Abstract
(1) Background: Despite the improvement of the in-hospital survival rate after aborted sudden cardiac death (SCD), cerebral anoxia may have severe neurologic consequences and may impair long-term outcome and quality of life of surviving patients. The aim of this study was to assess neurological outcomes at one year after resuscitated cardiac arrest; (2) Methods: This prospective, observational, and multicentre study included patients >18 yo admitted in the catheterisation laboratory for coronary angiography after aborted SCD between 1 May 2018 and 31 May 2020. Only patients who were discharged alive from hospital were evaluated. The primary endpoint was survival without neurological sequelae at one-year follow-up defined by a cerebral performance category (CPC) of one or two. Secondary end points included all-cause mortality, New York Heart Association (NYHA) functional class, neurologic evaluation at discharge, three-month and one-year follow-up using the CPC scale, and quality of life at 1 year using the Quality of Life after Brain Injury (QOLIBRI) questionnaire; (3) Results: Among 143 patients admitted for SCD within the study period, 61 (42.7%) were discharged alive from hospital, among whom 55 (90.1%) completed the one-year follow-up. No flow and low flow times were 1.9 ± 2.4 min and 16.5 ± 10.4 min, respectively. For 93.4% of the surviving patients, an initial shockable rhythm (n = 57) was observed and acute coronary syndrome was diagnosed in 75.4% of them (n = 46). At 1 year, survival rate without neurologic sequelae was 87.2% (n = 48). Patients with poor outcome were older (69.3 vs. 57.4 yo; p = 0.04) and had lower body mass index (22.4 vs. 26.7; p = 0.013) and a lower initial Left Ventricle Ejection Fraction (LVEF) (32.1% vs. 40.3%; p = 0.046). During follow-up, neurological status improved in 36.8% of patients presenting sequelae at discharge, and overall quality of life was satisfying for 66.7% of patients according to the QOLIBRI questionnaire; (4) Conclusions: Among patients admitted to the catheterisation laboratory for aborted SCD, mainly related to Acute Coronary Syndrom (ACS), less than a half of them were alive at discharge. However, the one-year survival rate without neurological sequelae was high and overall quality of life was good.
1. Introduction
Every year, about 350,000 sudden cardiac deaths (SCDs) are reported in the United States and 40,000–50,000 in France, mainly due to acute coronary syndrome (80%) and ventricular fibrillation (VF) [1,2,3]. Few studies have shown the benefit of immediate coronary angiography (CA) in survivors of out of hospital cardiac arrest (OHCA), especially in the setting of ST-segment elevation myocardial infarction (STEMI) [4,5]. The 2017 European Society of Cardiology Guidelines for the management of patients presenting with STEMI recommended direct admission to the catheterisation laboratory (cathlab) in comatose survivors of OHCA with electrocardiographic criteria for STEMI on the post-resuscitation electrocardiogram (ECG) (Class I, grade B) [6]. In the absence of STEMI criteria, admission to an intensive care unit first is recommended to exclude a non-coronary cause (Class IIa, grade B). Unconscious patients admitted to critical care units after SCD are at high risk for death, and neurologic deficits are common among the survivors [7]. Despite improvement in SCD management, the survival rate remains poor [8]. However, for the past few years, the number of immediate CA in patients with OHCA has increased, and prognosis was much more favourable with an 80% survival rate at discharge in a recent report [9]. During SCD, the brain suffers from temporary blood flow limitation, leading to hypoxic brain injury and cognitive impairment [7]. For survivors, the global anoxia can have severe neurological consequences [10]. While the survival rate seems to be well-known, neurological condition of this group of patients is poorly studied [11]. We therefore aimed to assess long-term neurological prognosis of survivors from SCD initially referred to the cathlab for CA.
2. Materials and Methods
This study was a prospective, observational, and multicentre registry. First, we checked all patients >18 yo who were admitted directly in the cathlab for out of hospital SCD between 1 May 2018 and 31 May 2020 in Montpellier and Nîmes University Hospitals. Then, we excluded patients who presented ventricular arrhythmia with immediate return of normal consciousness, patients without a return of spontaneous rhythm, or patients who died during the in-hospital stay. Therefore, only patients discharged alive from hospital were included for follow-up analysis. Nimes and Montpellier are university hospitals with intensive care units including cardiac monitoring, where appropriate invasive and non-invasive testing can be performed. A cardiovascular team, including interventional cardiology, electrophysiology, and cardiac surgery, are available. Nearly 150 cardiac arrests are admitted directly in the cathlab per year in these two centres.
The primary end point was the survival rate without significant neurological sequelae at one-year follow-up. Neurological outcome was assessed using the cerebral performance categories (CPC) of the Glasgow-Pittsburgh Outcome Categories: Category 1 is conscious and normal; Category 2 is conscious with moderate disability; Category 3 is conscious with severe disability; Category 4 is coma or vegetative state; and Category 5 is death [12,13,14]. We defined an absence of significant neurological sequelae as conscious, CPC 1 or 2, at one-year follow-up [15]. Secondary end points included total survival and survival without neurological sequelae (CPC 1 or 2) at 3 months, total survival at 1 year, New York Heart Associtation (NYHA) functional class at 3 months and 1 year, and quality of life at 1 year using the Quality of Life after Brain Injury (QOLIBRI) questionnaire. Quality of life was recorded by phone call or mail for SCD survivors without neurological sequelae at one-year follow-up. The QOLIBRI questionnaire is a novel health-related quality of life (HRQoL) instrument specifically developed for traumatic brain injury providing HRQoL in 6 fields [16,17]. This questionnaire has already been used to evaluate quality of life after an SCD [18]. It consists of 37 items in six scales summarised in Figure 1.
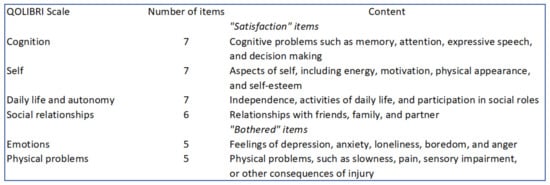
Figure 1.
QOLIBRI, Quality of Life after Brain Injury.
Patient characteristics, cardiopulmonary resuscitation (CPR) data (time, location, actors, and methods), and intra-hospital progress were collected at inclusion. If available (by phone call or through medical reports), an initial evaluation was performed at 3 months, with survival rate and cardiac and neurological evaluation. The cardiac and neurological status were also collected at one-year follow-up either by using DxCare software or by a phone call. A questionnaire was offered to all patients at one-year follow-up and was obtained by phone or by mail. At this moment, full study information was given and consent was obtained. No additional testing or biological samples were specifically required for the study.
Considering that our active patient file includes 150 cardiac arrests per year, of which 30 are discharged alive per year, we anticipated the inclusion of 60 patients discharged alive from hospital over a two-year period. Based on a previous study [13], we hypothesised that 10% of patients discharged alive from hospital would have severe neurological sequelae at one-year follow-up. Patient characteristics are presented using mean and standard deviation (SD) for continuous variables and frequencies and proportions for categorical variables. The chi-square test or Fisher’s exact test was used to compare categorical variables between groups (“good” and “poor” outcome). The Student’s t-test or the Wilcoxon–Mann–Whitney test was used to compare continuous variables. All analyses were conducted using R software (R Core Team, version 4.0.5, 2021, Vienna, Austria).
3. Results
3.1. Study Population
A flow chart is presented in Figure 2. A total of 143 patients were admitted directly to the cathlab for aborted SCD after return of spontaneous circulation (ROSC) between May 2018 and May 2020. In-hospital mortality was 57.3% (n = 82), 61 patients were therefore alive at discharge, with a mean age of 59.4 ± 14.1 yo and 77.0% (n = 47) being men. Baseline characteristics are presented in Table 1. Characteristics of six patients lost to follow-up were not different from our baseline population.
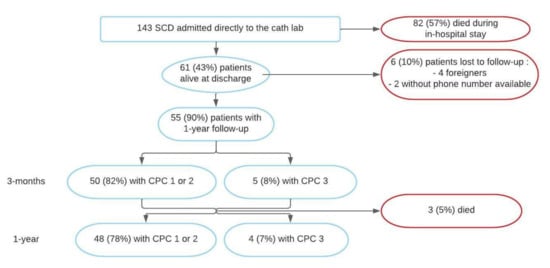
Figure 2.
Flow chart. CPC: Cerebral Performance Category; SCD: Sudden Cardiac Death.

Table 1.
Patients’ study baseline characteristics.
Regarding the cardiovascular risk factors, hypertension was the most frequently observed (n = 25, 40.9%). No-flow duration was 1.9 ± 2.4 min, low-flow duration was 16.5 ± 10.4 min, and an initial shockable rhythm was found in 93.4% of patients (n = 57). Twenty-nine patients (47.5%) of the survival patients at discharge had an SCD in the presence of a witness: for 18 patients (29.5%) in front of a doctor, for 8 patients (13.1%) in the emergency department, and for 3 patients (4.9%), SCD occurred during a hospital stay. The electrocardiogram immediately after the return of spontaneous circulation mostly showed a STEMI (n = 36, 59.0%). The CA found an artery occlusion in 27 patients (44%) and normal status in 15 patients (25%). At discharge, 29 patients (47%) had a left ventricular ejection fraction (LVEF) ≥ 50%. Rehabilitation was offered to all patients at discharge: 30% (n = 18), due to medical issues, were directly transferred from hospital to a general rehabilitation centre for neurological improvement, and the others went home to recover before benefiting from cardiac rehabilitation.
Regarding causes of SCD (Figure 3), 75.4% (n = 46) were related to ventricular tachycardia or fibrillation resulting from an acute ischemic syndrome. Of these, 78.2% (n = 36) were due to a STEMI and 21.7% (n = 10) resulted from a severe artery stenosis. Furthermore, 19.7% (n = 12) patients presented with a VT or VF resulting from a non-ischemic cardiopathy, 13.1% (n = 8) with dilated cardiomyopathy, and 6.6% (n = 4) with hypertrophic cardiomyopathy). The last three patients (4.9%) had an initial non-shockable rhythm from an extra-cardiac cause (one stroke, one pulmonary embolism, and one unknown aetiology).
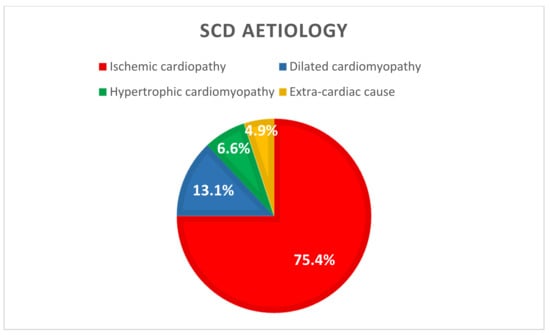
Figure 3.
SCD aetiology (n = 61). SCD: Sudden Cardiac Death.
3.2. Primary End Point
At one-year follow-up, clinical information was available for 55 patients (reports, phone call, or mail), representing 90.1% of our cohort. Among them, 48 (87.3%) patients were alive without neurologic sequelae as defined previously. A total of three patients (5.5%) died during follow-up and four (7.3%) had severe neurological sequelae (Category 3 on CPC). These seven patients (12.7%) were therefore classified in the “poor event” group (Figure 4).
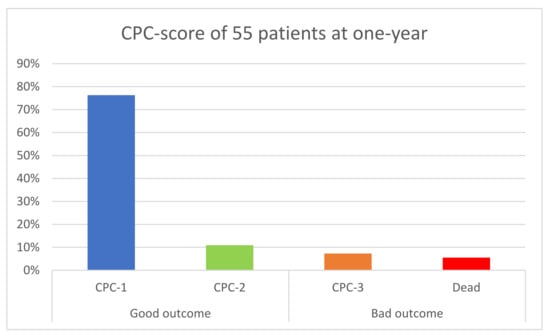
Figure 4.
Primary End Point in 55 patients; CPC: Cerebral Performance Category.
In the “good outcome” group, six patients (13%) were classified in CPC 2, meaning mild-to-moderate disabilities.
3.3. Secondary End Points
Secondary end points are listed in Table 2.

Table 2.
Secondary end points of 55 patients.
All patients were alive at three-month follow-up. Among the three patients who died at one-year follow-up, none died from cardiovascular causes (two died from sepsis and one from stroke). A total of 19 (35%) patients were hospitalised in the year following the SCD. At discharge, n = 6 (10%) patients were classified in CPC 3, n = 13 (21%) were CPC 2, and n = 42 (69%) were CPC 1. Among patients with CPC 2 or 3, n = 7 (37%) recovered during the follow-up: one from CPC 3 to CPC 2 and six from CPC 2 to CPC 1. Cardiac evolution was also favourable with mean LVEF ≥ 50% at 1 year (49.1% at 3 months versus 51.5% at 1 year, p = 0.037)). Only three patients (5%) experienced a recurrence of VT/FV and two (4%) suffered a new ischemic event. The number of patients with NYHA functional class I increased from 28 (51%) at 3 months to 36 (69%) at 1 year. We therefore compared characteristics between the two groups named “good outcome” and “bad outcome” (Table 3).

Table 3.
Univariate analysis.
In the “poor outcome” group, patients were significantly older (69.3 yo versus 57.4 yo, p = 0.036), with lower BMI (22.4 kg/m2 versus 26.7 kg/m2, p = 0.013), and had lower initial LVEF (32.1 ± 5.7 vs. 40.3 ± 12, p = 0.046). Regarding SCD characteristics, there was no difference between the two groups on no-flow or low-flow times and location of initial cardiopulmonary resuscitation (CPR).
3.4. Quality of Life
Among the 48 patients contacted to complete the QOLIBRI questionnaire, 36 (75%) returned the mail or accepted to answer by phone call. Results are represented in Table 4. Scores in “satisfaction items” were quite homogeneous with 23.25 ± 6.34 for cognition, 22.14 ± 5 for self, 23.53 ± 7.2 for daily life activities, and 19.56 ± 3.7 for social relationships. In “bothered items”, scores were also similar with 12 ± 4.6 for emotion and 10.9 ± 4 for physical problems.

Table 4.
Mean score for each item of QOLIBRI.
If we group the answers according to the scale of the questionnaire (from “not at all” to “very”), n = 18, 50% of patients were globally satisfied with their quality of life, and n = 20, 56% of patients did not report major health problems (Appendix A).
4. Discussion
Our study aimed to evaluate the survival rate and the neurological status of patients discharged alive from hospital after an aborted SCD referred for emergency CA with three main findings: (1) patients discharged alive were relatively young, with few cardiovascular risk factors and with an initial shockable rhythm (97%) mainly related to an ischemic aetiology (75%); (2) the survival rate without neurological sequelae at 1 year was high (87%); (3) younger age, lower BMI, and initial better LVEF were associated with survival with good neurological prognosis. For survivors, neurologic and psychological outcome were the main issues with a moderate impairment of quality of life at one-year follow-up.
Acute ischemic aetiology (with or without coronary occlusion) was identified in 75% of patients, and PCI was performed in most of them. We evaluated the outcome of successfully resuscitated patients who were discharged alive. The survival rate without neurological sequelae at 1 year was 87%, which is very encouraging. We selected patients admitted directly to the cathlab with suspicion of ACS and with hemodynamic success of CPR. According to guidelines, only patients with electrocardiographic criteria in favour of ischemic aetiology on the post-resuscitation electrocardiogram (ECG) may have emergency CA in our centres. As we also excluded patients who died during in-hospital stay (57% of patients), our population was highly selective and was relatively young, with normal BMI and a few cardiovascular risk factors. Moreover, time of no and low flow was relatively short, with the presence of a witness in near half of cases, explaining a sub-normal pH on admission. As we expected, almost all of our patients initially presented a shockable rhythm at the time of the SCD. The cardiac aetiology (ischemic and non-ischemic cardiopathy) of the SCD was predominant in our population and an initial shockable rhythm, observed in 93% of our study population, is a well-known strong factor of good prognosis after a cardiac arrest [19,20,21]. Therefore, these results are not generalisable to all patients, especially those with an extra-cardiac cause of SCD.
At 1 year, 87% of our patients were alive without neurological sequelae, which is consistent with previous studies [22,23,24]. In the COACT trial including the population without ST-segment elevation, 64.5% of patients in the immediate CA group were alive at 90 days [25]. An Israelian study found an 85% survival rate at 1 year among patients discharged alive from the intensive cardiology unit [26]. However, beyond mortality, possible anoxic brain injury, mental trauma from surviving a near-death experience, or a new or ongoing cardiac condition can make recovery after SCD difficult. Short-term studies suggest that these complications can lead to an increased physical and psychological burden for both survivors and their relatives [27,28,29]. The neurologic status of patients discharged with severe sequelae did not improve at 1 year (only one patient switched from CPC 3 to CPC 2). On the contrary, half of the patients with “mild-to-moderate” neurologic disabilities (CPC 2) completely recovered at 1 year (CPC 1), also showing progresses in neuro-cognitive care. On the other hand, despite the absence of neurological disorders, quality of life can be somewhat impaired in survivors of SCD. Most patients reported limitations in cognition, self-behaviour, and daily life activities explained by minor physical problems while others could also experience depression or persistent anxiety due to a post-traumatic state [11]. Rehabilitation has therefore been recommended to improve secondary physical and psychological consequences of SCD, but cooperation between cardiological and neurological rehabilitation teams is needed in case of cognitive consequences [30,31]. Despite many improvements in management, more knowledge is needed regarding expectations of OHCA survivors and their relatives. With a similar protocol in a much larger population and with more self-report outcome measurements, the Danish Cardiac Arrest Survivorship (DANCAS survey) will begin. Results will be used to identify the most prevalent problems suffered by OHCA survivors and their families and those at most risk of suffering them [32].
Characteristics of the two groups (good and poor outcome) were similar, except for age, body mass index (BMI), and initial LVEF. Surprisingly, overweight patients had a better neurologic outcome than normal weight patients. Found in previous studies, this phenomenon has been questioned [33,34]. Several explanations of this apparent paradox have been proposed including that higher BMI may allow for the use of higher doses of cardioprotective medications, such as β-adrenergic blockers, particularly in CAD patients, and conversely, lower BMI is traditionally associated with the increase in bleeding events with antithrombotic therapy required after ACS [35,36]. In a recent prospective trial, however, neurological status was assessed in 605 patients resuscitated from SCD. In this cohort, BMI was not associated with good neurologic and survival outcome at discharge [37].
No death during follow-up was due to cardiovascular cause and LVEF was near normal at 1 year, showing the effectiveness of actual management of ischemic heart disease and the beneficial effect of coronary revascularisation. After ACS, cardiac rehabilitation is known to decrease the mortality related to cardiac disease by 20% with the improvement of heart and lung function, socio-psychological status, and the quality of life. In addition, it delays the progression of atherosclerosis and decreases its severity, focusing on education aimed at a healthy lifestyle and improvement of exercise capacity [38,39]. This rehabilitation is safe and well-tolerated in patients with severe comorbidities, such as after aborted SCD [40]. Therefore, for patients without any neurologic sequelae at discharge, apparition of a new cardiac event or recurrence of initial arrhythmia seems not to be the main issue. In a recent study, Kubota et al. showed that the post-discharge mortality of ACS (STEMI or NSTEMI) patients with OHCA was comparable to that of patients without OHCA [41].
The first limitation of our study, even if we collected all patients discharged alive during the inclusion period, remains the small population evaluated. Multivariate analysis was not achievable due to the small number of patients in each subgroup and low incidence of events in the “poor outcome” group. Results of univariate analysis are thus to be taken with caution as we could not adjust with other parameters. Second, the CPC scale is a validated tool in the assessment of neurological status after SCD but it may not be as discriminant as other neurological tools. A CPC score of one or two is commonly regarded as a ‘good outcome’ but it includes subjects with ‘mild-to-moderate’ cognitive impairments, such as dysphasia and permanent memory or mental changes. Furthermore, the CPC seems insensitive to more subtle cognitive impairments. Therefore, the use of a more precise neuropsychological test would be more effective to obtain a precise outcome after SCD [42]. Finally, to assess quality of life, we used the QOLIBRI questionnaire which was initially validated in patients after a traumatic brain injury. Because this work focused on the consequences of neurological sequelae occurring after OHCA, we chose this questionnaire rather than the SF-36 to be more specific. Like others, the accuracy of the statements is uncertain. Moreover, patients’ answers can be modified by many events unrelated to the cardiac arrest. Here, the COVID-19 pandemic may have affected our results with perturbation of daily activities and social relationships, impacting the psychological state, whereas quality of life was good in our population.
5. Conclusions
Patients admitted directly to the cathlab after aborted SCD mainly related to ACS had a survival rate of 43% at discharge. For those patients, survival rate without neurological sequelae was excellent at 1 year with 87% of them alive without significant neurological sequelae but with persistent psychological impact in 44% of survivors. These results encourage us to further improve our practices, follow-up methods, and cardiac or multidisciplinary rehabilitation programs.
Author Contributions
Conceptualization, F.L., M.A. and Q.D.; methodology, F.L., M.A. and Q.D.; software, F.L., M.A. and Q.D.; validation, F.L., M.A. and Q.D.; formal analysis, F.L., M.A. and Q.D.; investigation, F.L., M.A., Q.D., G.C., B.L. and F.R.; resources, F.L., M.A. and Q.D.; data curation, Q.D.; writing—original draft preparation, Q.D. and F.L.; writing—review and editing, F.L., M.A. and Q.D.; visualization, F.L., M.A. and Q.D.; supervision, F.L., M.A., Q.D., G.C., B.L. and F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Montpellier University Hospital (IRB-MTP_2020_05_202000478, 28 May 2020).
Informed Consent Statement
Informed consent was obtained verbally from all subjects involved in the study.
Data Availability Statement
Datas are available in department of cardiology, University of Montpellier, France.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Patients answer of QOLIBRI.
Table A1.
Patients answer of QOLIBRI.
| Patient Answer (n = 36) | Satisfaction | Health Problems | ||||||
|---|---|---|---|---|---|---|---|---|
| Cognition | Self | Daily Life | Social Relationships | Total | Emotion | Physical Problems | Total | |
| “Not at all” no. (%) | 3 (8.3) | 0 (0) | 4 (11.1) | 1 (2.8) | 2 (5.6) | 18 (50) | 18 (50) | 20 (55.6) |
| “Slightly” no. (%) | 7 (19.4) | 15 (41.7) | 9 (25) | 8 (22.2) | 10 (27.8) | 5 (13.9) | 9 (25) | 5 (13.9) |
| “Quite” no. (%) | 16 (44.4) | 16 (44.4) | 5 (13.9) | 22 (61.1) | 18 (50) | 11 (30.6) | 9 (25) | 11 (30.6) |
| “Very” no. (%) | 10 (27.8) | 5 (13.9) | 18 (50) | 5 (13.9) | 6 (16.7) | 2 (5.6) | 0 (0) | 0 (0) |
References
- Luc, G.; Baert, V.; Escutnaire, J.; Genin, M.; Vilhelm, C.; Di Pompéo, C.; El Khoury, C.; Segal, N.; Wiel, E.; Adnet, F.; et al. Epidemiology of out-of-hospital cardiac arrest: A French national incidence and mid-term survival rate study. Anaesth. Crit. Care Pain Med. 2019, 38, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Reinier, K.; Teodorescu, C.; Evanado, A.; Kehr, E.; Al Samara, M.; Mariani, R.; Gunson, K.; Jui, J. Epidemiology of Sudden Cardiac Death: Clinical and Research Implications. Prog. Cardiovasc. Dis. 2008, 51, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Kuriachan, V.P.; Sumner, G.L.; Mitchell, L.B. Sudden Cardiac Death. Curr. Probl. Cardiol. 2015, 40, 133–200. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.M.; Joly, L.-M.; Rosenberg, A.; Monchi, M.; Weber, S.N.; Dhainaut, J.-F.A.; Carli, P. Immediate Coronary Angiography in Survivors of Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 1997, 336, 1629–1633. [Google Scholar] [CrossRef]
- Leclercq, F.; Lonjon, C.; Marin, G.; Akodad, M.; Roubille, F.; Macia, J.-C.; Cornillet, L.; Gervasoni, R.; Schmutz, L.; Ledermann, B.; et al. Post resuscitation electrocardiogram for coronary angiography indication after out-of-hospital cardiac arrest. Int. J. Cardiol. 2020, 310, 73–79. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Moulaert, V.R.; Verbunt, J.A.; van Heugten, C.M.; Wade, D.T. Cognitive impairments in survivors of out-of-hospital cardiac arrest: A systematic review. Resuscitation 2009, 80, 297–305. [Google Scholar] [CrossRef]
- Sasson, C.; Rogers, M.A.; Dahl, J.; Kellermann, A.L. Predictors of Survival From Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 63–81. [Google Scholar] [CrossRef]
- Patel, N.; Patel, N.J.; Macon, C.J.; Thakkar, B.; Desai, M.; Rengifo-Moreno, P.; Alfonso, C.E.; Myerburg, R.J.; Bhatt, D.L.; Cohen, M. Trends and Outcomes of Coronary Angiography and Percutaneous Coronary Intervention After Out-of-Hospital Cardiac Arrest Associated With Ventricular Fibrillation or Pulseless Ventricular Tachycardia. JAMA Cardiol. 2016, 1, 890–899. [Google Scholar] [CrossRef]
- Daubin, C.; Quentin, C.; Allouche, S.; Etard, O.; Gaillard, C.; Seguin, A.; Valette, X.; Parienti, J.-J.; Prevost, F.; Ramakers, M.; et al. Serum neuron-specific enolase as predictor of outcome in comatose cardiac-arrest survivors: A prospective cohort study. BMC Cardiovasc. Disord. 2011, 11, 48. [Google Scholar] [CrossRef]
- Haydon, G.; Van Der Riet, P.; Inder, K. A systematic review and meta-synthesis of the qualitative literature exploring the experiences and quality of life of survivors of a cardiac arrest. Eur. J. Cardiovasc. Nurs. J. Work Group Cardiovasc. Nurs. Eur. Soc. Cardiol. 2017, 16, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Cummins, R.O.; Chamberlain, D.A.; Abramson, N.S.; Allen, M.; Baskett, P.J.; Becker, L.; Bossaert, L.; Delooz, H.H.; Dick, W.F.; Eisenberg, M.S. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: The Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation 1991, 84, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Phelps, R.J.; Rea, T.; Maynard, C.; Dumas, F. Cerebral Performance Category and Long-Term Prognosis in Cardiac Arrest Survivors. Circulation 2011, 124 (Suppl. S21), A179. [Google Scholar] [CrossRef]
- Edgren, E.; Hedstrand, U.; Kelsey, S.; Sutton-Tyrrell, K.; Safar, P. BRCTI Study Group Assessment of neurological prognosis in comatose survivors of cardiac arrest. Lancet 1994, 343, 1055–1059. [Google Scholar] [CrossRef]
- Martinell, L.; Nielsen, N.; Herlitz, J.; Karlsson, T.; Horn, J.; Wise, M.P.; Undén, J.; Rylander, C. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit. Care 2017, 21, 96. [Google Scholar] [CrossRef]
- Von Steinbuechel, N.; Wilson, L.; Gibbons, H.; Hawthorne, G.; Höfer, S.; Schmidt, S.; Bullinger, M.; Maas, A.I.; Neugebauer, E.; Powell, J.; et al. Quality of Life after Brain Injury (QOLIBRI): Scale Validity and Correlates of Quality of Life. J. Neurotrauma 2010, 27, 1157–1165. [Google Scholar] [CrossRef]
- Von Steinbüchel, N.; Wilson, L.; Gibbons, H.; Hawthorne, G.; Hofer, S.; Schmidt, S.; Bullinger, M.; Maas, A.I.; Neugebauer, E.; Powell, J.; et al. Quality of Life after Brain Injury (QOLIBRI): Scale Development and Metric Properties. J. Neurotrauma 2010, 27, 1167–1185. [Google Scholar] [CrossRef]
- Middelkamp, W.; Moulaert, V.R.; Verbunt, J.A.; Van Heugten, C.M.; Bakx, W.G.; Wade, D.T. Life after survival: Long-term daily life functioning and quality of life of patients with hypoxic brain injury as a result of a cardiac arrest. Clin. Rehabil. 2007, 21, 425–431. [Google Scholar] [CrossRef]
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Donnino, M.W.; Granfeldt, A. In-Hospital Cardiac Arrest: A Review. JAMA 2019, 321, 1200–1210. [Google Scholar] [CrossRef]
- Nolan, J.P.; Soar, J.; Smith, G.B.; Gwinnutt, C.; Parrott, F.; Power, S.; Harrison, D.; Nixon, E.; Rowan, K. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation 2014, 85, 987–992. [Google Scholar] [CrossRef]
- Pasupula, D.K.; Bhat, A.G.; Meera, S.J.; Malleshappa, S.K.S. Influence of comorbidity on survival after out-of-hospital cardiac arrest in the United States. Resuscitation 2019, 145, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Bunch, T.J.; White, R.D.; Gersh, B.J.; Meverden, R.A.; Hodge, D.O.; Ballman, K.V.; Hammill, S.C.; Shen, W.-K.; Packer, D.L. Long-Term Outcomes of Out-of-Hospital Cardiac Arrest after Successful Early Defibrillation. N. Engl. J. Med. 2003, 348, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Kalbag, A.; Kotyra, Z.; Richards, M.; Spearpoint, K.; Brett, S. Long-term survival and residual hazard after in-hospital cardiac arrest. Resuscitation 2006, 68, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Pleskot, M.; Hazukova, R.; Parizek, P.; Cermakova, E.; Tacheci, I. A seven-year follow-up of discharged patients after out-of-hospital cardiac arrest with respect to ST-segment elevation myocardial infarction. Signa Vitae J. Intesive Care Emerg. Med. 2012, 7, 33–39. [Google Scholar]
- Lemkes, J.S.; Janssens, G.N.; van der Hoeven, N.W.; Jewbali, L.S.; Dubois, E.A.; Meuwissen, M.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; Bleeker, G.B.; et al. Coronary Angiography after Cardiac Arrest without ST-Segment Elevation. N. Engl. J. Med. 2019, 380, 1397–1407. [Google Scholar] [CrossRef]
- Antonelli, D.; Koren, O.; Nahir, M.; Rozner, E.; Freedberg, N.A.; Turgeman, Y. Long-Term Survival of Discharged Patients Admitted to Intensive Coronary Care Unit after Out-of-Hospital Cardiac Arres. Isr. Med. Assoc. J. 2017, 19, 751–755. [Google Scholar]
- Lilja, G.; Nielsen, N.; Bro-Jeppesen, J.; Dunford, H.; Friberg, H.; Hofgren, C.; Horn, J.; Insorsi, A.; Kjaergaard, J.; Nilsson, F.; et al. Return to Work and Participation in Society After Out-of-Hospital Cardiac Arrest. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e003566. [Google Scholar] [CrossRef]
- Van Wijnen, H.G.; Mc Rasquin, S.; Van Heugten, C.M.; Verbunt, J.A.; Moulaert, V.R. The impact of cardiac arrest on the long-term wellbeing and caregiver burden of family caregivers: A prospective cohort study. Clin. Rehabil. 2017, 31, 1267–1275. [Google Scholar] [CrossRef]
- Lilja, G. Follow-Up of Cardiac Arrest Survivors: Why, How, and When? A Practical Approach. Semin Neurol. 2017, 37, 88–93. [Google Scholar] [CrossRef]
- Schaaf, K.P.W.; Artman, L.K.; Peberdy, M.A.; Walker, W.C.; Ornato, J.P.; Gossip, M.R.; Kreutzer, J.S. Anxiety, depression, and PTSD following cardiac arrest: A systematic review of the literature. Resuscitation 2013, 84, 873–877. [Google Scholar] [CrossRef]
- Boyce, L.W.; Goossens, P.H.; Moulaert, V.R.; Pound, G.; Van Heugten, C.M. Out-of-hospital cardiac arrest survivors need both cardiological and neurological rehabilitation! Curr. Opin. Crit. Care 2019, 25, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.L.; Tang, L.H.; Borregaard, B.; Zinckernagel, L.; Mikkelsen, T.B.; Taylor, R.S.; Christiansen, S.R.; Nielsen, J.F.; Zwisler, A.D. Long-term physical and psychological outcomes after out-of-hospital cardiac arrest—protocol for a national cross-sectional survey of survivors and their relatives (the DANCAS survey). BMJ Open 2021, 11, e045668. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Alhussami, I.; Nanchal, R.; Wunderink, R.G.; Pellis, T.; Wittebole, X.; Martin-Loeches, I.; François, B.; Leone, M.; Vincent, J.-L. Being Overweight Is Associated With Greater Survival in ICU Patients: Results From the Intensive Care Over Nations Audit. Crit. Care Med. 2015, 43, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Bunch, T.J.; White, R.D.; Lopez-Jimenez, F.; Thomas, R. Association of body weight with total mortality and with ICD shocks among survivors of ventricular fibrillation in out-of-hospital cardiac arrest. Resuscitation 2008, 77, 351–355. [Google Scholar] [CrossRef]
- Mak, K.-H.; Bhatt, D.L.; Shao, M.; Haffner, S.M.; Hamm, C.W.; Hankey, G.; Johnston, S.C.; Montalescot, G.; Steg, P.G.; Steinhubl, S.R.; et al. The influence of body mass index on mortality and bleeding among patients with or at high-risk of atherothrombotic disease. Eur. Heart J. 2009, 30, 857–865. [Google Scholar] [CrossRef]
- Matinrazm, S.; Ladejobi, A.; Pasupula, D.K.; Javed, A.; Durrani, A.; Ahmad, S.; Munir, M.B.; Adelstein, E.; Jain, S.K.; Saba, S. Effect of body mass index on survival after sudden cardiac arrest. Clin. Cardiol. 2018, 41, 46–50. [Google Scholar] [CrossRef]
- Lee, H.; Oh, J.; Kang, H.; Lim, T.H.; Ko, B.S.; Choi, H.J.; Park, S.M.; Jo, Y.H.; Lee, J.S.; Park, Y.S.; et al. Association between the body mass index and outcomes of patients resuscitated from out-of-hospital cardiac arrest: A prospective multicentre registry study. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 24. [Google Scholar] [CrossRef]
- Witt, B.J.; Jacobsen, S.; Weston, S.A.; Killian, J.M.; Meverden, R.A.; Allison, T.G.; Reeder, G.S.; Roger, V.L. Cardiac rehabilitation after myocardial infarction in the community. J. Am. Coll. Cardiol. 2004, 44, 988–996. [Google Scholar] [CrossRef]
- González-Salvado, V.; Rodríguez-Núñez, A.; González-Juanatey, J.R. From Prevention to Rehabilitation: Toward a Comprehensive Approach to Tackling Cardiac Arrest. Rev. Espanola Cardiol. 2019, 72, 3–6. [Google Scholar] [CrossRef]
- Kim, C.; Jung, H.; Choi, H.E.; Kang, S.H. Cardiac Rehabilitation After Acute Myocardial Infarction Resuscitated From Cardiac Arrest. Ann. Rehabil. Med. 2014, 38, 799–804. [Google Scholar] [CrossRef][Green Version]
- Kubota, T.; Komukai, K.; Miyanaga, S.; Shirasaki, K.; Oki, Y.; Yoshida, R.; Fukushima, K.; Kamba, T.; Okuyama, T.; Maehara, T.; et al. Out-of-Hospital Cardiac Arrest Does Not Affect Post-Discharge Survival in Patients With Acute Myocardial Infarction. Circ. Rep. 2021, 3, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Moulaert, V.R.; Verbunt, J.A.; Van Heugten, C.M.; Bakx, W.G.; Gorgels, A.P.; Bekkers, S.C.; De Krom, M.C.; Wade, D.T. Activity and Life After Survival of a Cardiac Arrest (ALASCA) and the effectiveness of an early intervention service: Design of a randomised controlled trial. BMC Cardiovasc. Disord. 2007, 7, 26. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).