Abstract
Electromagnetic fields are emerging as a therapeutic option for patients with spasticity. They have been applied at brain or peripheral level. The effects of electromagnetic fields applied to the brain have been extensively studied for years in spasticity, but not so at the peripheral level. Therefore, the purpose of our work is to analyze the effects of electromagnetic fields, applied peripherally to spasticity. A systematic review was conducted resulting in 10 clinical trials. The frequency ranged from 1 Hz to 150 Hz, with 25 Hz being the most commonly used and the intensity it was gradually increased but there was low homogeneity in how it was increased. Positive results on spasticity were found in 80% of the studies: improvements in stretch reflex threshold, self questionnaire about difficulties related to spasticity, clinical spasticity score, performance scale, Ashworth scale, spastic tone, Hmax/Mmax Ratio and active and passive dorsal flexion. However, results must be taken with caution due to the large heterogeneity and the small number of articles. In future studies, it would be interesting to agree on the parameters to be used, as well as the way of assessing spasticity, to be more objective in the study of their effectiveness.
1. Introduction
The Spasticity is described as a speed-dependent increase in muscle tone and repetitive, uncontrolled involuntary contractions of skeletal muscles [1] and it arises from upper motor neuron lesions due to a lesion in the pyramidal tracts [2].
The most common symptoms of spasticity are: increased muscle tone, pain and decreased functional abilities with severe consequences are in lessen joint mobility and diminished muscle flexibility [3].
This sensory-motor disorder is observed in patients of all ages [4] affecting about 85% of patients with multiple sclerosis, 65–78% with spinal cord injury and 30% with stroke [5], among other neurological pathologies [6], such as cerebral palsy [7].
Electromagnetic fields are emerging as a therapeutic option for these patients [8]. This therapy can produce electromagnetic biological effects such as regenerative effects on the peripheral nervous system [9] with the possibility of penetrating deep into the tissues [10]. Moreover, it a safe and painless tool that may help in restoring motor control through activation of sensory proprioceptive fibers [11].
They have been applied at brain or peripheral level, using different frequencies and amplitudes. The following can be applied: Repetitive peripheral magnetic stimulation (RPMS), Pulsed electromagnetic field therapy (PEMF) and Transcranial magnetic stimulation (TMS).
RPMS is a system that produces eddy currents through electromagnetic induction activating peripheral nerves and muscles without stimulating skin nociceptors [12]. These electromagnetic fields target neuromuscular tissue and induce electrical currents that depolarize neurons and cause concentric muscle contractions. They have a deep penetration with an anti-spastic effect. As well, electromagnetic field increases blood perfusion of the exposed region, leading to circulatory and trophic improvement [13].
On the other hand, PEMF uses electromagnetic fields, creating small electric fields in the tissues, with a pulsing effect to produce athermal effects that promote tissue healing, relieve pain and inflammation [14,15].
And finally, TMS is a neurostimulation and neuromodulation technique that has provided over two decades of data in focal and non-invasive brain stimulation based on the principles of electromagnetic induction with minimal risk and excellent tolerability [16].
The effects of electromagnetic fields applied to the brain using TMS have been extensively studied for years in spasticity [17,18,19,20,21,22], but not so at the peripheral level, where most research has focused on the effect of electromagnetic fields on bone regeneration [23,24,25,26].
Therefore, the purpose of our work is to analyze the effects of electromagnetic fields, applied peripherally, on spasticity.
2. Materials and Methods
2.1. Search Strategy
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [27] guidelines were followed to perform this systematic review (Appendix A). The search protocol was registered in the PROSPERO database of prospectively registered systematic reviews (CRD 42022301773). The literature search was performed between December 2021 to February 2022 in the following electronic databases: Web of Science (WoS), Scopus, PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials, SciELO, Physiotherapy Evidence Database (PEDro), LILACs and ScienceDirect. Medical Subjects Headings (MeSH) descriptors and other keywords combined with Boolean operators were used. The terms were: “spasticity”, “muscle spasticity”, “electromagnetic stimulation”, “electromagnetic therapy”, “pulsed electromagnetics”, “electromagnetics fields” and “magnetic field therapies”.
The search was filtered to full-text clinical trials papers. No date and language filters were applied. Table 1 shows the different search combinations.

Table 1.
Search combinations.
The PICOS (Population, Intervention, Comparison, Outcomes and Study design) model [28] was used to establish the inclusion criteria: (I) Population: humans with spasticity; (II) Intervention: Treatment with electromagnetic fields administered to the lower or upper limbs or spine; (III) Comparison: placebo, no treatment, a different electrotherapy modality or any other intervention; (IV) Outcomes: related to spasticity; (V) Study design: controlled clinical trials. Articles where participants were people with spasticity, but the outcome data were not provided or those where transcranial therapy was used, were excluded.
2.2. Study Selection Process and Data Extraction
First, a search was carried out by combining the keywords in the different databases. Duplicate articles were then removed using the Rayyan tool (https://www.rayyan.ai/, accessed on 27 February 2022). Subsequently, studies were selected or excluded. Two reviewers (M.J.V.-G. and G.G.-M.) carried out the process of study selection, review and systematic data extraction. A third reviewer (R.M.-V.) was involved in reaching consensus in case of controversy.
The following information was extracted from each article included in the review: authors, type of intervention, disease or pathology causing spasticity, number of subjects, frequency of sessions per week, time of each session, total duration of the intervention, outcome measures, measurement instrument, device used for the application of magnetic fields, parameters used and results obtained.
2.3. Risk of Bias and Assessment of the Methodological Quality of the Included Studies
The risk of bias was calculated for each selected study using the Cochrane Collaboration tool [29]. The following types of bias were assessed: selection bias, performance bias, detection bias, attrition bias, reporting bias and other biases.
In order to assess the quality of the articles used for the systematic review, the PEDro scale [30] was used. This scale consists of 10 items: randomization, concealed allocation, comparability at baseline, blinding of subjects, blinding of therapists, blinding of assessors, more than 85% follow-up for at least one key outcome, intention-to-treat analysis, statistical comparison between groups, and point and variability measures for at least one key outcome. Items are scored as yes (1) or no (0), and the maximum score is 10 points. An additional criterion (item 1: selection criteria) that relates to external validity (applicability of the test) is included to complete the Delphi list, but this criterion is not used for the calculation of the scale score [31]. Taking into account the established criteria, a study with a PEDro score of 6 or higher is considered as evidence level 1 (6–8: good; 9–10: excellent), and a study with a score of 5 or lower is considered as evidence level 2 (4–5: acceptable; <4: poor).
3. Results
3.1. Selection of Studies
Once the database searches were completed, by combining the different key words, a total of 521 documents were obtained, of which 10 studies were finally included in the systematic review [32,33,34,35,36,37,38,39,40,41]. Figure 1 shows the flow chart of the search process.
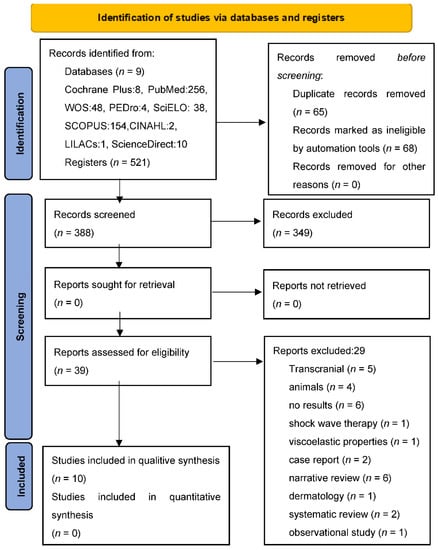
Figure 1.
PRISMA 2020 flow diagram. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: n 71. doi: 10.1136/bmj.n71.
A meta-analysis was attempted with the EPIDAT program of the four studies [32,33,40,41] that provided numerical data for its performance; however, given the methodological, clinical and statistical heterogeneity, it was not possible.
3.2. Data Extraction
3.2.1. Characteristics of the Subjects
There were a total of 460 participants with an age ranging from 32 [38] to 76 years [40], out of which 40% were men.
The largest sample studied was that of Lappin et al. [34] with a total of 117 subjects and the smallest was that of Serag et al. [37] with 26. In 70% of the studies the sample was between 26 and 38 years of age [32,33,35,36,37,39,40] and 63% were women.
Regarding the pathology that had originated the spasticity, 50% of the studies were multiple sclerosis [32,33,34,35,37], although in the study by Krause et al. other spinal diseases were also included [35] and the other 50% of the trials dealt with stroke [36,38,39,40,41], although in the article by Krewer, subjects with traumatic brain injury were also included [38].
3.2.2. Main Characteristics of the Studies
Table 2 shows the main characteristics of the interventions performed in the different studies that make up the present review. We can highlight that 80% of the trials used sham stimulation in the control group [32,33,34,35,36,37,38,39] and in the other 20% in the intervention group RPMS was applied in the antagonists and agonists and in the intervention group only in the antagonists. In two of the trials there was a control group with healthy individuals [35,39]. Concerning the way the electromagnetic field was falsely applied, sometimes the device was used switched on but without applying any intensity [32,33,34,35,37,38,39] or it was applied without intensity and in another location [36].

Table 2.
Principal studies characteristics.
In 3 articles [36,38,40], both the intervention and control groups also had a complementary physiotherapy program.
In 3 of the 10 trials the electromagnetic fields were administered in the form of PEMP [33,34,36] and in the other 7 the therapy was RPMS [32,35,37,38,39,40,41].
There was much heterogeneity in terms of the device used, in two of the articles it was portable [33,34]. The oldest trial (1997) used an oil-cooled coil [32] and the most recent [40,41] (2018 and 2022) used an inductive system. There were articles in which the brand of the device was mentioned: Enermed [33,34], Magstim Rapid [35,39], BTL-6000 [40], Dantec-Maglite [37], P-Stim 160 b [38].
Some were placed on the spine [32,35,36,37] and others on the upper limbs [38,39,40,41]. In one trial, the device was placed on an empirically determined acupuncture point on the spine, shoulder or hip [33].
Some articles specified that they were placed directly on the skin [33,34] and in other cases, there was no contact [35,37,40].
In terms of frequency, the most commonly used frequency was 25 Hz [32,34,38]. In addition, 1 Hz [37], 4–13 Hz [33], 20 Hz [35], 50 Hz [39] and 25–150 Hz [40] were used.
The time of application ranged from 8 min [39,40] to 24 h a day [33,34], although 9 min [41], 20 min [36,38] or 25 min were also applied [32].
The intensity was gradually increased up to 0.7 Tesla [32], increased by around 20% for the stimulation series [35], was increased or decreased by patient’s tolerance [40] or was set at 10% above the level that evoked a movement [38].
The sessions were performed once a day but sometimes twice a day [32,38] or during 24 h [33] and the total number ranged from 1 session [39] to 10 sessions [40]. Concerning the treatment time it was very variable: 1 day [39], 7 days [32], 10 days [40], 14 days [37,38], 56 days [33] or 70 days [34].
As for how to assess spasticity, the Asworth scale [32,35,37,40,41] was most commonly used, followed by electromyographic parameters [32,36,39]. Among them, the Hmax/Mmax ratio [36] was recorded. The Watenberg’s pendulum test [35] and other scales such as the Modified Tardie Scale [38] were also used.
There were also self-reported questionnaires on difficulties encountered in activities of daily live [32] or on performance [33] due to spasticity and a self-reported spasm frequency [37]. The Barthel Index [40,41] and other more specific multiple sclerosis scales such as the Expanded Disability Status Scale (EDSS) [33] and the Multiple Sclerosis Quality of Life Inventory (MSQLI) [34] were also used for the same purpose.
Other variables measured were upper limb functionality measured by the Fugl-Meyer Assessment [38] and ankle functionality through passive and active range of motion measured with a goniometer, sometric muscle strength and resistance of plantar flexors to stretch with a dynamometer [39] or walking speed through the 25 feet walking test [37].
In addition, a quantitative electroencephalografic during a language task [33] and a TMS were used to see the ipsilateral cortical motor representation [39].
Regarding the results obtained in terms of spasticity, in 80% of the studies were positive. Improvements were found in stretch reflex threshold [32,35,39], self questionnaire about difficulties related to spasticity [32], clinical spasticity score [32], performance scale [33], Ashworth scale [32,35,37,40,41], spastic tone [35], Hmax/Mmax Ratio [36] and active and passive dorsal flexion [39].
Only 2 articles found mixed results [34] or a limited effect [38] for spasticity.
Regarding other measured variables, there was improvement in Barthel index [40,41], fatigue [33,34], quality of life [34] and sensory function [38] but not gait speed [37].
3.3. Methodological Quality Assessment
80% of the studies were of good quality [32,33,34,36,37,38,39,41] and the other 20% [35,40] were of acceptable quality. Table 3 shows the score for each study.

Table 3.
Methodological quality assessment (PEDro Scale).
3.4. Risk of Bias Assessment
The results of the risk of bias can be observed in Figure 2. It should be noted that the risk of bias is low in relation with selection bias referring to random sequence generation as in only one article it was not fulfilled [35] and another had uncertain risk [33]. Only one article specified allocation concealment [37]. 80% of the articles had a low risk of bias in relation with performance bias [32,33,34,35,36,37,38,39]. With respect to reporting bias all of them were unclear risk because none of the articles specified whether they had registered the clinical trial in a database before (Figure 3).
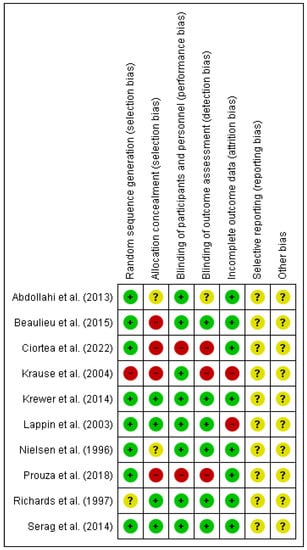
Figure 2.
Risk of bias summary [32,33,34,35,36,37,38,39,40,41].
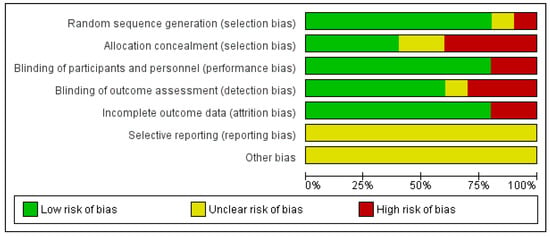
Figure 3.
Risk of bias graph.
4. Discussion
A systematic review has been carried out to synthesize the scientific evidence regarding the use of electromagnetic fields, applied peripherally, in the treatment of the spasticity.
With regard to the characteristics of the sample, it was homogeneous in terms of the pathologies studied, as half were in sclerosis and the other half in stroke. It should be borne in mind that, although the incidence and prevalence figures for spasticity vary [42], multiple sclerosis and stroke are two of the most prevalent pathologies, with an estimated 80% [43] and 42.6% [44], respectively.
It would be advisable to study the effect of magnetic fields in other diseases where prevalence is also high, such as spinal cord injuries where it is estimated that between 40 and 78% have spasticity [45] or in cerebral palsy where the percentage is even higher (72–91%) [46].
Studies have been carried out on rats with induced spinal cord injuries with good results demonstrating the viability and efficacy of this therapeutic strategy for spasticity; however their results have not yet been demonstrated in humans [47].
Regarding the type of electromagnetic fields used in the treatment of spasticity, the most investigated therapy has been transcranial [17,18,19,20,21,22]. In our extensive literature search on peripherally delivered electromagnetic fields, only three of the articles [33,34,36] studied the efficacy of PEMF in spasticity. This type of therapy has been most studied in osteogenesis stimulation [48] and in muscleskeleton disorders such as osteoarthritis [49], fibromyalgia [50], rotator cuff tendinitis [51] and lateral epycondilitis [52].In neurology its use has focused on diabetic peripheral neuropathy [53]. The rationale for this therapy is that it has a stimulating effect on biological processes [54].
The other 7 articles [32,35,37,38,39,40,41] used RPMS. This system activates peripheral nerves and muscles without stimulating skin nociceptors while limiting pain [12].
When compared to other types of electrotherapy that have been shown to be effective in improving spasticity such as neuromuscular electrical stimulation NMES [55,56], RPMS-induced pain is significantly less than NMES-induced pain, even when using the same stimulation intensity [57]. Therefore, RPMS simultaneously provides stronger stimulation than NMES and limits pain [58]. A review of non-pharmacological interventions used for the treatment of spasticity in people with multiple sclerosis, there was no evidence for the use of NMES. Furthermore, its depth of stimulation is very shallow and it has some adverse effects including skin burns, dermatitis and pain [59]. However the authors found that magnetic stimulation and electromagnetic therapies were beneficial although with a ‘low level’ of evidence [60].
Therefore, an important aspect of this type of therapy is that there are no known adverse effects; only one of the articles mentions [32] that magnetic stimulation evoked contraction of the mid-thoracic paravertebral and intercostal muscles, causing a sensation of tension around the chest but without cardiac involvement, recommending for future studies a more careful placement of the magnetic coil.
With respect to the number and duration of sessions, device used, doses, intensity and place of therapy administration, there is no clear pattern. All these parameters have been inhomogeneous.
Concerning the measuring instruments, they were very heterogeneous, but it must be taken into account that the term “spasticity” is multifactorial, which makes it difficult to evaluate, and there are different measurement methods that can be divided into non-instrumental and instrumental methods, based on neurophysiological studies of spinal reflexes [61]. In the articles of our review, the most commonly used method was the modified Ashowrth scale [32,35,37,40,41], which is in agreement with the scientific literature, as it does not require any tools and is easy to apply [42], but it is not sensitive enough for measuring the characteristics that distinguish spasticity from other tone alterations [62]. The Tardie scale is considered a better option as it compares muscle reaction to passive movement at different speeds [63], although it was only used in one of the articles in our review to measure spasticity in stroke [38]; however it is most reliable in cerebral palsy [64]. Other indirect clinical assessment methods would be those aimed at measuring the impact of spasticity on the individual. The articles in our review used the Fugl-Meyer scale to measure limb functionality [38], the Barthel Index [40,41] to assess functionality in daily living, and gait scales such as the 25 feet walking test [33] to assess walking speed. There were also more specific multiple sclerosis questionnaires such as the Expanded Disability Status Scale (EDSS) [33] and the Multiple Sclerosis Quality of Life Inventory (MSQLI) [34].
As for quantitative or instrumental methods, the most accurate would be electromyography and the pendulum test [42]. In our study, only two studies used these more objective methods, in one of the articles the pendulum test [36] and the other the Hmax/Mmax ratio [35]. Given the wide heterogeneity in the measurement of spasticity, it would be advisable to be more specific in its measurement, using more objective instrumental methods that could be supported by more specific, reliable and validated non-instrumental methods according to the pathology studied. Gómez-Soriano et al. recommended a combination of the different assessment tools such as the scales, neurophysiological measures, biomechanical methods to know the degree of spasticity present in the patient [6].
Regarding the results, in most of the articles there is an improvement of spasticity, in agreement with what has been found in the scientific literature where there have been trials in rats [47], observational studies [65] or clinical case studies [66,67,68]. What is not clear are the mechanisms of action and the maintenance time of the antispasticity effect. In relation to the mechanisms of action, it would be useful to acquire more knowledge in order to be able to be more specific in the treatment [48]. According to the maintenance time of the anti-spasticity effect, it is stated that it did not outlast 24 h; however, in the articles reviewed in this study, this effect lasted longer, up to 30 days [41].
The present study has several strengths, including the broad and easily reproducible search strategy applied to nine major medical databases. In addition, studies have a good or acceptable quality. It should be noted that in physiotherapy studies it is difficult to blind subjects but with this type of therapy this has been achieved in most studies.
However, some limitations need to be addressed before drawing conclusions from the results of the present analysis. Despite the extensive literature search was carried out, with no date limit, only a few articles were found. The first one in 1996 and the last one in 2021. The scientific evidence of electromagnetic fields on spasticity has been written about for more than two decades, and there are few randomized clinical trials.
Another limitation was related to the great heterogeneity among the different studies. It was so extensive that a meta-analysis could not be performed. There was little uniformity in device used, time of application, duration, frequency, intensity and how and where it was applied. Furthermore, different pathologies and types of electromagnetic fields applied in the periphery were analyzed. In addition, the measurement instruments were not very objective and very heterogeneous.
Large, multi-center, double-blind, controlled studies are needed to draw conclusions for the therapeutic management of spastic patients, as well as comparative studies of treatment protocols with standardized methodology should be carried out.
5. Conclusions
Based on the studies included in this review, it appears that the peripheral application of electromagnetic fields is beneficial in spasticity. Improvements have been found in stretch reflex threshold, self-questionnaire about difficulties related to spasticity, clinical spasticity score, performance scale, Ashworth scale, spastic tone, Hmax/Mmax Ratio and active and passive dorsal flexion. However, results must be taken with caution due the small number of articles and to the large heterogeneity in terms of the device used, application site, treatment time, intensity, number of sessions and duration of therapy. The most commonly used form of application was RPMS and the frequency was 25 Hz. In future studies, it would be interesting to define and agree on the parameters to be used, as well as the way of assessing spasticity, in order to be able to make a more objective comparison of its efficacy compared to other therapeutic alternatives.
Author Contributions
Conceptualization, M.J.V.-G., M.R.-H. and R.M.-V.; methodology, M.J.V.-G., R.M.-V. and G.G.-M.; software, R.M.-V.; validation, F.J.M.-V.; formal analysis, M.J.V.-G., G.G.-M. and R.M.-V.; investigation, F.J.M.-V. and M.R.-H.; resources, C.G.-M.; data curation, C.G.-M.; writing—original draft preparation, M.J.V.-G.; writing—review and editing, M.R.-H. and M.J.V.-G.; visualization, M.J.V.-G. and C.G.-M.; supervision, M.J.V.-G. and R.M.-V.; project administration, M.J.V.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
PRISMA 2020 Checklist.
Table A1.
PRISMA 2020 Checklist.
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Pag. 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Pag. 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Pag. 1–2 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Pag. 2 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 2–3 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 3 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | 3 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 4 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 4 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 4 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | ||
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | 4 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 4 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | ||
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | ||
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | ||
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | ||
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | ||
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | 4 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | ||
| Study characteristics | 17 | Cite each included study and present its characteristics. | Table 2 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Figure 2 and Figure 3 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Table 2 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | Table 3, Figure 2 and Figure 3 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was carried out, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | ||
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | ||
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | ||
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | Figure 2 and Figure 3. Pag. 15 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 14 |
| 23b | Discuss any limitations of the evidence included in the review. | 16 | |
| 23c | Discuss any limitations of the review processes used. | 16 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 16 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Pag. 2 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | ||
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | ||
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | |
| Competing interests | 26 | Declare any competing interests of review authors. | Pag. 1 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | |
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372, n71. doi: 10.1136/bmj.n71.
References
- Chang, E.; Ghosh, N.; Yanni, D.; Lee, S.; Alexandru, D.; Mozaffar, T. A Review of Spasticity Treatments: Pharmacological and Interventional Approaches. Crit. Rev. Phys. Rehabil. Med. 2013, 25, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ozcakir, S.; Sivrioglu, K. Botulinum Toxin in Poststroke Spasticity. Clin. Med. Res. 2007, 5, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Taricco, M.; Adone, R.; Pagliacci, C.; Telaro, E. Pharmacological Interventions for Spasticity Following Spinal Cord Injury. Cochrane Database Syst. Rev. 2000, 2, CD001131. [Google Scholar] [CrossRef] [PubMed]
- Awaad, Y.; Rizk, T.; Siddiqui, I.; Roosen, N.; Mcintosh, K.; Waines, G.M. Complications of Intrathecal Baclofen Pump: Prevention and Cure. ISRN Neurol. 2012, 2012, 575168. [Google Scholar] [CrossRef]
- Thibaut, A.; Chatelle, C.; Ziegler, E.; Bruno, M.A.; Laureys, S.; Gosseries, O. Spasticity after Stroke: Physiology, Assessment and Treatment. Brain Inj. 2013, 27, 1093–1105. [Google Scholar] [CrossRef]
- Gómez-Soriano, J.; Cano-de la Cuerda, R.; Munóz-Hellin, E.; Ortiz-Gutierrez, R.; Taylor, J.S. Valoración y Cuantificación de La Espasticidad: Revisión de Los Métodos Clínicos, Biomecánicos y Neurofisiológicos. Rev. Neurol. 2012, 55, 217–226. [Google Scholar] [CrossRef]
- Shamsoddini, A.; Amirsalari, S.; Hollisaz, M.T.; Rahimniya, A.; Khatibi-Aghda, A. Management of Spasticity in Children with Cerebral Palsy. Iran. J. Pediatr. 2014, 24, 345–351. [Google Scholar] [CrossRef]
- Oluigbo, C.O.; Rezai, A.R. Addressing Neurological Disorders with Neuromodulation. IEEE Trans. Biomed. Eng. 2011, 58, 1907–1917. [Google Scholar] [CrossRef]
- Huang, P.; Xu, L.; Xie, Y. Biomedical Applications of Electromagnetic Detection: A Brief Review. Biosensors 2021, 11, 225. [Google Scholar] [CrossRef]
- Sakai, K.; Yasufuku, Y.; Kamo, T.; Ota, E.; Momosaki, R. Repetitive Peripheral Magnetic Stimulation for Patients after Stroke. Stroke 2020, 51, E105–E106. [Google Scholar] [CrossRef]
- Struppler, A.; Binkofski, F.; Angerer, B.; Bernhardt, M.; Spiegel, S.; Drzezga, A.; Bartenstein, P. A Fronto-Parietal Network Is Mediating Improvement of Motor Function Related to Repetitive Peripheral Magnetic Stimulation: A PET-H2O15 Study. Neuroimage 2007, 36, T174–T186. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.T. An Introduction to the Basic Principles of Magnetic Nerve Stimulation. J. Clin. Neurophysiol. 1991, 8, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, D.; Kazalakova, K. Repetitive Peripheral Magnetic Stimulation as Pain Management Solution in Musculoskeletal and Neurological Disorders—A Pilot Study. Int. J. Physiother. 2016, 3, 671–675. [Google Scholar] [CrossRef]
- Özgüçlü, E.; Çetin, A.; Çetin, M.; Calp, E. Additional Effect of Pulsed Electromagnetic Field Therapy on Knee Osteoarthritis Treatment: A Randomized, Placebo-Controlled Study. Clin. Rheumatol. 2010, 29, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Rioja-Toro, J.; Estévez-Poy, P.J.; De Prada-Espinel, J.; González-Rebollo, A. Los Campos Magnéticos de Baja Amplitud y de Frecuencia Extremadamente Baja Para El Tratamiento de La Gonalgia Crónica. Rehabilitacion 2008, 42, 127–136. [Google Scholar] [CrossRef]
- Rajapakse, T.; Kirton, A. Non-Invasive Brain Stimulation in Children: Applications and Future Directions. Transl. Neurosci. 2013, 4, 217–233. [Google Scholar] [CrossRef]
- Valle, A.C.; Dionisio, K.; Pitskel, N.B.; Pascual-Leone, A.; Orsati, F.; Ferreira, M.J.L.; Boggio, P.S.; Lima, M.C.; Rigonatti, S.P.; Fregni, F. Low and High Frequency Repetitive Transcranial Magnetic Stimulation for the Treatment of Spasticity. Dev. Med. Child Neurol. 2007, 49, 534–538. [Google Scholar] [CrossRef]
- McIntyre, A.; Mirkowski, M.; Thompson, S.; Burhan, A.M.; Miller, T.; Teasell, R. A Systematic Review and Meta-Analysis on the Use of Repetitive Transcranial Magnetic Stimulation for Spasticity Poststroke. PMR 2018, 10, 293–302. [Google Scholar] [CrossRef]
- Kumru, H.; Murillo, N.; Vidal Samso, J.; Valls-Sole, J.; Edwards, D.; Pelayo, R.; Valero-Cabre, A.; Tormos, J.M.; Pascual-Leone, A. Reduction of Spasticity with Repetitive Transcranial Magnetic Stimulation in Patients with Spinal Cord Injury. Neurorehabil. Neural Repair 2010, 24, 435–441. [Google Scholar] [CrossRef]
- Mori, F.; Ljoka, C.; Magni, E.; Codecà, C.; Kusayanagi, H.; Monteleone, F.; Sancesario, A.; Bernardi, G.; Koch, G.; Foti, C.; et al. Transcranial Magnetic Stimulation Primes the Effects of Exercise Therapy in Multiple Sclerosis. J. Neurol. 2011, 258, 1281–1287. [Google Scholar] [CrossRef]
- Centonze, D.; Koch, G.; Versace, V.; Mori, F.; Rossi, S.; Brusa, L.; Grossi, K.; Torelli, F.; Prosperetti, C.; Cervellino, A.; et al. Repetitive Transcranial Magnetic Stimulation of the Motor Cortex Ameliorates Spasticity in Multiple Sclerosis. Neurology 2007, 68, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, F.; Lanza, G.; Grasso, A.A.; Pennisi, G.; Bella, R.; Paulus, W.; Pennisi, M. Repetitive Transcranial Magnetic Stimulation in Stroke Rehabilitation: Review of the Current Evidence and Pitfalls. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419878317. [Google Scholar] [CrossRef] [PubMed]
- Griffin, X.L.; Costa, M.L.; Parsons, N.; Smith, N. Electromagnetic Field Stimulation for Treating Delayed Union or Non-Union of Long Bone Fractures in Adults. Cochrane Database Syst. Rev. 2011, 4, CD008471. [Google Scholar] [CrossRef] [PubMed]
- Handoll, H.H.G.; Elliott, J. Rehabilitation for Distal Radial Fractures in Adults. Cochrane Database Syst. Rev. 2015, 2015, CD003324. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.; Shomura, K.; Soejima, K.; Ito, G. Effects of Pulsed Electromagnetic Field (PEMF) Stimulation on Bone Tissue Like Formation Are Dependent on the Maturation Stages of the Osteoblasts. Bioelectromagnetics 2002, 23, 398–405. [Google Scholar] [CrossRef]
- Gossling, H.R.; Bernstein, R.A.; Abbott, J. Treatment of Ununited Tibial Fractures: A Comparison of Surgery and Pulsed Electromagnetic Fields (PEMF). Orthopedics 1992, 15, 711–719. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 10, 89. [Google Scholar] [CrossRef]
- Brennan, S.E.; Cumpston, M.S.; McKenzie, J.E.; Thomas, J. The Use of ‘PICO for Synthesis’ and Methods for Synthesis without Meta-Analysis: Protocol for a Survey of Current Practice in Systematic Reviews of Health Interventions. F1000Research 2021, 9, 678. [Google Scholar] [CrossRef]
- Green, S.; Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions—Version 5.0.2; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Foley, N.C.; Bhogal, S.K.; Teasell, R.W.; Bureau, Y.; Speechley, M.R. Estimates of Quality and Reliability with the Physiotherapy Evidence-Based Database Scale to Assess the Methodology of Randomized Controlled Trials of Pharmacological and Nonpharmacological Interventions. Phys. Ther. 2006, 86, 817–824. [Google Scholar] [CrossRef]
- Verhagen, A.P.; De Vet, H.C.W.; De Bie, R.A.; Kessels, A.G.H.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Nielsen, J.F.; Sinkjaer, T.; Jakobsen, J. Treatment of Spasticity with Repetitive Magnetic Stimulation; a Double-Blind Placebo-Controlled Study. Mult. Scler. 1996, 2, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.L.; Lappin, M.S.; Acosta-Urquidi, J.; Kraft, G.H.; Heide, A.C.; Lawrie, F.W.; Merrill, T.E.; Melton, G.B.; Cunningham, C.A. Double-Blind Study of Pulsing Magnetic Field Effects on Multiple Sclerosis. J. Altern. Complement. Med. 1997, 3, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.S.; Lawrie, F.W.; Richards, T.L.; Kramer, E.D. Effects of a Pulsed Electromagnetic Therapy on Multiple Sclerosis Fatigue and Quality of Life: A Double-Blind, Placebo Controlled Trial. Altern. Ther. Health Med. 2003, 9, 38–48. [Google Scholar] [PubMed]
- Krause, P.; Edrich, T.; Straube, A. Lumbar Repetitive Magnetic Stimulation Reduces Spastic Tone Increase of the Lower Limbs. Spinal Cord 2004, 42, 67–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdollahi, M.; Bahrpeyma, F.; Forough, B. Investigation of the Effects of Spinal Pulsed Electromagnetic Field on Spasticity of Lower Extremity and Alpha Motoneuron Excitability in Hemiplegic Patients Using Hmax/Mmax Ratio. Cerebrovasc. Dis. 2013, 36, 18–19. [Google Scholar]
- Serag, H.; Abdelgawad, D.; Emara, T.; Moustafa, R.; El-Nahas, N.; Haroun, M. Effects of Para-Spinal Repetitive Magnetic Stimulation on Multiple Sclerosis Related Spasticity. Int. J. Phys. Med. Rehabil. 2014, 2, 2. [Google Scholar] [CrossRef]
- Krewer, C.; Hartl, S.; Müller, F.; Koenig, E. Effects of Repetitive Peripheral Magnetic Stimulation on Upper-Limb Spasticity and Impairment in Patients with Spastic Hemiparesis: A Randomized, Double-Blind, Sham-Controlled Study. Arch. Phys. Med. Rehabil. 2014, 95, 1039–1047. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Massé-Alarie, H.; Brouwer, B.; Schneider, C. Noninvasive Neurostimulation in Chronic Stroke: A Double-Blind Randomized Sham-Controlled Testing of Clinical and Corticomotor Effects. Top. Stroke Rehabil. 2015, 22, 8–17. [Google Scholar] [CrossRef]
- Prouza, O.; Kouloulas, E.; Zarkovic, D. High-Intensity Electromagnetic Stimulation Can Reduce Spasticity in Post-Stroke Patients. Int. J. Physiother. 2018, 5, 87–91. [Google Scholar] [CrossRef]
- Ciortea, V.M.; Motoașcă, I.; Borda, I.M.; Ungur, R.A.; Bondor, C.I.; Iliescu, M.G.; Ciubean, A.D.; Lazăr, I.; Bendea, E.; Irsay, L. Effects of High-Intensity Electromagnetic Stimulation on Reducing Upper Limb Spasticity in Post-Stroke Patients. Appl. Sci. 2022, 12, 2125. [Google Scholar] [CrossRef]
- Sainz-Pelayo, M.P.; Albu, S.; Murillo, N.; Benito-Penalva, J. Spasticity in Neurological Pathologies. An Update on the Pathophysiological Mechanisms, Advances in Diagnosis and Treatment. Rev. Neurol. 2020, 70, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Patejdl, R.; Zettl, U.K. Spasticity in Multiple Sclerosis: Contribution of Inflammation, Autoimmune Mediated Neuronal Damage and Therapeutic Interventions. Autoimmun. Rev. 2017, 16, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Manack, A.; Brainin, M. Toward an Epidemiology of Poststroke Spasticity. Neurology 2013, 80, S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Levi, R.; Seiger, S. The Stockholm Spinal Cord Injury Study: 1. Medical Problems in a Regional SCI Population. Paraplegia 1995, 33, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Odding, E.; Roebroeck, M.E.; Stam, H.J. The Epidemiology of Cerebral Palsy: Incidence, Impairments and Risk Factors. Disabil. Rehabil. 2006, 28, 183–191. [Google Scholar] [CrossRef]
- Hou, J.; Nelson, R.; Mohammad, N.; Mustafa, G.; Plant, D.; Thompson, F.J.; Bose, P. Effect of Simultaneous Combined Treadmill Training and Magnetic Stimulation on Spasticity and Gait Impairments after Cervical Spinal Cord Injury. J. Neurotrauma 2020, 37, 1999–2013. [Google Scholar] [CrossRef]
- Waldorff, E.I.; Zhang, N.; Ryaby, J.T. Pulsed Electromagnetic Field Applications: A Corporate Perspective. J. Orthop. Transl. 2017, 9, 60–68. [Google Scholar] [CrossRef]
- Yang, X.; He, H.; Ye, W.; Perry, T.A.; He, C. Effects of Pulsed Electromagnetic Field Therapy on Pain, Stiffness, Physical Function, and Quality of Life in Patients with Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Phys. Ther. 2020, 100, 1118–1131. [Google Scholar] [CrossRef]
- Sutbeyaz, S.T.; Sezer, N.; Koseoglu, F.; Kibar, S. Low-Frequency Pulsed Electromagnetic Field Therapy in Fibromyalgia. Clin. J. Pain 2009, 25, 722–728. [Google Scholar] [CrossRef]
- Binder, A.; Parr, G.; Hazleman, B.; Fitton-Jackson, S. Pulsed Electromagnetic Field Therapy of Persistent Rotator Cuff Tendinitis. A Double-Blind Assessment. Lancet 1984, 323, 695–698. [Google Scholar] [CrossRef]
- Uzunca Murat Birtane Nurettin Taştekin, K. Effectiveness of Pulsed Electromagnetic Field Therapy in Lateral Epicondylitis. Clin. Rheumatol. 2007, 26, 69–74. [Google Scholar] [CrossRef]
- Pieber, K.; Herceg, M.; Paternostro-Sluga, T. Electrotherapy for the Treatment of Painful Diabetic Peripheral Neuropathy—A Review. J. Rehabil. Med. 2010, 42, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sadlonovas, J.; Korpas, J.; Vrabec, M.; Salat, D.; Buchancova, J.; Kudlicka, J. The Effect of the Pulsatile Electromagnetic Field in Patients Suffering from Chronic Obstructive Pulmonary Disease and Bronchial Asthma. Bratisl. Lek. Listy 2002, 103, 260–265. [Google Scholar]
- Cobo-Vicente, F.; San Juan, A.F.; Larumbe-Zabala, E.; Estévez-González, A.J.; Donadio, M.V.F.; Pérez-Ruiz, M. Neuromuscular Electrical Stimulation Improves Muscle Strength, Biomechanics of Movement, and Functional Mobility in Children with Chronic Neurological Disorders: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab170. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Fritsch, C.G.; Robinson, C.; Sbruzzi, G.; Plentz, R.D.M. Effects of Electrical Stimulation in Spastic Muscles After Stroke: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Stroke 2015, 46, 2197–2205. [Google Scholar] [CrossRef]
- Han, T.R.; Shin, H.I.; Kim, I.S. Magnetic Stimulation of the Quadriceps Femoris Muscle: Comparison of Pain with Electrical Stimulation. Am. J. Phys. Med. Rehabil. 2006, 85, 593–599. [Google Scholar] [CrossRef]
- Kagaya, H.; Ogawa, M.; Mori, S.; Aoyagi, Y.; Shibata, S.; Inamoto, Y.; Mori, H.; Saitoh, E. Hyoid Bone Movement at Rest by Peripheral Magnetic Stimulation of Suprahyoid Muscles in Normal Individuals. Neuromodulation 2019, 22, 593–596. [Google Scholar] [CrossRef]
- Kinoshita, S.; Ikeda, K.; Yasuno, S.; Takahashi, S.; Yamada, N.; Okuyama, Y.; Sasaki, N.; Hada, T.; Kuriyama, C.; Suzuki, S.; et al. Dose-Response of RPMS for Upper Limb Hemiparesis after Stroke. Medicine 2020, 99, e20752. [Google Scholar] [CrossRef]
- Amatya, B.; Khan, F.; La Mantia, L.; Demetrios, M.; Wade, D.T. Non Pharmacological Interventions for Spasticity in Multiple Sclerosis. Cochrane Database Syst. Rev. 2013, 2013, CD009974. [Google Scholar] [CrossRef]
- Aloraini, S.M.; Gäverth, J.; Yeung, E.; MacKay-Lyons, M. Assessment of Spasticity after Stroke Using Clinical Measures: A Systematic Review. Disabil. Rehabil. 2015, 37, 2313–2323. [Google Scholar] [CrossRef]
- Petek Balci, B. Spasticty Measurement. Arch. Neuropsychiatry 2018, 55, S49. [Google Scholar] [CrossRef] [PubMed]
- Van Wijck, F.M.J.; Pandyan, A.D.; Johnson, G.R.; Barnes, M.P. Assessing Motor Deficits in Neurological Rehabilitation: Patterns of Instrument Usage. Neurorehabil. Neural Repair 2001, 15, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Numanoǧlu, A.; Günel, M.K. Intraobserver Reliability of Modified Ashworth Scale and Modified Tardieu Scale in the Assessment of Spasticity in Children with Cerebral Palsy. Acta Orthop. Traumatol. Turc. 2012, 46, 196–200. [Google Scholar] [CrossRef]
- Kinoshita, S.; Ikeda, K.; Hama, M.; Suzuki, S.; Abo, M. Repetitive Peripheral Magnetic Stimulation Combined with Intensive Physical Therapy for Gait Disturbance after Hemorrhagic Stroke: An Open-Label Case Series. Int. J. Rehabil. Res. 2020, 43, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.; Struppler, A. First Steps in Functional Magnetic Stimulation (FMS)-Movements of Forearm and Fingers Induced by Closed-Loop Controlled FMS. Acta Physiol. Pharmacol. Bulg. 2001, 26, 185–188. [Google Scholar] [PubMed]
- Flamand, V.H.; Schneider, C. Noninvasive and Painless Magnetic Stimulation of Nerves Improved Brain Motor Function and Mobility in a Cerebral Palsy Case. Arch. Phys. Med. Rehabil. 2014, 95, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, M.; Angerer, B.; Buss, M.; Struppler, A. Neural Observer Based Spasticity Quantification during Therapeutic Muscle Stimulation. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, New York, NY, USA, 30 August–3 September 2006; pp. 4897–4900. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).