Positive Effect of Manipulated Virtual Kinematic Intervention in Individuals with Traumatic Stiff Shoulder: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population
2.3. Measurement Tools
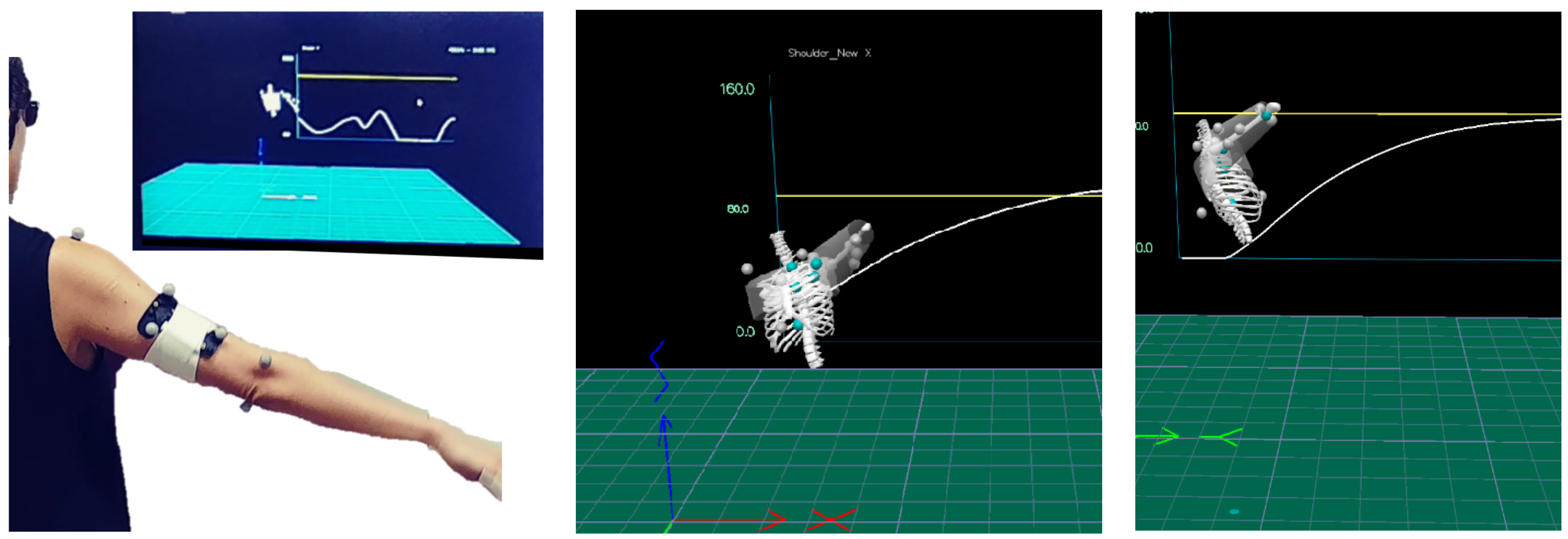
2.4. The Intervention
2.5. Study Protocol
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Itoi, E.; Arce, G.; Bain, G.I.; Diercks, R.L.; Guttmann, D.; Imhoff, A.B.; Mazzocca, A.D.; Sugaya, H.; Yoo, Y.S. Shoulder Stiffness: Current Concepts and Concerns. Arthroscopy 2016, 32, 1402–1414. [Google Scholar] [CrossRef]
- Pogorzelski, J.; Imhoff, A.B.; Degenhardt, H.; Siebenlist, S. Primary (idiopathic) shoulder stiffness: Definition, disease progression, epidemiology and etiology. Unfallchirurg 2019, 122, 917–924. [Google Scholar] [CrossRef] [PubMed]
- De Groef, A.; Van Kampen, M.; Dieltjens, E.; Christiaens, M.R.; Neven, P.; Geraerts, I.; Devoogdt, N. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: A systematic review. Arch. Phys. Med. Rehabil. 2015, 96, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Lim, H.K.; Yang, J.L. Effect of shoulder tightness on glenohumeral translation, scapular kinematics, and scapulohumeral rhythm in subjects with stiff shoulders. J. Orthop. Res. 2006, 24, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.B.Y.; Pua, P.Y.; How, C.H. Physical therapy in the management of frozen shoulder. Singap. Med. J. 2017, 58, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Donatelli, R.; Ruivo, R.M.; Thurner, M.; Ibrahim, M.I. New concepts in restoring shoulder elevation in a stiff and painful shoulder patient. Phys. Ther. Sport 2014, 15, 3–14. [Google Scholar] [CrossRef]
- Bhargav, D.; Murrell, G.A. Shoulder stiffness: Management. Aust. Fam. Physician 2004, 33, 149–152. [Google Scholar]
- Hanchard, N.C.A.; Goodchild, L.; Brealey, S.D.; Lamb, S.E.; Rangan, A. Physiotherapy for primary frozen shoulder in secondary care: Developing and implementing stand-alone and post operative protocols for UK FROST and inferences for wider practice. Physiotherapy 2020, 107, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Brealey, S.; Northgraves, M.; Kottam, L.; Keding, A.; Corbacho, B.; Goodchild, L.; Srikesavan, C.; Rex, S.; Charalambous, C.P.; Hanchard, N.; et al. Surgical treatments compared with early structured physiotherapy in secondary care for adults with primary frozen shoulder: The UK FROST three-arm RCT. Health Technol. Assess. 2020, 24, 1–161. [Google Scholar] [CrossRef]
- Qian, J.; McDonough, D.J.; Gao, Z. The Effectiveness of Virtual Reality Exercise on Individual’s Physiological, Psychological and Rehabilitative Outcomes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4133. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Jan, Y.K.; El Sayed, W.H.; Wanis, M.E.A.; Yamany, A.A. Dynamic scapular recognition exercise improves scapular upward rotation and shoulder pain and disability in patients with adhesive capsulitis: A randomized controlled trial. J. Man. Manip. Ther. 2020, 28, 146–158. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lin, C.Y.; Tsai, M.J.; Chuang, T.Y.; Lee, O.K.S. Wearable Motion Sensor Device to Facilitate Rehabilitation in Patients with Shoulder Adhesive Capsulitis: Pilot Study to Assess Feasibility. J. Med. Internet Res. 2020, 22, e17032. [Google Scholar] [CrossRef]
- Muñoz, G.F.; Mollineda, R.A.; Casero, J.G.; Pla, F. A RGBD-Based Interactive System for Gaming-Driven Rehabilitation of Upper Limbs. Sensors 2019, 19, 3478. [Google Scholar] [CrossRef] [Green Version]
- Kiper, P.; Szczudlik, A.; Agostini, M.; Opara, J.; Nowobilski, R.; Ventura, L.; Tonin, P.; Turolla, A. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 834–842.e4. [Google Scholar] [CrossRef]
- Kapşigay, B.; Sari, Z.; Kavlak, B.; Aras, I.; Tanhan, A. Effects of virtual rehabilitation on shoulder periarthritis. Ann. Rheum. Dis. 2017, 76, 1496. [Google Scholar]
- Lee, S.H.; Yeh, S.C.; Chan, R.C.; Chen, S.; Yang, G.; Zheng, L.R. Motor Ingredients Derived from a Wearable Sensor-Based Virtual Reality System for Frozen Shoulder Rehabilitation. Biomed. Res. Int. 2016, 2016, 7075464. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Yeh, S.C.; Lee, S.H. Wearable Sensors Integrated with Virtual Reality: A Self-Guided Healthcare System Measuring Shoulder Joint Mobility for Frozen Shoulder. J. Healthc. Eng. 2019, 2019, 7681237. [Google Scholar] [CrossRef] [Green Version]
- Lamontagne, A.; Fung, J.; McFadyen, B.J.; Faubert, J. Modulation of walking speed by changing optic flow in persons with stroke. J. Neuroeng. Rehabil. 2007, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Adamovich, S.V.; Fluet, G.G.; Tunik, E.; Merians, A.S. Sensorimotor training in virtual reality: A review. NeuroRehabilitation 2009, 25, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Kolber, M.J.; Mdt, C.; Hanney, W.J. The Reliability AND Concurrent Validity of Shoulder Mobility Measurements Using a Digital Inclinometer and Goniometer: A Technical Report. Int. J. Sports Phys. Ther. 2012, 7, 306. [Google Scholar]
- Elgendy, M.H.; El-khalek, W.O.A.A. Validity and Intra-Rater Reliability of Laser Goniometer versus Electro-Goniometer in Measuring Shoulder Range of Motion. Int. J. Physiother. 2019, 6, 169–176. [Google Scholar] [CrossRef]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Green, S.; Buchbinder, R.; Hetrick, S.E. Physiotherapy interventions for shoulder pain. Cochrane Database Syst. Rev. 2003, 2003. [Google Scholar] [CrossRef]
- Roy, J.S.; Macdermid, J.C.; Woodhouse, L.J. Measuring shoulder function: A systematic review of four questionnaires. Arthritis Rheum. 2009, 61, 623–632. [Google Scholar] [CrossRef]
- Wajngarten, D.; Campos, J.; Garcia, P. The Disabilities of the Arm, Shoulder and Hand scale in the evaluation of disability—A literature review. Med. Lav. 2017, 108, 314–323. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Susan Muir, C.; Muir, S.W.; Luciak Corea, C.; Beaupre, L. Evaluating Change in Clinical Status: Reliability and Measures of Agreement for the Assessment of Glenohumeral Range of Motion. N. Am. J. Sports Phys. Ther. 2010, 5, 98. [Google Scholar]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Hobden, E.; Stiell, I.G.; Wells, G.A. Clinically important change in the visual analog scale after adequate pain control. Acad. Emerg. Med. 2003, 10, 1128–1130. [Google Scholar] [CrossRef]
- Limanowski, J.; Friston, K. Active inference under visuo-proprioceptive conflict: Simulation and empirical results. Sci. Rep. 2020, 10, 4010. [Google Scholar] [CrossRef] [Green Version]
- Oosterwijk, A.M.; Nieuwenhuis, M.K.; Van der Schans, C.P.; Mouton, L.J. Shoulder and elbow range of motion for the performance of activities of daily living: A systematic review. Physiother. Theory Pract. 2018, 34, 505–528. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.K.; Shanahan, E.M.; Tucker, G.R.; Buchbinder, R.; Hill, C.L. Shoulder range of movement in the general population: Age and gender stratified normative data using a community-based cohort. BMC Musculoskelet. Disord. 2020, 21, 676. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, P.M.; Reynolds, J.F. The association of scapular kinematics and glenohumeral joint pathologies. J. Orthop. Sports Phys. Ther. 2009, 39, 90–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neviaser, A.S.; Neviaser, R.J. Adhesive capsulitis of the shoulder. J. Am. Acad. Orthop. Surg. 2011, 19, 536–542. [Google Scholar] [CrossRef]
- Gleyze, P.; Flurin, P.H.; Laprelle, E.; Katz, D.; Toussaint, B.; Benkalfate, T.; Solignac, N.; Lévigne, C. Pain management in the rehabilitation of stiff shoulder: Prospective multicenter comparative study of 193 cases. Orthop. Traumatol. Surg. Res. 2011, 97, S195–S203. [Google Scholar] [CrossRef] [Green Version]
- Rossettini, G.; Latini, T.M.; Palese, A.; Jack, S.M.; Ristori, D.; Gonzatto, S.; Testa, M. Determinants of patient satisfaction in outpatient musculoskeletal physiotherapy: A systematic, qualitative meta-summary, and meta-synthesis. Disabil. Rehabil. 2020, 42, 460–472. [Google Scholar] [CrossRef]
- Oh-Young, C.; Gordon, H.R.D.; Xing, X.; Filler, J. Meta-Analytic Procedures for Career and Technical Education Post-secondary Researchers and Practitioners. J. Res. Tech. Careers 2018, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Pease, B.; Ross, M. Defining subgroups of patients with a stiff and painful shoulder: An analytical model using cluster analysis. Disabil. Rehabil. 2021, 43, 537–544. [Google Scholar] [CrossRef]

| Characteristic | M-Group (n = 6) | NM-Group (n = 7) | p |
|---|---|---|---|
| Age (years) | 60.2 ± 6.1 | 63.3 ± 7.3 | 0.518 |
| Sex | 5 females, 1 male | 7 females | 0.261 |
| Injured shoulder | 2 left, 4 right | 4 left, 3 right | 0.391 |
| Weeks from injury | 6.2 ± 2.2 | 8.7 ± 5.1 | 0.563 |
| Characteristic | M-Group (n = 6) | NM-Group (n = 7) | p | r |
|---|---|---|---|---|
| Passive flexion ROM (°) | 90.0 (70.0–100.0) | 97.0 (92.0–105.0) | 0.197 | −0.358 |
| Passive abduction ROM (°) | 64.0 (47.5–75.0) | 70.0 (60.0–80.0) | 0.428 | −0.220 |
| Active flexion ROM (°) | 61.5 (38.8–77.5) | 82.0 (78.0–85.0) | 0.053 | −0.536 |
| Active abduction ROM (°) | 52.5 (39.5–59.0) | 48.0 (44.0–60.0) | 0.830 | −0.060 |
| VAS (0–10) | 3.5 (0.8–5.1) | 3.0 (0.0–5.0) | 0.942 | −0.020 |
| DASH (0–100) | 88.6 (72.7–108.6) | 96.0 (81.1–104.3) | 0.668 | −0.119 |
| Characteristic | M-Group (n = 6) | NM-Group (n = 7) | p | r |
|---|---|---|---|---|
| Passive flexion ROM (%) | 127.8 (113.3–173.2) | 125.6 (106.0–152.5) | 0.391 | −0.238 |
| Passive abduction ROM (%) | 134.5 (115.8–191.4) | 146.4 (137.3–180.0) | 1.000 | 0 |
| Active flexion ROM (%) | 197.1 (140.5–425.0) | 142.5 (139.1–151.3) | 0.046 | −0.555 |
| Active abduction ROM (%) | 150.0 (124.8–191.2) | 162.5 (129.2–187.5) | 1.000 | 0 |
| VAS (0–10) | 75.0 (12.5–106.8) | 26.7 (5.0–120.8) | 0.916 | −0.034 |
| DASH (%) | 67.7 (52.8–86.2) | 89.7 (83.8–98.3) | 0.022 | −0.634 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, I.; Safran, O.; Karniel, N.; Abel, M.; Berko, A.; Seyres, M.; Tsoar, T.; Portnoy, S. Positive Effect of Manipulated Virtual Kinematic Intervention in Individuals with Traumatic Stiff Shoulder: A Pilot Study. J. Clin. Med. 2022, 11, 3919. https://doi.org/10.3390/jcm11133919
Schwartz I, Safran O, Karniel N, Abel M, Berko A, Seyres M, Tsoar T, Portnoy S. Positive Effect of Manipulated Virtual Kinematic Intervention in Individuals with Traumatic Stiff Shoulder: A Pilot Study. Journal of Clinical Medicine. 2022; 11(13):3919. https://doi.org/10.3390/jcm11133919
Chicago/Turabian StyleSchwartz, Isabella, Ori Safran, Naama Karniel, Michal Abel, Adina Berko, Martin Seyres, Tamir Tsoar, and Sigal Portnoy. 2022. "Positive Effect of Manipulated Virtual Kinematic Intervention in Individuals with Traumatic Stiff Shoulder: A Pilot Study" Journal of Clinical Medicine 11, no. 13: 3919. https://doi.org/10.3390/jcm11133919
APA StyleSchwartz, I., Safran, O., Karniel, N., Abel, M., Berko, A., Seyres, M., Tsoar, T., & Portnoy, S. (2022). Positive Effect of Manipulated Virtual Kinematic Intervention in Individuals with Traumatic Stiff Shoulder: A Pilot Study. Journal of Clinical Medicine, 11(13), 3919. https://doi.org/10.3390/jcm11133919







