Clinical Characteristics of Patients with Ossification of the Posterior Longitudinal Ligament and a High OP Index: A Multicenter Cross-Sectional Study (JOSL Study)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
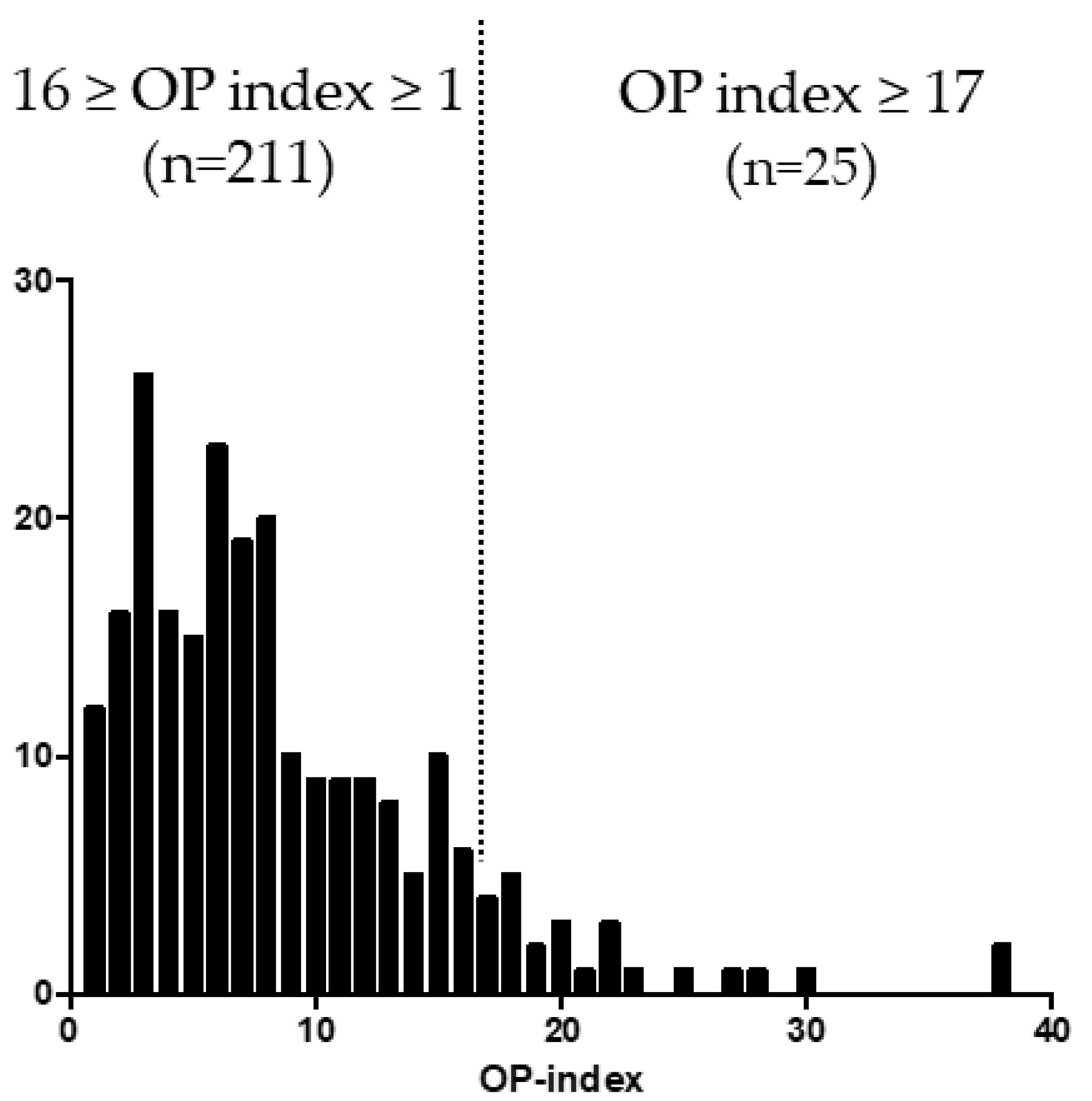
3.1. Distribution of OP-Index Values in Patients with OPLL
3.2. Demographic Characteristics in the High and Moderate/Low OP Index Groups
3.3. Deterioration of Lumbar Spine and Lower Extremity Function in the High OP-Index Group
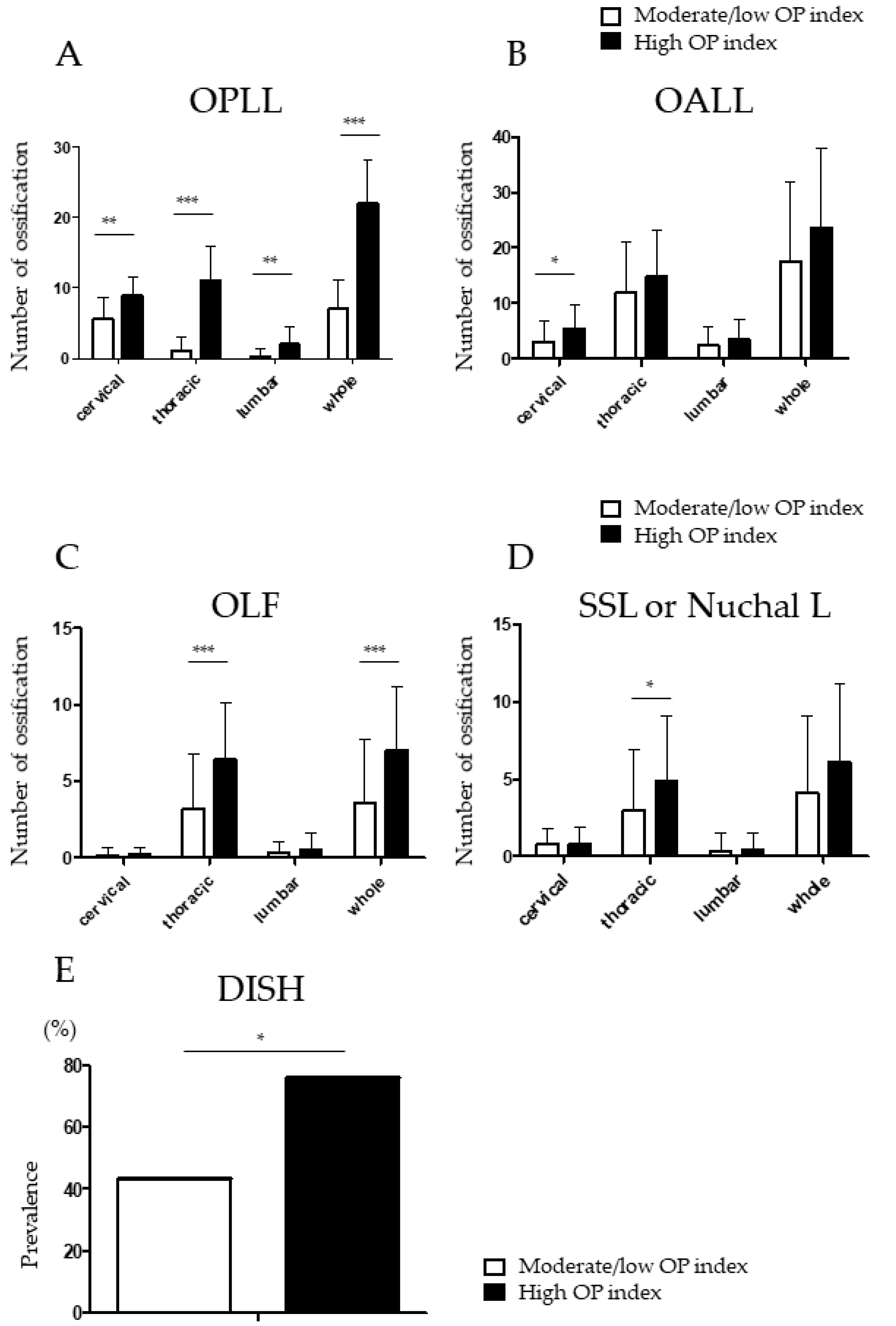
3.4. Higher Prevalence of Ossified Thoracic Lesions in Patients with a High OP Index
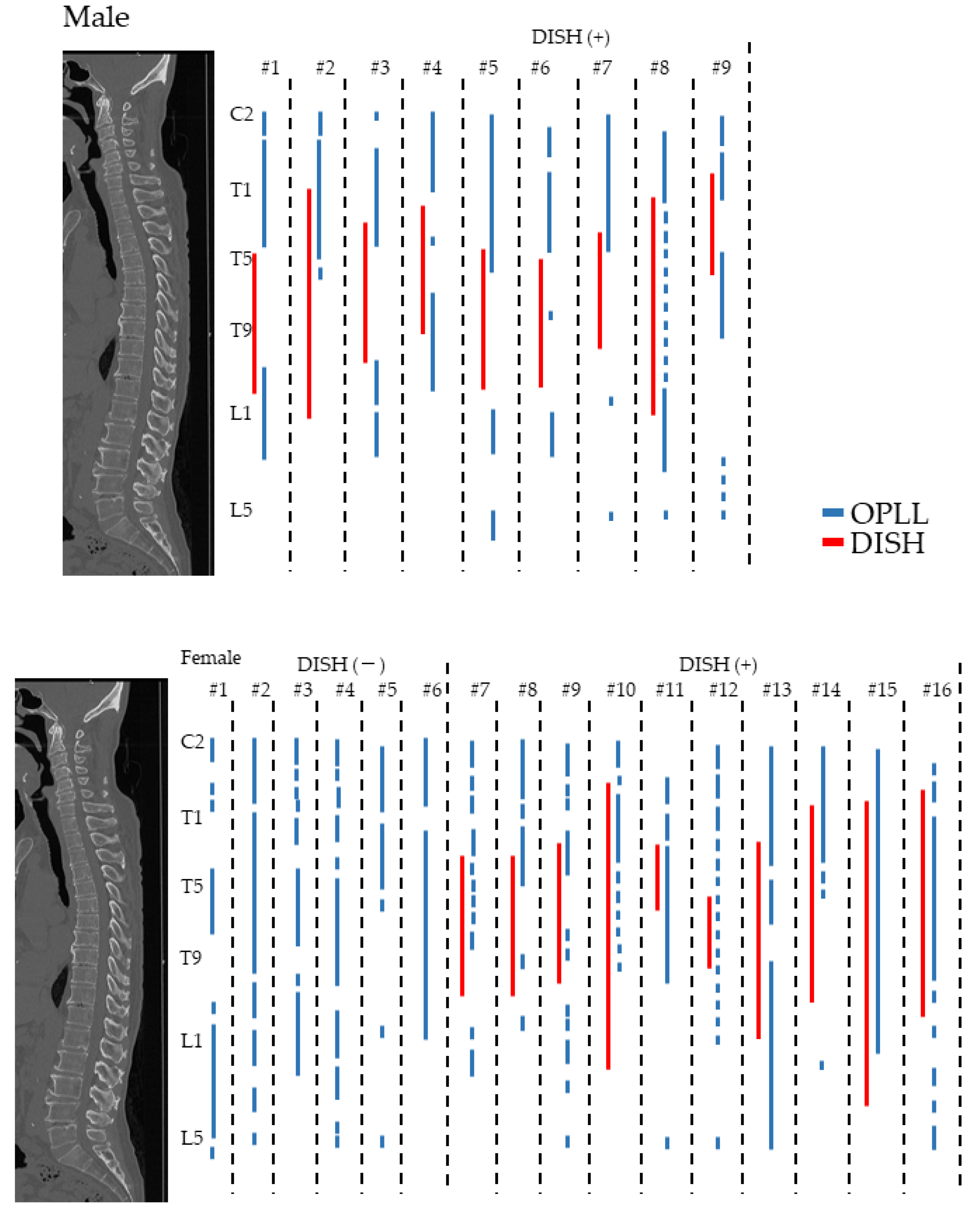
3.5. Distribution of OPLL and DISH in the High OP Index Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsunaga, S.; Nakamura, K.; Seichi, A.; Yokoyama, T.; Toh, S.; Ichimura, S.; Satomi, K.; Endo, K.; Yamamoto, K.; Kato, Y.; et al. Radiographic predictors for the development of myelopathy in patients with ossification of the posterior longitudinal ligament: A multicenter cohort study. Spine 2008, 33, 2648–2650. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Imai, S.; Kasahara, T.; Nishizawa, K.; Mimura, T.; Matsusue, Y. Prevalence, distribution, and morphology of thoracic ossification of the posterior longitudinal ligament in Japanese: Results of CT-based cross-sectional study. Spine 2014, 39, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Russell, W.J.; Kudo, S.; Kuroiwa, Y.; Takamori, M.; Motomura, S.; Murakami, J. Ossification of the thoracic posterior longitudinal ligament in a fixed population. Radiological and neurological manifestations. Radiology 1982, 143, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Takahashi, A.; Tsuji, T.; Karasugi, T.; Baba, H.; Uchida, K.; Kawabata, S.; Okawa, A.; Shindo, S.; Takeuchi, K.; et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 2014, 46, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Sakai, K.; Hirai, T.; Yamada, T.; Inose, H.; Kato, T.; Enomoto, M.; Tomizawa, S.; Kawabata, S.; Arai, Y.; et al. Anterior decompression with fusion versus posterior decompression with fusion for massive cervical ossification of the posterior longitudinal ligament with a ≥50% canal occupying ratio: A multicenter retrospective study. Spine J. 2016, 16, 1351–1357. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshii, T.; Egawa, S.; Sakai, K.; Inose, H.; Yuasa, M.; Yamada, T.; Ushio, S.; Kato, T.; Arai, Y.; et al. Increased height of fused segments contributes to early-phase strut dislodgement after anterior cervical corpectomy with fusion for multilevel ossification of the posterior longitudinal ligament. Spine Surg. Relat. Res. 2020, 4, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Imagama, S.; Ando, K.; Takeuchi, K.; Kato, S.; Murakami, H.; Aizawa, T.; Ozawa, H.; Hasegawa, T.; Matsuyama, Y.; Koda, M.; et al. Perioperative Complications After Surgery for Thoracic Ossification of Posterior Longitudinal Ligament: A Nationwide Multicenter Prospective Study. Spine 2018, 43, E1389–E1397. [Google Scholar] [CrossRef]
- Boody, B.S.; Lendner, M.; Vaccaro, A.R. Ossification of the posterior longitudinal ligament in the cervical spine: A review. Int. Orthop. 2019, 43, 797–805. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshii, T.; Ushio, S.; Hashimoto, J.; Mori, K.; Maki, S.; Katsumi, K.; Nagoshi, N.; Takeuchi, K.; Furuya, T.; et al. Associations between Clinical Symptoms and Degree of Ossification in Patients with Cervical Ossification of the Posterior Longitudinal Ligament: A Prospective Multi-Institutional Cross-Sectional Study. J. Clin. Med. 2020, 9, 4055. [Google Scholar] [CrossRef]
- Fujimori, T.; Watabe, T.; Iwamoto, Y.; Hamada, S.; Iwasaki, M.; Oda, T. Prevalence, Concomitance, and Distribution of Ossification of the Spinal Ligaments: Results of Whole Spine CT Scans in 1500 Japanese Patients. Spine 2016, 41, 1668–1676. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Nakano, M.; Yasuda, T.; Seki, S.; Hori, T.; Kimura, T. Ossification of the posterior longitudinal ligament in not only the cervical spine, but also other spinal regions: Analysis using multidetector computed tomography of the whole spine. Spine 2013, 38, E1477–E1482. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Yoshii, T.; Iwanami, A.; Takeuchi, K.; Mori, K.; Yamada, T.; Wada, K.; Koda, M.; Matsuyama, Y.; Takeshita, K.; et al. Prevalence and Distribution of Ossified Lesions in the Whole Spine of Patients with Cervical Ossification of the Posterior Longitudinal Ligament A Multicenter Study (JOSL CT study). PLoS ONE 2016, 11, e0160117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshii, T.; Hirai, T.; Iwanami, A.; Nagoshi, N.; Takeuchi, K.; Mori, K.; Yamada, T.; Seki, S.; Tsuji, T.; Fujiyoshi, K.; et al. Co-existence of ossification of the nuchal ligament is associated with severity of ossification in the whole spine in patients with cervical ossification of the posterior longitudinal ligament—A multi-center CT study. J. Orthop. Sci. 2019, 24, 35–41. [Google Scholar] [CrossRef]
- Mori, K.; Yoshii, T.; Hirai, T.; Maki, S.; Katsumi, K.; Nagoshi, N.; Nishimura, S.; Takeuchi, K.; Ushio, S.; Furuya, T.; et al. The characteristics of the young patients with cervical ossification of the posterior longitudinal ligament of the spine: A multicenter cross-sectional study. J. Orthop. Sci. 2021, in press. [CrossRef] [PubMed]
- Katsumi, K.; Hirai, T.; Yoshii, T.; Maki, S.; Mori, K.; Nagoshi, N.; Nishimura, S.; Takeuchi, K.; Ushio, S.; Furuya, T.; et al. The impact of ossification spread on cervical spine function in patients with ossification of the posterior longitudinal ligament. Sci. Rep. 2021, 11, 14337. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Yoshii, T.; Nagoshi, N.; Takeuchi, K.; Mori, K.; Ushio, S.; Iwanami, A.; Yamada, T.; Seki, S.; Tsuji, T.; et al. Distribution of ossified spinal lesions in patients with severe ossification of the posterior longitudinal ligament and prediction of ossification at each segment based on the cervical OP index classification: A multicenter study (JOSL CT study). BMC Musculoskelet. Disord. 2018, 19, 107. [Google Scholar] [CrossRef] [Green Version]
- Hirai, T.; Okawa, A.; Arai, Y.; Takahashi, M.; Kawabata, S.; Kato, T.; Enomoto, M.; Tomizawa, S.; Sakai, K.; Torigoe, I.; et al. Middle-term results of a prospective comparative study of anterior decompression with fusion and posterior decompression with laminoplasty for the treatment of cervical spondylotic myelopathy. Spine 2011, 36, 1940–1947. [Google Scholar] [CrossRef]
- Fukui, M.; Chiba, K.; Kawakami, M.; Kikuchi, S.; Konno, S.; Miyamoto, M.; Seichi, A.; Shimamura, T.; Shirado, O.; Taguchi, T.; et al. JOA Back Pain Evaluation Questionnaire (JOABPEQ)/JOA Cervical Myelopathy Evaluation Questionnaire (JOACMEQ). The report on the development of revised versions. April 16, 2007. The Subcommittee of the Clinical Outcome Committee of the Japanese Orthopaedic Association on Low Back Pain and Cervical Myelopathy Evaluation. J. Orthop. Sci. 2009, 14, 348–365. [Google Scholar] [CrossRef]
- Mori, K.; Yoshii, T.; Hirai, T.; Iwanami, A.; Takeuchi, K.; Yamada, T.; Seki, S.; Tsuji, T.; Fujiyoshi, K.; Furukawa, M.; et al. Prevalence and distribution of ossification of the supra/interspinous ligaments in symptomatic patients with cervical ossification of the posterior longitudinal ligament of the spine: A CT-based multicenter cross-sectional study. BMC Musculoskelet. Disord. 2016, 17, 492. [Google Scholar] [CrossRef]
- Nishimura, S.; Nagoshi, N.; Iwanami, A.; Takeuchi, A.; Hirai, T.; Yoshii, T.; Takeuchi, K.; Mori, K.; Yamada, T.; Seki, S.; et al. Prevalence and distribution of diffuse idiopathic skeletal hyperostosis on whole-spine computed tomography in patients with cervical ossification of the poosterior longitudinal ligament: A multicenter study. Clin. Spine Surg. 2018, 31, E460–E465. [Google Scholar] [CrossRef]
- Fujimori, T.; Nakajima, N.; Sugiura, T.; Ikegami, D.; Sakaura, H.; Kaito, T.; Iwasaki, M. Epidemiology of symptomatic ossification of the posterior longitudinal ligament: A nationwide registry survey. J. Spine Surg. 2021, 7, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Nagata, K.; Muraki, S.; Oka, H.; Yoshida, M.; Enyo, Y.; Kagotani, R.; Hashizume, H.; Yamada, H.; Ishimoto, Y.; et al. Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: A 3-year follow-up of the ROAD study. Osteoporos. Int. 2014, 25, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Jin, Z.; Shi, L.; Zhao, Y.; Zhou, S.; Chen, D.; Tang, D.; Yang, L.; Chen, X. Prevalence of Ossification of Posterior Longitudinal Ligament in Patients With Degenerative Cervical Myelopathy: Cervical Spine 3D CT Observations in 7210 Cases. Spine 2020, 45, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Yoshii, T.; Ushio, S.; Mori, K.; Maki, S.; Katsumi, K.; Nagoshi, N.; Takeuchi, K.; Furuya, T.; Watanabe, K.; et al. Clinical characteristics in patients with ossification of the posterior longitudinal ligament: A prospective multi-institutional cross-sectional study. Sci. Rep. 2020, 10, 5532. [Google Scholar] [CrossRef]
- Haddas, R.; Patel, S.; Arakal, R.; Boah, A.; Belanger, T.; Ju, K.L. Spine and lower extremity kinematics during gait in patients with cervical spondylotic myelopathy. Spine J. 2018, 18, 1645–1652. [Google Scholar] [CrossRef]
- Lee, B.J.; Park, J.H.; Jeon, S.R.; Rhim, S.C.; Roh, S.W. Clinically significant radiographic parameter for thoracic myelopathy caused by ossification of the ligamentum flavum. Eur. Spine J. 2019, 28, 1846–1854. [Google Scholar] [CrossRef]
- Yamada, T.; Shindo, S.; Yoshii, T.; Ushio, S.; Kusano, K.; Miyake, N.; Arai, Y.; Otani, K.; Okawa, A.; Nakai, O. Surgical outcomes of the thoracic ossification of ligamentum flavum: A retrospective analysis of 61 cases. BMC Musculoskelet. Disord. 2021, 22, 7. [Google Scholar] [CrossRef]
- Chen, G.; Fan, T.; Yang, X.; Sun, C.; Fan, D.; Chen, Z. The prevalence and clinical characteristics of thoracic spinal stenosis: A systematic review. Eur. Spine J. 2020, 29, 2164–2172. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Furushima, K.; Sugimori, K.; Inoue, I.; Kimura, T. Association between polymorphism of the transforming growth factor-beta1 gene with the radiologic characteristic and ossification of the posterior longitudinal ligament. Spine 2003, 28, 1424–1426. [Google Scholar] [CrossRef]
- Komm, B.S.; Terpening, C.M.; Benz, D.J.; Graeme, K.A.; Gallegos, A.; Korc, M.; Greene, G.L.; O’Malley, B.W.; Haussler, M.R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science 1988, 241, 81–84. [Google Scholar] [CrossRef]
- Eriksen, E.F.; Colvard, D.S.; Berg, N.J.; Graham, M.L.; Mann, K.G.; Spelsberg, T.C.; Riggs, B.L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science 1988, 241, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nakajima, A.; Aiba, A.; Koda, M.; Okawa, A.; Takahashi, K.; Yamazaki, M. Association between serum leptin and bone metabolic markers, and the development of heterotopic ossification of the spinal ligament in female patients with ossification of the posterior longitudinal ligament. Eur. Spine J. 2011, 20, 1450–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivieri, I.; D’Angelo, S.; Palazzi, C.; Padula, A.; Mader, R.; Khan, M.A. Diffuse idiopathic skeletal hyperostosis: Differentiation from ankylosing spondylitis. Curr. Rheumatol. Rep. 2009, 11, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kuperus, J.S.; Waalwijk, J.F.; Regan, E.A.; van der Horst-Bruinsma, I.E.; Oner, F.C.; de Jong, P.A.; Verlaan, J.J. Simultaneous occurrence of ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis: A systematic review. Rheumatology 2018, 57, 2120–2128. [Google Scholar] [CrossRef]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Hirai, T.; Nishimura, S.; Yoshii, T.; Nagoshi, N.; Hashimoto, J.; Mori, K.; Maki, S.; Katsumi, K.; Takeuchi, K.; Ushio, S.; et al. Associations between Clinical Findings and Severity of Diffuse Idiopathic Skeletal Hyperostosis in Patients with Ossification of the Posterior Longitudinal Ligament. J. Clin. Med. 2021, 10, 4137. [Google Scholar] [CrossRef]
- Nishimura, S.; Hirai, T.; Nagoshi, N.; Yoshii, T.; Hashimoto, J.; Mori, K.; Maki, S.; Katsumi, K.; Takeuchi, K.; Ushio, S.; et al. Association between Severity of Diffuse Idiopathic Skeletal Hyperostosis and Ossification of Other Spinal Ligaments in Patients with Ossification of the Posterior Longitudinal Ligament. J. Clin. Med. 2021, 10, 4690. [Google Scholar] [CrossRef]
- Nguyen, T.C.T.; Yahara, Y.; Yasuda, T.; Seki, S.; Suzuki, K.; Watanabe, K.; Makino, H.; Kamei, K.; Mori, K.; Kawaguchi, Y. Morphological characteristics of DISH in patients with OPLL and its association with high-sensitivity CRP: Inflammatory DISH. Rheumatology 2022, keac051. [Google Scholar] [CrossRef]
| High OP Index (n = 25) | Moderate/Low OP Index (n = 211) | p-Value | |
|---|---|---|---|
| Age (years) | 59.0 ± 14.0 | 64.5 ± 12.0 | 0.07 |
| Male sex (%) | 32.0 | 73.5 | <0.001 *** |
| Body mass index | 27.4 ± 5.8 | 25.7 ± 4.3 | 0.17 |
| Diabetes mellitus (%) | 28.0 | 24.6 | 0.82 |
| Cervical JOA score | 11.5 (6–17) | 12.4 (−2, 17) | 0.12 |
| High OP Index (n = 25) | Moderate/Low OP Index (n = 211) | p-Value | |
|---|---|---|---|
| Prevalence of symptoms (%) | |||
| Neck pain | 60.0 | 60.2 | 0.98 |
| Back pain | 32.0 | 28.0 | 0.67 |
| Low back pain | 68.0 | 52.6 | 0.14 |
| JOA-CMEQ score | |||
| Cervical spine function | 59.8 ± 33.8 | 66.6 ± 27.9 | 0.34 |
| Upper extremity function | 74.5 ± 21.6 | 80.8 ± 21.5 | 0.18 |
| Lower extremity function | 46.1 ± 35.7 | 68.3 ± 29.3 | 0.006 ** |
| Bladder function | 73.0 ± 20.2 | 74.7 ± 22.3 | 0.69 |
| Quality of life | 40.4 ± 20.2 | 51.0 ± 19.6 | 0.02 * |
| JOA-BPEQ score | |||
| Lumbar spine function | 54.0 ± 29.3 | 69.7 ± 31.6 | 0.02 * |
| Social dysfunction | 43.4 ± 32.8 | 57.9 ± 28.7 | 0.04 * |
| Mentality | 40.8 ± 18.6 | 50.1 ± 19.9 | 0.03 * |
| Locomotive function | 45.6 ± 37.1 | 66.4 ± 34.5 | 0.01 * |
| Body pain | 58.2 ± 39.1 | 72.3 ± 32.7 | 0.09 |
| VAS score | |||
| Neck pain | 40.2 ± 31.3 | 38.6 ± 31.2 | 0.81 |
| Upper extremity numbness | 47.5 ± 34.5 | 44.8 ± 33.2 | 0.71 |
| Chest constriction | 13.0 ± 27.9 | 9.9 ± 21.0 | 0.60 |
| Numbness below the chest | 60.1 ± 28.9 | 34.2 ± 33.6 | <0.001 *** |
| Low back pain | 44.1 ± 32.9 | 25.7 ± 27.9 | 0.01 * |
| Lower extremity numbness | 54.4 ± 34.9 | 28.1 ± 32.6 | <0.01 ** |
| Lower extremity pain | 37.8 ± 33.6 | 21.4 ± 29.4 | 0.03 * |
| CNR Grade | High OP Index (n = 25) | Moderate/Low OP Index (n = 211) | p-Value |
|---|---|---|---|
| Grade 1 | 2 (8%) | 70 (33.2%) | |
| 2 | 8 (32%) | 71 (33.6%) | |
| 3 | 12 (48%) | 56 (26.5%) | |
| 4 | 3 (12%) | 14 (6.6%) | |
| 0.03 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirai, T.; Yoshii, T.; Hashimoto, J.; Ushio, S.; Mori, K.; Maki, S.; Katsumi, K.; Nagoshi, N.; Takeuchi, K.; Furuya, T.; et al. Clinical Characteristics of Patients with Ossification of the Posterior Longitudinal Ligament and a High OP Index: A Multicenter Cross-Sectional Study (JOSL Study). J. Clin. Med. 2022, 11, 3694. https://doi.org/10.3390/jcm11133694
Hirai T, Yoshii T, Hashimoto J, Ushio S, Mori K, Maki S, Katsumi K, Nagoshi N, Takeuchi K, Furuya T, et al. Clinical Characteristics of Patients with Ossification of the Posterior Longitudinal Ligament and a High OP Index: A Multicenter Cross-Sectional Study (JOSL Study). Journal of Clinical Medicine. 2022; 11(13):3694. https://doi.org/10.3390/jcm11133694
Chicago/Turabian StyleHirai, Takashi, Toshitaka Yoshii, Jun Hashimoto, Shuta Ushio, Kanji Mori, Satoshi Maki, Keiichi Katsumi, Narihito Nagoshi, Kazuhiro Takeuchi, Takeo Furuya, and et al. 2022. "Clinical Characteristics of Patients with Ossification of the Posterior Longitudinal Ligament and a High OP Index: A Multicenter Cross-Sectional Study (JOSL Study)" Journal of Clinical Medicine 11, no. 13: 3694. https://doi.org/10.3390/jcm11133694
APA StyleHirai, T., Yoshii, T., Hashimoto, J., Ushio, S., Mori, K., Maki, S., Katsumi, K., Nagoshi, N., Takeuchi, K., Furuya, T., Watanabe, K., Nishida, N., Nishimura, S., Watanabe, K., Kaito, T., Kato, S., Nagashima, K., Koda, M., Nakashima, H., ... Kawaguchi, Y. (2022). Clinical Characteristics of Patients with Ossification of the Posterior Longitudinal Ligament and a High OP Index: A Multicenter Cross-Sectional Study (JOSL Study). Journal of Clinical Medicine, 11(13), 3694. https://doi.org/10.3390/jcm11133694










