.343 |
| E | Abandoned leads (yes/no) | 2.169 | 1.376–3.425 | <0.001 | 1.524 | 0.596–3.891 | 0.378 |
| E | HV lead before TLE (yes/no) | 1.493 | 1.057–2.110 | 0.023 | 3.040 | 1.761–5.236 | <0.001 |
| E | CS lead before TLE (yes/no) | 1.613 | 1.087–2.392 | 0.017 | 0.535 | 0.270–1.059 | 0.072 |
| E | Number of previous procedures (by one) | 1.946 | 1.608–2.347 | <0.001 | 0.896 | 0.682–1.178 | 0.430 |
| E | History of early CIED intervention (yes/no) | 3.704 | 1.618–8.475 | 0.002 | 2.137 | 0.852–5.348 | 0.105 |
| E | Upgrading or additional lead implantation (yes/no) | 2.500 | 1.572–3.968 | <0.001 | 1.147 | 0.639–2.058 | 0.646 |
| E | Dwell time of oldest lead per patient during the last procedure before TLE (by one year) | 1.157 | 1.117–1.200 | <0.001 | 1.091 | 1.031–1.153 | 0.003 |
| E | Any procedure other than first system implantation (yes/no) | 6.711 | 4.651–9.709 | <0.001 | 4.016 | 2.304–6.993 | <0.001 |
| E | Presence of CoNS (yes/no) | 2.817 | 1.129–6.993 | 0.026 | 3.984 | 1.344–11.776 | 0.012 |
| Isolated pocket infection | |||||||
| E | CRT-D (yes/no) | 5.376 | 1.151–25.00 | 0.031 | 7.299 | 1.078–50.00 | 0.040 |
| E | Number of leads in the system before TLE (by one) | 1.695 | 1.012–2.833 | 0.043 | 1.271 | 0.455–3.546 | 0.645 |
| E | Number of leads in the heart before TLE (by one) | 1.650 | 1.043–2.611 | 0.031 | 0.765 | 0.316–1.848 | 0.549 |
| E | Number of previous procedures (by one) | 2.141 | 1.397–3.279 | <0.001 | 0.768 | 0.440–1.340 | 0.350 |
| E | Dwell time of oldest lead per patient during the last procedure before TLE (by one year) | 1.166 | 1.085–1.253 | <0.001 | 1.003 | 0.995–1.011 | 0.477 |
| E | Any procedure other than first system implantation (by one) | 8.547 | 4.202–17.54 | <0.001 | 10.00 | 3.378–29.41 | <0.001 |
| Pocket infection with concomitant infective endocarditis | |||||||
| E | CRT-D (yes/no) | 2.513 | 1.056–5.988 | 0.036 | 1.427 | 0.233–8.772 | 0.699 |
| E | Number of leads in the system before TLE (by one) | 1.812 | 1.160–2.833 | 0.009 | 2.538 | 0.608–10.64 | 0.199 |
| L | Number of leads in the heart before TLE (by one) | 2.092 | 1.425–3.077 | <0.001 | 0.583 | 0.162–2.101 | 0.406 |
| E | Abandoned leads (yes/no) | 4.329 | 1.880–10.00 | <0.001 | 1.815 | 0.244–13.51 | 0.558 |
| E | CS leads before TLE (yes/no) | 2.646 | 1.269–5.495 | 0.009 | 0.839 | 0.165–4.274 | 0.832 |
| E | Number of previous procedures (by one) | 3.279 | 2.174–4.926 | <0.001 | 1.506 | 0.858–2.646 | 0.152 |
| E | Upgrading or additional lead implantation (yes/no) | 6.849 | 2.532–18.52 | <0.001 | 3.745 | 0.653–21.28 | 0.136 |
| E | Upgrading or downgrading with lead abandonment (yes/no) | 5.495 | 1.550–19.61 | 0.008 | 0.479 | 0.044–5.208 | 0.543 |
| E | Dwell time of oldest lead per patient during the last procedure before TLE (by one year) | 1.203 | 1.126–1.287 | <0.001 | 1.053 | 0.955–1.160 | 0.301 |
| E | Any procedure other than first system implantation (yes/no) | 9.091 | 4.762–17.54 | 0.000 | 3.155 | 1.285–7.752 | 0.012 |
| E | Staph epidermidis (yes/no) | 2.415 | 1.200–4.854 | 0.013 | 2.326 | 0.993–5.435 | 0.051 |
| Isolated lead-related infective endocarditis | |||||||
| L | CRT-D (yes/no) | 0.366 | 0.133–1.002 | 0.049 | 1.366 | 0.305–6.117 | 0.682 |
| E | Number of leads in the system before TLE (by one) | 1.661 | 1.086–2.545 | 0.019 | 2.037 | 0.929–4.464 | 0.074 |
| L | Number of leads in the heart before TLE (by one) | 0.692 | 0.491–0.974 | 0.034 | 1.128 | 0.573–2.218 | 0.726 |
| E | HV leads before TLE (by one) | 1.908 | 1.029–3.546 | 0.039 | 3.367 | 1.401–8.130 | 0.006 |
| L | Number of previous procedures (by one) | 0.766 | 0.593–0.989 | 0.040 | 1.357 | 0.808–2.280 | 0.246 |
| E | Dwell time of oldest lead per patient during the last procedure before TLE (by one year) | 1.091 | 1.030–1.153 | 0.003 | 1.110 | 1.000–1.232 | 0.049 |
| E | Any procedure other than first system implantation (yes/no) | 3.058 | 1.653–5.650 | <0.001 | 2.475 | 0.895–6.849 | 0.079 |
| E | Presence of CoNS (yes/no) | 5.952 | 1.054–33.33 | 0.042 | 10.00 | 1.429–71.43 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polewczyk, A.; Jacheć, W.; Polewczyk, M.; Szczęśniak-Stańczyk, D.; Kutarski, A. Early, Delayed and Late Cardiac Implantable Electronic Device Infections: Do the Timing of Onset and Pathogens Matter? J. Clin. Med. 2022, 11, 3929. https://doi.org/10.3390/jcm11143929
Polewczyk A, Jacheć W, Polewczyk M, Szczęśniak-Stańczyk D, Kutarski A. Early, Delayed and Late Cardiac Implantable Electronic Device Infections: Do the Timing of Onset and Pathogens Matter? Journal of Clinical Medicine. 2022; 11(14):3929. https://doi.org/10.3390/jcm11143929
Chicago/Turabian StylePolewczyk, Anna, Wojciech Jacheć, Maciej Polewczyk, Dorota Szczęśniak-Stańczyk, and Andrzej Kutarski. 2022. "Early, Delayed and Late Cardiac Implantable Electronic Device Infections: Do the Timing of Onset and Pathogens Matter?" Journal of Clinical Medicine 11, no. 14: 3929. https://doi.org/10.3390/jcm11143929
APA StylePolewczyk, A., Jacheć, W., Polewczyk, M., Szczęśniak-Stańczyk, D., & Kutarski, A. (2022). Early, Delayed and Late Cardiac Implantable Electronic Device Infections: Do the Timing of Onset and Pathogens Matter? Journal of Clinical Medicine, 11(14), 3929. https://doi.org/10.3390/jcm11143929






