Effect of Iron Depletion by Bloodletting vs. Observation on Oxidative Stress Biomarkers of Women with Functional Hyperandrogenism Taking a Combined Oral Contraceptive: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
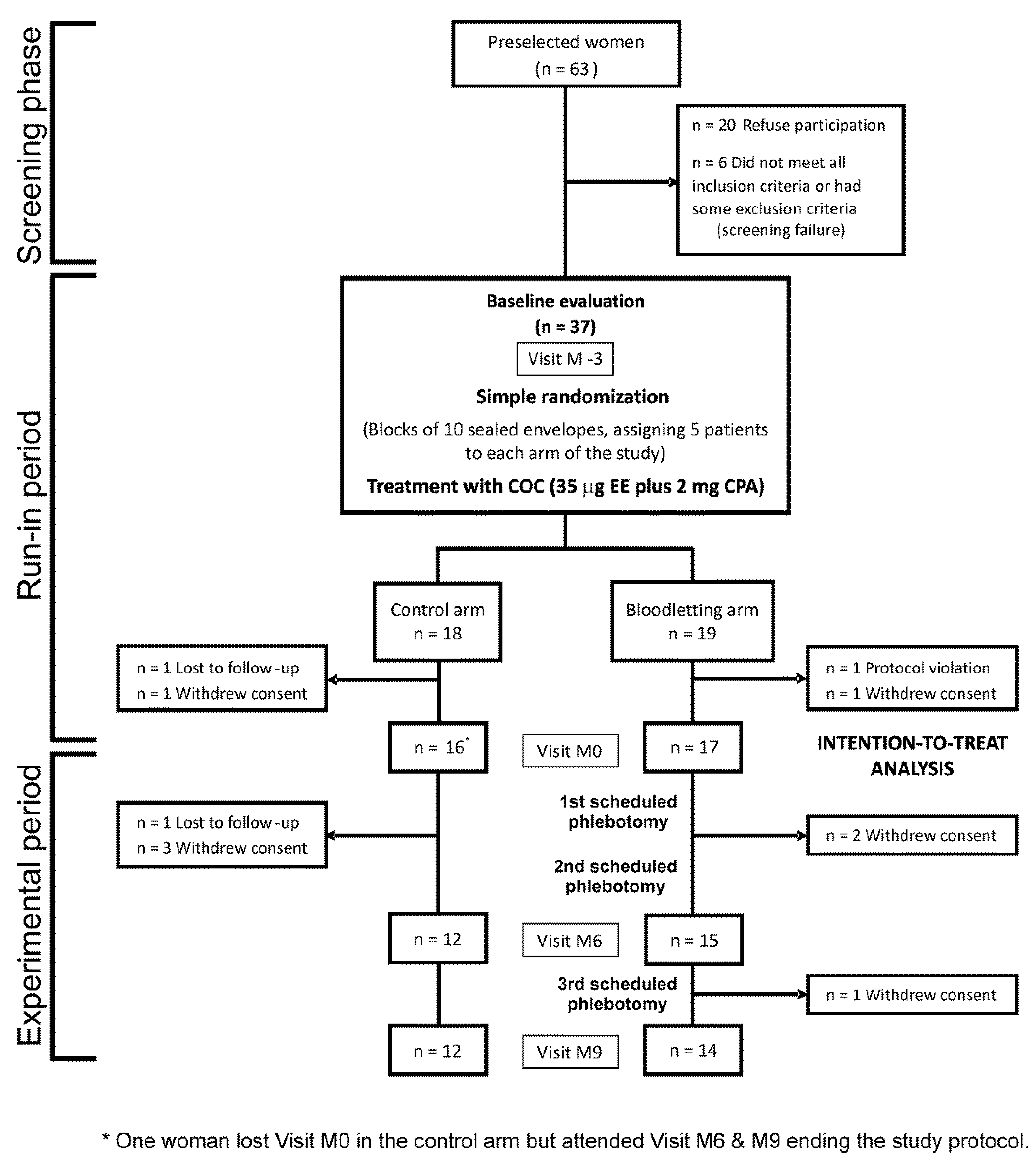
2.1. Study Design
2.2. Patients
2.3. Randomization
2.4. Intervention
2.5. Outcomes Assessment
2.6. Biochemical and Hormonal Assays
2.7. Power Calculation
2.8. Statistical Analysis
3. Results
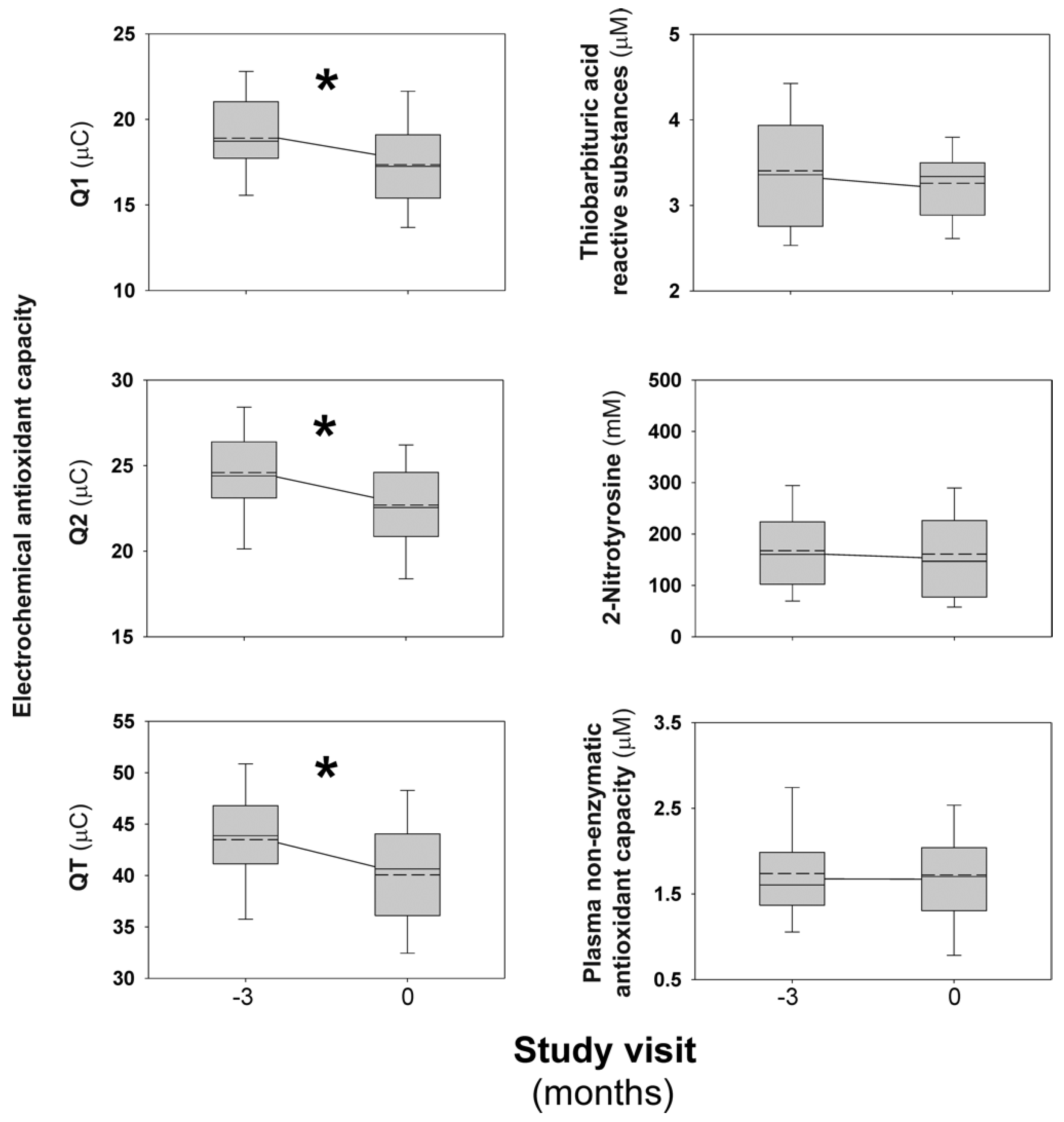
3.1. Short-Term Effects of Combined Oral Contraceptives on Oxidative Stress Biomarkers
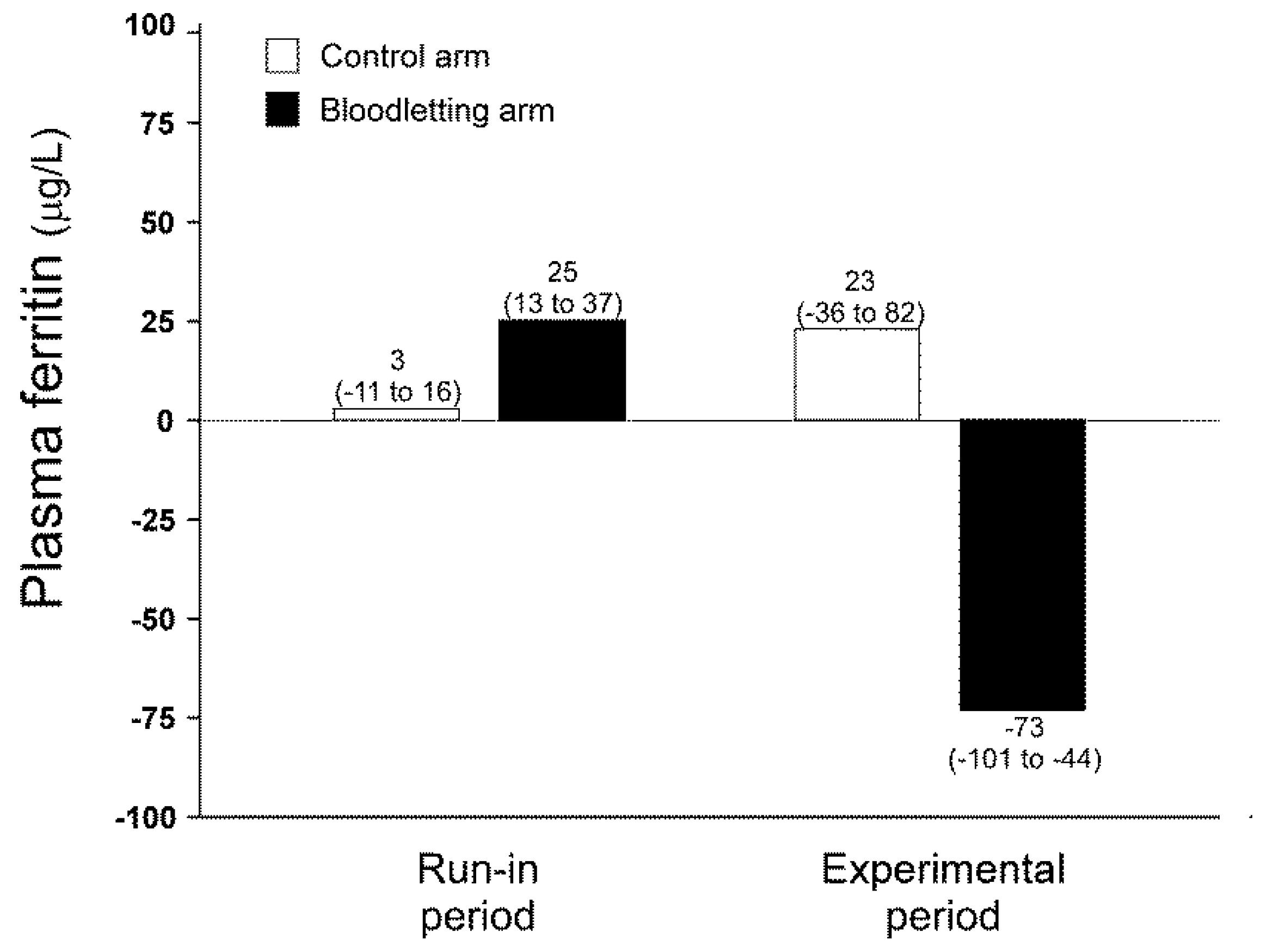
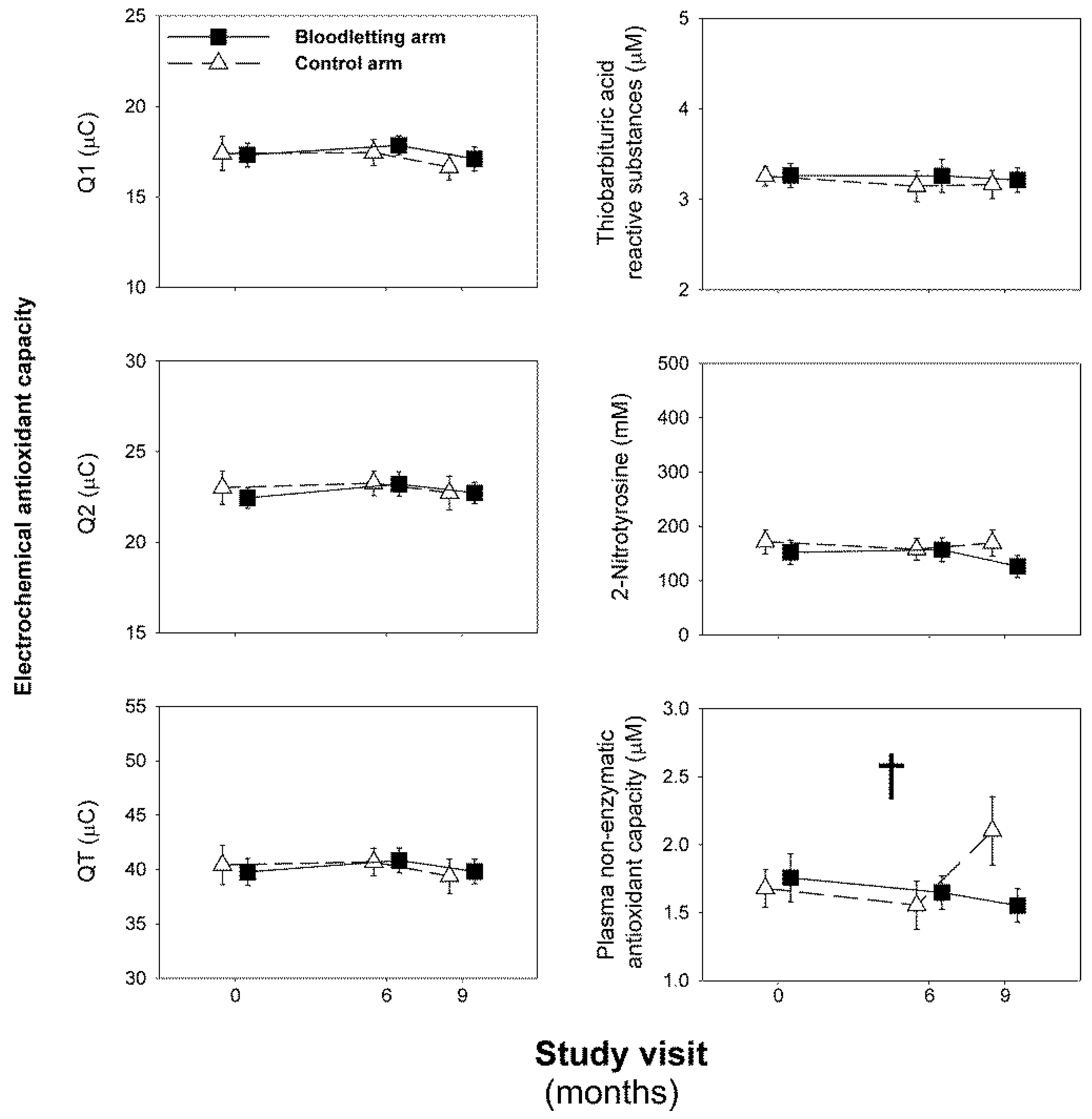
3.2. Effect of Iron Depletion by Bloodletting on Oxidative Stress Biomarkers
3.3. Determinants of Changes in Oxidative Stress Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skiba, M.A.; Islam, R.M.; Bell, R.J.; Davis, S.R. Understanding variation in prevalence estimates of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2018, 24, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Luque-Ramirez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rocha, M.; Bañuls, C.; Sanchez-Serrano, M.; Sola, E.; Gomez, M.; Hernandez-Mijares, A. Mitochondrial complex I impairment in leukocytes from polycystic ovary syndrome patients with insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 3505–3512. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Guo, X.; Jia, W.; Liu, G.; Zhang, J.; Li, J.; Cui, P.; Sferruzzi-Perri, A.N.; Han, Y.; et al. Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E794–E809. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; Bañuls, C.; Alvarez, A.; de Pablo, C.; Sanchez-Serrano, M.; Gomez, M.; Hernandez-Mijares, A. Induction of oxidative stress and human leukocyte/endothelial cell interactions in polycystic ovary syndrome patients with insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, 3115–3122. [Google Scholar] [CrossRef]
- González, F.; Sia, C.L.; Bearson, D.M.; Blari, H.E. Hyperandrogenism induces a proinflammatory TNFα response to glucose ingestion in a receptor-dependent fashion. J. Clin. Endocrinol. Metab. 2014, 99, E848–E854. [Google Scholar] [CrossRef][Green Version]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; González, F. Circulating inflammatory markers in polycystic ovary syndrome: A systematic review and metaanalysis. Fertil. Steril. 2011, 95, 1048–1058. [Google Scholar] [CrossRef]
- González, F.; Considine, R.V.; Abdelhadi, O.A.; Acton, A.J. Oxidative Stress in Response to Saturated Fat Ingestion Is Linked to Insulin Resistance and Hyperandrogenism in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 5360–5371. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Kirwan, J.P.; Sia, C.L.; González, F. Glucose-stimulated oxidative stress in mononuclear cells is related to pancreatic β-cell dysfunction in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Sia, C.L.; Shepard, M.K.; Rote, N.S.; Minium, J. Hyperglycemia-induced oxidative stress is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. Hum. Reprod. 2012, 27, 3560–3568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Han, B.; Fan, M.; Wang, N.; Wang, H.; Zhu, H.; Cheng, T.; Zhao, S.; Song, H.; Qiao, J. Oxidative stress increases the 17,20-lyase-catalyzing activity of adrenal P450c17 through p38α in the development of hyperandrogenism. Mol. Cell Endocrinol. 2019, 484, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, S.; Liu, H.; Bai, H.; Hu, K.; Zhang, R.; Liu, Q.; Fan, P. Oxidative stress promotes hyperandrogenism by reducing sex hormone-binding globulin in polycystic ovary syndrome. Fertil. Steril. 2021, 22, 01806–01809. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Luque-Ramírez, M.; Álvarez-Blasco, F.; Botella-Carretero, J.I.; Sancho, J.; San Millan, J.L. Body iron stores are increased in overweight and obese women with polycystic ovary syndrome. Diabetes Care 2005, 28, 2042–2044. [Google Scholar] [CrossRef]
- Martínez-García, M.A.; Luque-Ramírez, M.; San-Millán, J.L.; Escobar-Morreale, H.F. Body iron stores and glucose intolerance in premenopausal women: Role of hyperandrogenism, insulin resistance, and genomic variants related to inflammation, oxidative stress, and iron Metab.olism. Diabetes Care 2009, 32, 1525–1530. [Google Scholar] [CrossRef]
- Adamska, A.; Łebkowska, A.; Krentowska, A.; Adamski, M.; Kowalska, I. The Association Between Serum Ferritin Concentration and Visceral Adiposity Estimated by Whole-Body DXA Scan in Women With Polycystic Ovary Syndrome. Front. Endocrinol. (Lausanne) 2019, 10, 873. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Manco, M. Effects of iron overload on chronic Metab.olic diseases. Lancet Diabetes Endocrinol. 2014, 2, 513–526. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Broch, M.; Ricart, W.; Casamitjana, R.; Gutierrez, C.; Vendrell, J.; Richart, C. Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes 1998, 47, 1757–1762. [Google Scholar] [CrossRef]
- Suárez-Ortegón, M.F.; McLachlan, S.; Price, A.H.; Fernández-Balsells, M.; Franch-Nadal, J.; Mata-Cases, M.; Barrot-de la Puente, J.; Mundet-Tudurí, X.; Mauricio, D.; Ricart, W.; et al. Decreased iron stores are associated with cardiovascular disease in patients with type 2 diabetes both cross-sectionally and longitudinally. Atherosclerosis 2018, 272, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Balla, J.; Vercellotti, G.M.; Jeney, V.; Yachie, A.; Varga, Z.; Jacob, H.S.; Eaton, J.W.; Balla, G. Heme, heme oxygenase, and ferritin: How the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid. Redox Signal. 2007, 9, 2119–2137. [Google Scholar] [CrossRef] [PubMed]
- Rineau, E.; Gueguen, N.; Procaccio, V.; Geneviève, F.; Reynier, P.; Henrion, D.; Lasocki, S. Iron Deficiency without Anemia Decreases Physical Endurance and Mitochondrial Complex I Activity of Oxidative Skeletal Muscle in the Mouse. Nutrients 2021, 13, 1056. [Google Scholar] [CrossRef]
- Orme, M.L. The third S.K. & F. Prize lecture, University of London, December 1981. The clinical pharmacology of oral contraceptive steroids. Br. J. Clin. Pharm. 1982, 14, 31–42. [Google Scholar]
- Stanczyk, F.Z.; Archer, D.F.; Bhavnani, B.R. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: Pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87, 706–727. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Vanbelle, S.; Gaspard, U.; Collette, G.; Haleng, J.; Cheramy-Bien, J.P.; Charlier, C.; Chapelle, J.P.; Giet, D.; Albert, A.; et al. Effect of different contraceptive methods on the oxidative stress status in women aged 40 48 years from the ELAN study in the province of Liege, Belgium. Hum. Reprod. 2007, 22, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Cauci, S.; Xodo, S.; Buligan, C.; Colaninno, C.; Barbina, M.; Barbina, G.; Francescato, M.P. Oxidative Stress Is Increased in Combined Oral Contraceptives Users and Is Positively Associated with High-Sensitivity C-Reactive Protein. Molecules 2021, 26, 1070. [Google Scholar] [CrossRef]
- Venter, G.; van der Berg, C.L.; van der Westhuizen, F.H.; Erasmus, E. Health Status Is Affected, and Phase I/II Biotransformation Activity Altered in Young Women Using Oral Contraceptives Containing Drospirenone/Ethinyl Estradiol. Int J. Environ. Res. Public Health 2021, 18, 10607. [Google Scholar] [CrossRef]
- Ortiz-Flores, A.E.; Martínez-García, M.; Nattero-Chávez, L.; Álvarez-Blasco, F.; Fernández-Durán, E.; Quintero-Tobar, A.; Escobar-Morreale, H.F.; Luque-Ramírez, M. Iron Overload in Functional Hyperandrogenism: In a Randomized Trial, Bloodletting Does Not Improve Metabolic Outcomes. J. Clin. Endocrinol. Metab. 2021, 19, e1559–e1573. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Carmina, E.; Dewailly, D.; Gambineri, A.; Kelestimur, F.; Moghetti, P.; Pugeat, M.; Qiao, J.; Wijeyaratne, C.N.; Witchel, S.F.; et al. Epidemiology, diagnosis and management of hirsutism: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2013, 19, 146–170. [Google Scholar] [CrossRef][Green Version]
- Templeton, G.F. Approach for transforming continuous variables to normal: Implications and recommendations for IS research. Commun. Assoc. Inf. Syst. 2011, 28. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, H.; Bai, H.; Zhang, Y.; Liu, Q.; Guan, L.; Fan, P. Oxidative stress status in Chinese women with different clinical phenotypes of polycystic ovary syndrome. Clin. Endocrinol. 2017, 86, 88–96. [Google Scholar] [CrossRef] [PubMed]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Nikiforov, N.G.; Eid, A.H.; Nedosugova, L.V.; Starodubova, A.V.; Popkova, T.V.; Bezsonov, E.E.; Orekhov, A.N. Mitochondrial Dysfunction and Chronic Inflammation in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2021, 22, 3923. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Millán, J.L. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol. Metab. 2007, 18, 266–272. [Google Scholar] [CrossRef]
- Liu, S.; Navarro, G.; Mauvais-Jarvis, F. Androgen excess produces systemic oxidative stress and predisposes to beta-cell failure in female mice. PLoS ONE 2010, 5, e11302. [Google Scholar]
- Lobysheva, I.I.; van Eeckhoudt, S.; Dei Zotti, F.; Rifahi, A.; Pothen, L.; Beauloye, C.; Balligand, J.L. Heme-nitrosylated hemoglobin and oxidative stress in women consuming combined contraceptives. Clinical application of the EPR spectroscopy. Free Radic. Biol. Med. 2017, 108, 524–532. [Google Scholar] [CrossRef]
- Swanepoel, A.C.; Bester, J.; Emmerson, O.; Soma, P.; Beukes, D.; van Reenen, M.; Loots, D.T.; du Preez, I. Serum Metab.olome Changes in Relation to Prothrombotic State Induced by Combined Oral Contraceptives with Drospirenone and Ethinylestradiol. Omics 2020, 24, 404–414. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, A.; Ortiz-Balderas, E.; Cardozo-Saldaña, G.; Diaz-Diaz, E.; El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. 2014, 126, 19–29. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Biophys. Acta 2014, 1840, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Rey Alonso, S.C. Study of the Redox Balance in Cellular Lines: Influcence of Used Technology; Universidad de Oviedo: Oviedo, Spain, 2018. [Google Scholar]
- Kowalska, K.; Milnerowicz, H. Pro/antioxidant status in young healthy women using oral contraceptives. Environ. Toxicol. Pharm. 2016, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ramírez, M.; Mendieta-Azcona, C.; del Rey Sánchez, J.M.; Matíes, M.; Escobar-Morreale, H.F. Effects of an antiandrogenic oral contraceptive pill compared with metformin on blood coagulation tests and endothelial function in women with the polycystic ovary syndrome: Influence of obesity and smoking. Eur. J. Endocrinol. 2009, 160, 469–480. [Google Scholar] [CrossRef]
- Sharma, G.S.; Bhattacharya, R.; Singh, L.R. Functional inhibition of redox regulated heme proteins: A novel mechanism towards oxidative stress induced by homocysteine. Redox Biol. 2021, 46, 102080. [Google Scholar] [CrossRef]
- Luque-Ramírez, M.; Álvarez-Blasco, F.; Alpañés, M.; Escobar-Morreale, H.F. Role of decreased circulating hepcidin concentrations in the iron excess of women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2011, 96, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, Y.; Li, T.; Li, M.; Li, J.; Li, R.; Liu, P.; Yu, Y.; Qiao, J. Metab.olism alteration in follicular niche: The nexus among intermediary Metab.olism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic. Biol. Med. 2015, 86, 295–307. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kowdley, K.V. Hereditary hemochromatosis and diabetes mellitus: Implications for clinical practice. Nat. Rev. Endocrinol. 2010, 6, 26–33. [Google Scholar] [CrossRef]
- Ong, S.Y.; Gurrin, L.C.; Dolling, L.; Dixon, J.; Nicoll, A.J.; Wolthuizen, M.; Wood, E.M.; Anderson, G.J.; Ramm, G.A.; Allen, K.J.; et al. Reduction of body iron in HFE-related haemochromatosis and moderate iron overload (Mi-Iron): A multicentre, participant-blinded, randomised controlled trial. Lancet Haematol. 2017, 4, e607–e614. [Google Scholar] [CrossRef]
- Shizukuda, Y.; Bolan, C.D.; Nguyen, T.T.; Botello, G.; Tripodi, D.J.; Yau, Y.Y.; Waclawiw, M.A.; Leitman, S.F.; Rosing, D.R. Oxidative stress in asymptomatic subjects with hereditary hemochromatosis. Am. J Hematol. 2007, 82, 249–250. [Google Scholar] [CrossRef]
- Distante, S.; Eikeland, J.; Pawar, T.; Skinnes, R.; Høie, K.; You, P.; Mørkrid, L.; Eide, L. Blood removal therapy in hereditary hemochromatosis induces a stress response resulting in improved genome integrity. Transfusion 2016, 56, 1435–1441. [Google Scholar] [CrossRef]
- Kaito, M.; Iwasa, M.; Kobayashi, Y.; Fujita, N.; Tanaka, H.; Gabazza, E.C.; Adachi, Y.; Kojima, Y.; Nakagawa, N.; Watanabe, S. Iron reduction therapy by phlebotomy reduces lipid peroxidation and oxidative stress in patients with chronic hepatitis C. J. Gastroenterol. 2006, 41, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Lainé, F.; Reymann, J.M.; Morel, F.; Langouët, S.; Perrin, M.; Guillygomarc’h, A.; Brissot, P.; Turmel, V.; Mouchel, C.; Pape, D.; et al. Effects of phlebotomy therapy on cytochrome P450 2e1 activity and oxidative stress markers in dysMetab.olic iron overload syndrome: A randomized trial. Aliment. Pharm. 2006, 24, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Gulyani, S.; Earley, C.J.; Cutler, R.G.; Mattson, M.P.; Rifkind, J.M. Iron-deficiency anaemia enhances red blood cell oxidative stress. Free Radic. Res. 2008, 42, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Total antioxidant capacity: Appraisal of a concept. J. Nutr. 2007, 137, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Schwedhelm, E.; Frölich, J.C. Methodological considerations on the detection of 3-nitrotyrosine in the cardiovascular system. Circ. Res. 2002, 90, E70. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moral, M.P.; Kannan, K. How stable is oxidative stress level? An observational study of intra- and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environ. Int. 2019, 123, 382–389. [Google Scholar] [CrossRef]
- Moore, K.; Roberts, L.J., 2nd. Measurement of lipid peroxidation. Free Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef]
| Intention-to-Treat Population | |||
|---|---|---|---|
| All Women | Bloodletting Arm | Control Arm | |
| (n = 33) | (n = 17) | (n = 16) | |
| Age (years) | 25 ± 6 | 25 ± 7 | 25 ± 6 |
| (23 to 27) | (21 to 29) | (22 to 28) | |
| Body mass index (kg/m2) | 28.9 ± 8.0 | 29.6 ± 8.1 | 28.3 ± 8.1 |
| (26.1 to 31.7) | (25.4 to 33.8) | (24.0 to 32.6) | |
| Waist circumference (cm) | 89 ± 18 | 90 ± 16 | 87 ± 20 |
| (83 to 95) | (82 to 98) | (76 to 98) | |
| Frequency of obesity, n (%) | 14 (42) | 8 (47) | 6 (38) |
| (27 to 59) | (26 to 69) | (19 to 61) | |
| Total testosterone (nmol/L) | 2.75 ± 1.04 | 2.6 ± 1.1 | 2.9 ± 1.0 |
| (2.4 to 3.1) | (2.1 to 3.2) | (2.3 to 3.4) | |
| Calculated free testosterone (pmol/L) | 52 ± 22 | 52 ± 25 | 51 ± 20 |
| (44 to 60) | (39 to 65) | (40 to 62) | |
| Dehydroepiandrosterone-sulphate (μmol/L) | 7.56 ± 2.92 | 7.3 ± 2.2 | 7.8 ± 3.9 |
| (6.53 to 8.60) | (6.2 to 8.5) | (5.7 to 9.9) | |
| Homeostasis model assessment of insulin resistance | 2.8 ± 2.5 (1.9 to 3.6) | 2.7 ± 2.5 (1.4 to 4.0) | 2.8 ± 2.5 (1.5 to 4.1) |
| Electrochemical antioxidant capacity (μC) | |||
| Q1 | 18.9 ± 2.6 | 19.3 ± 2.2 | 18.5 ± 3.0 |
| (18.0 to 19.8) | (18.1 to 20.4) | (16.8 to 20.2) | |
| Q2 | 24.6 ± 2.7 | 24.9 ± 1.8 | 24.3 ± 3.4 |
| (23.6 to 25.6) | (24.0 to 25.8) | (22.4 to 26.2) | |
| QT | 43.5 ± 5.0 | 44.2 ± 3.4 | 42.7 ± 6.3 |
| (41.7 to 45.3) | (42.5 to 46.0) | (39.2 to 46.2) | |
| Thiobarbituric acid reactive substances (μM) | 3.40 ± 0.69 | 3.35 ± 0.68 | 3.47 ± 0.72 |
| (3.16 to 3.65) | (3.00 to 3.70) | (3.09 to 3.85) | |
| 2-Nitrotyrosine (mM) | 168 ± 83 | 166 ± 86 | 169 ± 83 |
| (138 to 197) | (122 to 211) | (125 to 213) | |
| Non-enzymatic antioxidant capacity (μM) | 1.74 ± 0.57 | 1.71 ± 0.55 | 1.77 ± 0.60 |
| (1.54 to 1.94) | (1.43 to 1.99) | (1.44 to 2.10) | |
| Q1 (μC) | Q2 (μC) | QT (μC) | |
|---|---|---|---|
| Body mass index (kg/m2) | r: −0.086 | r: −0.129 | r: −0.141 |
| p = 0.638 | p = 0.481 | p = 0.441 | |
| Waist circumference (cm) | r: 0.086 | r: 0.136 | r: 0.196 |
| p = 0.274 | p = 0.465 | p = 0.290 | |
| Fat mass (%) | r: −0.190 | r: −0.155 | r: −0.192 |
| p = 0.298 | p = 0.396 | p = 0.293 | |
| Free testosterone (pmol/L) | r: 0.170 | r: 0.169 | r: 0.156 |
| p = 0.352 | p = 0.355 | p = 0.394 | |
| Dehydroepiandrosterone-sulphate (μmol/L) | r: 0.004 | r: 0.051 | r: 0.048 |
| p = 0.983 | p = 0.781 | p = 0.795 | |
| Fasting glucose (mmol/L) | r: −0.158 | r: −0.152 | r: −0.182 |
| p = 0.389 | p = 0.408 | p = 0.318 | |
| Fasting insulin (pmol/L) | r: −0.296 | r: −0.287 | r: −0.328 |
| p = 0.106 | p = 0.117 | p = 0.072 | |
| Homeostasis model assessment of insulin resistance | r: −0.339 | r: −0.335 | r: −0.374 |
| p = 0.062 | p = 0.065 | p = 0.038 | |
| Ferritin (μg/L) | r: 0.116 | r: 0.023 | r: 0.100 |
| p = 0.528 | p = 0.901 | p = 0.586 | |
| Total iron binding capacity (μg/dL) | r: −0.345 | r: −0.216 | r: −0.302 |
| p = 0.053 | p = 0.236 | p = 0.093 | |
| Transferrin Saturation (%) | r: −0.154 | r: −0.416 | r: −0.262 |
| p = 0.401 | p = 0.018 | p = 0.147 | |
| Plasma hemoglobin (g/L) | r: −0.401 | r: −0.229 | r: −0.340 |
| p = 0.023 | p = 0.207 | p = 0.057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque-Ramírez, M.; Ortiz-Flores, A.E.; Martínez-García, M.Á.; Insenser, M.; Quintero-Tobar, A.; De Lope Quiñones, S.; Fernández-Durán, E.; Nattero-Chávez, M.L.; Álvarez-Blasco, F.; Escobar-Morreale, H.F. Effect of Iron Depletion by Bloodletting vs. Observation on Oxidative Stress Biomarkers of Women with Functional Hyperandrogenism Taking a Combined Oral Contraceptive: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 3864. https://doi.org/10.3390/jcm11133864
Luque-Ramírez M, Ortiz-Flores AE, Martínez-García MÁ, Insenser M, Quintero-Tobar A, De Lope Quiñones S, Fernández-Durán E, Nattero-Chávez ML, Álvarez-Blasco F, Escobar-Morreale HF. Effect of Iron Depletion by Bloodletting vs. Observation on Oxidative Stress Biomarkers of Women with Functional Hyperandrogenism Taking a Combined Oral Contraceptive: A Randomized Clinical Trial. Journal of Clinical Medicine. 2022; 11(13):3864. https://doi.org/10.3390/jcm11133864
Chicago/Turabian StyleLuque-Ramírez, Manuel, Andrés E. Ortiz-Flores, María Ángeles Martínez-García, María Insenser, Alejandra Quintero-Tobar, Sara De Lope Quiñones, Elena Fernández-Durán, María Lía Nattero-Chávez, Francisco Álvarez-Blasco, and Héctor Francisco Escobar-Morreale. 2022. "Effect of Iron Depletion by Bloodletting vs. Observation on Oxidative Stress Biomarkers of Women with Functional Hyperandrogenism Taking a Combined Oral Contraceptive: A Randomized Clinical Trial" Journal of Clinical Medicine 11, no. 13: 3864. https://doi.org/10.3390/jcm11133864
APA StyleLuque-Ramírez, M., Ortiz-Flores, A. E., Martínez-García, M. Á., Insenser, M., Quintero-Tobar, A., De Lope Quiñones, S., Fernández-Durán, E., Nattero-Chávez, M. L., Álvarez-Blasco, F., & Escobar-Morreale, H. F. (2022). Effect of Iron Depletion by Bloodletting vs. Observation on Oxidative Stress Biomarkers of Women with Functional Hyperandrogenism Taking a Combined Oral Contraceptive: A Randomized Clinical Trial. Journal of Clinical Medicine, 11(13), 3864. https://doi.org/10.3390/jcm11133864







