Incidence, Outcome, and Risk Factors of Cardiovascular Surgery-Associated Disseminated Intravascular Coagulation: A Single-Center Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
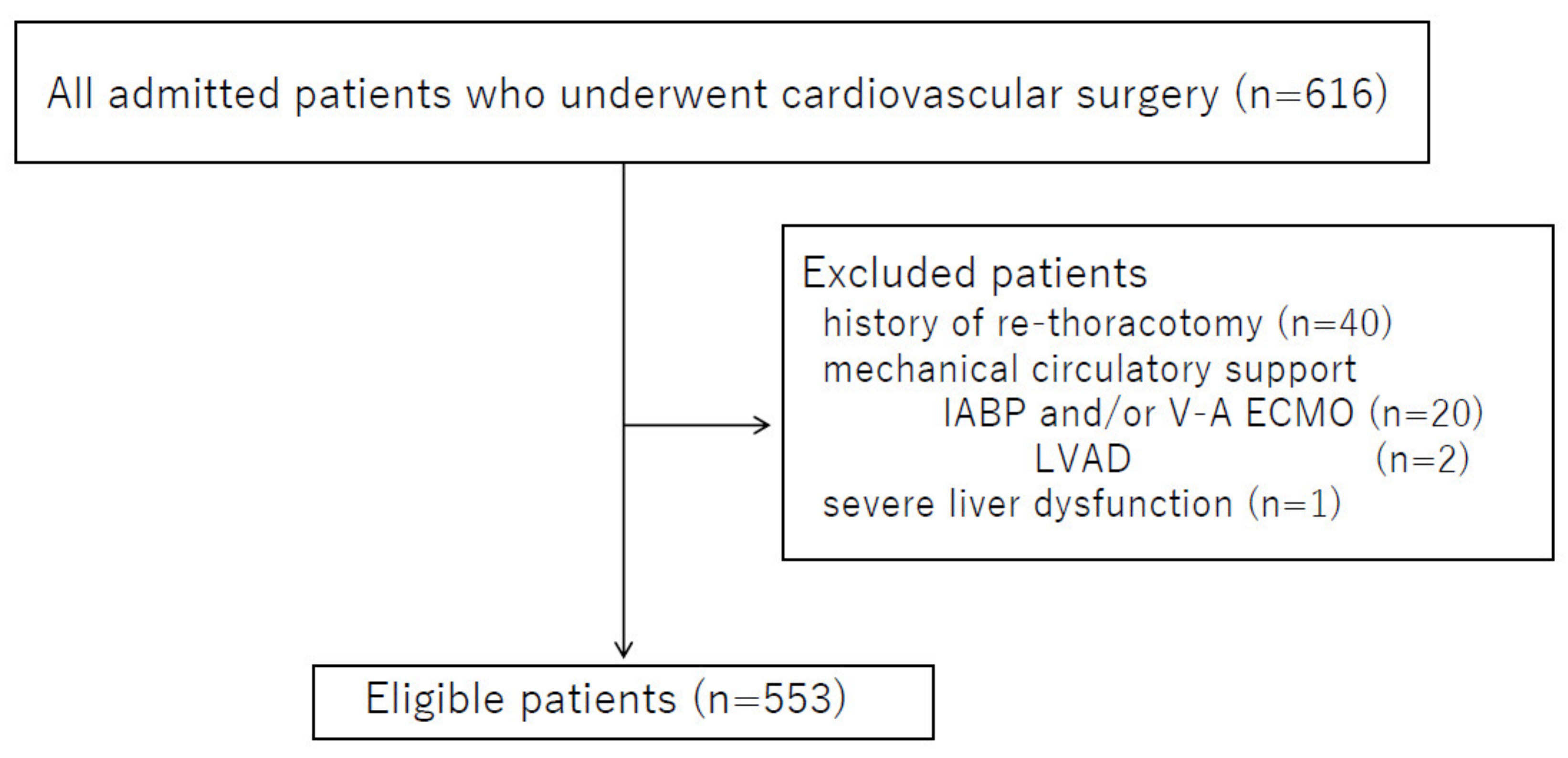
3.1. Patient Characteristics
3.2. Incidence of DIC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gando, S.; Iba, T.; Eguchi, Y.; Ohtomo, Y.; Okamoto, K.; Koseki, K. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit. Care Med. 2006, 34, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Saitoh, D.; Ogura, H.; Mayumi, T.; Koseki, K.; Ikeda, T. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: Results of a multicenter, prospective survey. Crit. Care Med. 2008, 36, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunclicova, M.; Simurda, T.; Zolkova, J.; Sterankova, M.; Skornova, I.; Dobrotova, M. Heterogeneity of Genotype-Phenotype in congenital hypofibrinogenemia- A review of case reports associated with bleeding and thrombosis. J. Clin. Med. 2022, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Simurda, T.; Asselta, R.; Zolkova, J.; Brunclikova, M.; Dobrotova, M.; Kolkova, Z. Congenital Afibrinogenemia and Hypofibrinogenemia: Laboratory and Genetic Testing in Rare Bleeding Disorders with Life-Threatening Clinical Manifestations and Challenging Management. Diagnostics 2021, 11, 2140. [Google Scholar] [CrossRef] [PubMed]
- Levi, M. Disseminated intravascular coagulation. Crit. Care Med. 2007, 35, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernandez-Mondejar, E. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit. Care 2013, 17, R76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornblith, L.Z.; Moore, H.B.; Cohen, M.J. Trauma-induced coagulopathy: The past, present, and future. J. Thromb. Haemost. 2019, 17, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Meesters, M.I.; von Heymann, C. Optimizing perioperative blood and coagulation management during cardiac surgery. Anesthesiology 2019, 37, 713–728. [Google Scholar]
- Zhang, Y.; Li, C.; Shen, M.; Liu, B.; Zeng, X.; Shen, T. Aortic aneurysm and chronic disseminated intravascular coagulation: A retrospective study of 235 patients. Front. Med. 2017, 11, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ismail, M.Y.; Sridhar, D.C.; Nayak, L. Estrogen and thrombosis: A bench to bedside review. Thromb. Res. 2020, 192, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Demma, L.J.; Faraoni, D.; Winkler, A.M.; Iba, T.; Levy, J.H. Predicting mortality in patients with disseminated intravascular coagulation after cardiopulmonary bypass surgery by utilizing two scoring systems. Blood Coagul. Fibrinolysis 2019, 30, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Grafender, J.; Krychtiuk, K.A.; Buchtele, N.; Schoergenhofer, C.; Gelbenegger, G.; Lenz, M. The ISTH DIC score predicts outcome in non-septic patients admitted to a cardiovascular intensive care unit. Eur. J. Intern. Med. 2020, 79, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Maruyama, I.; Shimazaki, S.; Yamamoto, Y.; Aikawa, N.; Ohno, R. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: Results of a PhaseIII, randomized, double-blind clinical trial. J. Thromb. Haemost. 2007, 5, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Kienast, J.; Juers, M.; Widermann, C.J.; Hoffmann, J.N.; Ostermann, H.; Strauss, R. Treatment effects of high-dose antithrombin Ⅲ without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J. Thromb. Haemost. 2006, 4, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Iyama, S.; Sato, T.; Murase, K.; Kamihara, Y.; Ono, K.; Kikuchi, S. Intermittent administration of recombinant human thrombomodulin successfully controlled chronic disseminated intravascular coagulation in a patient with dissecting aortic aneurysm on an outpatient basis. Blood Coagul. Fibrinolysis 2012, 23, 548–550. [Google Scholar] [CrossRef] [PubMed]
| Systemic Inflammatory Response Syndrome Criteria | Score |
|---|---|
| ≥3 | 1 |
| 0–2 | 0 |
| Platelet counts (×104/μL) | |
| <8.0, or a ≥50% decrease within 24 h | 3 |
| ≥8.0 and <12.0, or a ≥30% decrease within 24 h | 1 |
| ≥12.0 | 0 |
| PT-INR | |
| ≥1.2 | 1 |
| <1.2 | 0 |
| FDP (μg/mL) | |
| ≥25 | 3 |
| ≥10 and <25 | 1 |
| <10 | 0 |
| DIC diagnosis | |
| ≥4 points |
| Variables | n = 553 |
|---|---|
| Age (year) | 72 (64–79) |
| Sex (male/female), n | 342/211 |
| APACHE-II score at POD0 | 14 (10–17) |
| SOFA score at POD0 | 7 (4–9) |
| Preoperative JAAM DIC score | 0 (0–1) |
| Mechanical ventilation (y/n) | 393/160 |
| ICU length of stay (days) | 4 (3–6) |
| CRRT (y/n) | 74/479 |
| 90-day mortality, n | 7 |
| Variables | n = 553 | |
|---|---|---|
| Type of operation | Valve surgery | 178 (32%) |
| Thoracic aortic surgery | 128 (23%) | |
| CABG surgery | 91 (17%) | |
| Abdominal aortic surgery | 105 (19%) | |
| Stent-graft surgery | 32 (6%) | |
| Other procedures | 19 (3%) | |
| Operation time (min) | 310 (239–401) | |
| Anesthesia time (min) | 449 (357–543) | |
| Use of CPB (n) | 299 | |
| CPB time (min) | 148 (72–210) | |
| Intraoperative blood loss (mL) | 580 (300–1120) |
| Total (n) | DIC Group (n) | No-DIC Group (n) | Comparison of Intraoperative Blood Loss between the Two Groups | |
|---|---|---|---|---|
| POD1 | 536 | 228 | 308 | p = 0.0058 |
| POD3 | 360 | 141 | 219 | p = 0.0013 |
| POD7 | 127 | 66 | 61 | p = 0.19 (n.s) |
| Variables | DIC Group (n = 66) | No-DIC Group (n = 61) | p Value |
|---|---|---|---|
| Age (years) | 76 (65–80) | 74 (63–80) | 0.348 |
| Sex (M/F) | 36/30 | 41/20 | 0.144 |
| APACHE-II at POD0 | 17 (14–22) | 16 (12–19) | 0.337 |
| SOFA at POD0 | 11 (8–12) | 9 (6–12) | 0.038 |
| Preoperative JAAM DIC score | 2 (1–4) | 1 (0–3) | 0.026 |
| Anesthesia time (min) | 488 (413–650) | 446 (347–525) | 0.011 |
| Intraoperative blood loss (mL) | 803 (470–1803) | 670 (320–1230) | 0.191 |
| Use of CPB (n) | 21 | 14 | 0.096 |
| 90-day mortality (n) | 3 | 1 | 0.369 |
| Variables | Coefficient | Odds Ratio | 95% CI | p Value |
|---|---|---|---|---|
| Females | 1.133 | 3.106 | 1.291–7.472 | 0.0113 |
| Preoperative JAAM DIC score | 0.297 | 1.323 | 1.060–1.651 | 0.0134 |
| Anesthesia time | 0.003 | 1.016 | 1.001–1.050 | 0.0165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, N.; Goto, K.; Kuribayashi, Y.; Ohchi, Y.; Kitano, T. Incidence, Outcome, and Risk Factors of Cardiovascular Surgery-Associated Disseminated Intravascular Coagulation: A Single-Center Retrospective Study. J. Clin. Med. 2022, 11, 3633. https://doi.org/10.3390/jcm11133633
Yasuda N, Goto K, Kuribayashi Y, Ohchi Y, Kitano T. Incidence, Outcome, and Risk Factors of Cardiovascular Surgery-Associated Disseminated Intravascular Coagulation: A Single-Center Retrospective Study. Journal of Clinical Medicine. 2022; 11(13):3633. https://doi.org/10.3390/jcm11133633
Chicago/Turabian StyleYasuda, Norihisa, Koji Goto, Yoshihide Kuribayashi, Yoshifumi Ohchi, and Takaaki Kitano. 2022. "Incidence, Outcome, and Risk Factors of Cardiovascular Surgery-Associated Disseminated Intravascular Coagulation: A Single-Center Retrospective Study" Journal of Clinical Medicine 11, no. 13: 3633. https://doi.org/10.3390/jcm11133633
APA StyleYasuda, N., Goto, K., Kuribayashi, Y., Ohchi, Y., & Kitano, T. (2022). Incidence, Outcome, and Risk Factors of Cardiovascular Surgery-Associated Disseminated Intravascular Coagulation: A Single-Center Retrospective Study. Journal of Clinical Medicine, 11(13), 3633. https://doi.org/10.3390/jcm11133633





