A Preliminary Study on Photic Driving in the Electroencephalogram of Children with Autism across a Wide Cognitive and Behavioral Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive and Behavioral Assessment
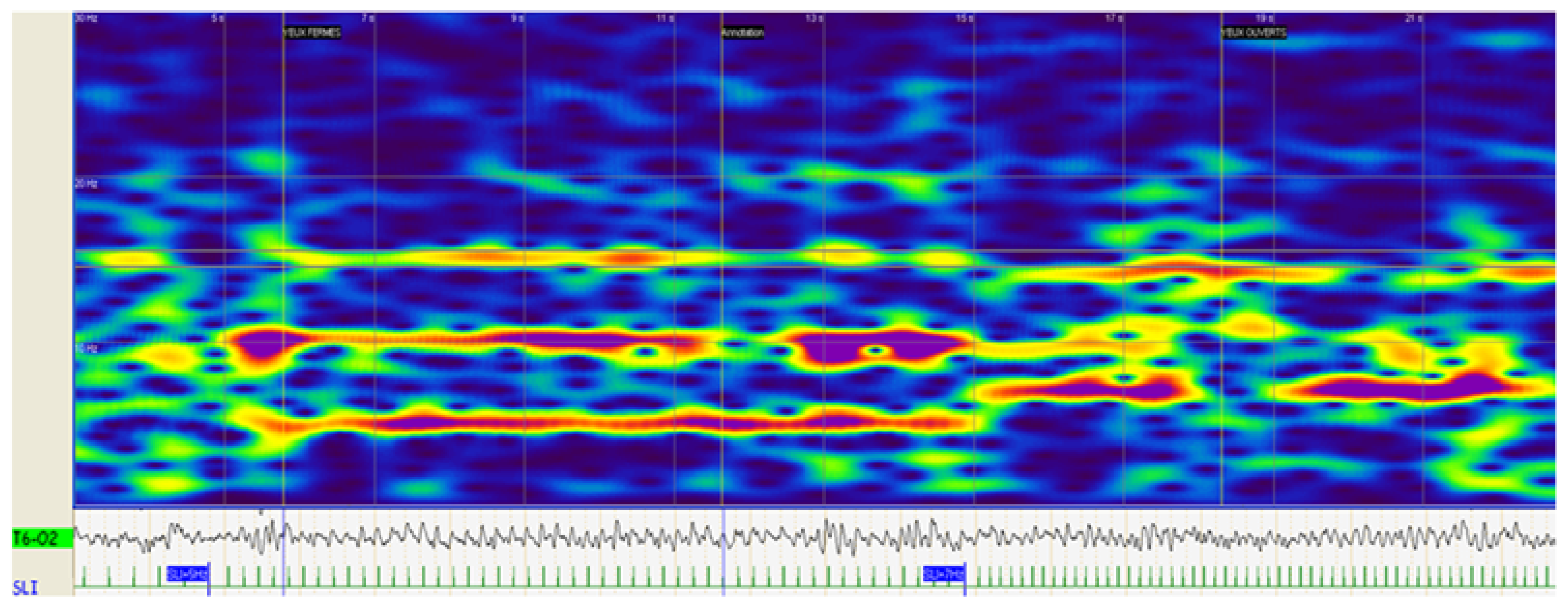
2.3. EEG Recording
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations and Implications for Treatment Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Zikopoulos, B.; Liu, X.; Tepe, J.; Trutzer, I.; John, Y.J.; Barbas, H. Opposite development of short-and long-range anterior cingulate pathways in autism. Acta Neuropathol. 2018, 136, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, D.H.; Levitt, P. Autism spectrum disorders: Developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007, 17, 103–111. [Google Scholar] [CrossRef]
- Niedermeyer, E.; da Silva, F.H.L. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Livanov, M.N. Spatial Organization of Cerebral Processes; John Wiley & Sons: Hoboken, NJ, USA, 1977. [Google Scholar]
- Chatrian, G.E. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr. Clin. Neurophysiol. 1974, 37, 538–548. [Google Scholar]
- Tyler, C.W.; Apkarian, P.; Nakayama, K. Multiple spatial-frequency tuning of electrical responses from human visual cortex. Exp. Brain Res. 1978, 33, 535–550. [Google Scholar] [CrossRef]
- Fedotchev, A.I.; Bondar, A.T.; Konovalov, V.F. Stability of resonance EEG reactions to flickering light in humans. Int. J. Psychophysiol. 1990, 9, 189–193. [Google Scholar] [CrossRef]
- Beydoun, A.; Schechter, S.H.; Nasreddine, W.; Drury, I. Responses to photic stimulation in patients with occipital spikes. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 13–17. [Google Scholar] [CrossRef]
- Lazarev, V.V.; Simpson, D.M.; Schubsky, B.M.; Deazevedo, L.C. Photic driving in the electroencephalogram of children and adolescents: Harmonic structure and relation to the resting state. Braz. J. Med. Biol. Res. 2001, 34, 1573–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, V.J.; Walter, W.G. The central effects of rhythmic sensory stimulation. Electroencephalogr. Clin. Neurophysiol. 1949, 1, 57–86. [Google Scholar] [CrossRef]
- Danilova, N.N. Functional States: Mechanisms and Diagnostics; Publishing House of Moscow State Univ.: Moscow, Russia, 1985. [Google Scholar]
- Kooi, K.A.; Tucker, R.P.; Marshall, R.E. Fundamentals of Electroencephalography; HarperCollins Publishers: New York, NY, USA, 1978. [Google Scholar]
- Kaiser, J.; Gruzelier, J.H. Timing of puberty and EEG coherence during photic stimulation. Int. J. Psychophysiol. 1996, 21, 135–149. [Google Scholar] [CrossRef]
- Lazarev, V.V.; Pontes, A.; deAzevedo, L.C. EEG photic driving: Right-hemisphere reactivity deficit in childhood autism. A pilot study. Int. J. Psychophysiol. 2009, 71, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.V.; Pontes, A.; Mitrofanov, A.A.; DeAzevedo, L.C. Interhemispheric asymmetry in EEG photic driving coherence in childhood autism. Clin. Neurophysiol. 2010, 121, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.V.; Pontes, A.; Mitrofanov, A.A.; Deazevedo, L.C. Reduced interhemispheric connectivity in childhood autism detected by electroencephalographic photic driving coherence. J. Autism Dev. Disord. 2015, 45, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.; DiStefano, C.; Lin, Y.-Y.; Scheffler, A.W.; Senturk, D.; Jeste, S.S. Interhemispheric alpha-band hypoconnectivity in children with autism spectrum disorder. Behav. Brain Res. 2018, 348, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Wechsler, D. Echelle D’Intelligence de Wechsler Pour la Période Pré-Scolaire ET Primaire—Quatrième ÉDition (WPPSI-IV); Pearson: London, UK, 2014. [Google Scholar]
- Wechsler, D. ÉChelle D’Intelligence de Wechsler Pour Enfants: WISC-IV; ECPA, les Éditions du centre de psychologie appliquée: Paris, France, 2005. [Google Scholar]
- Perron-Borelli, M. EDEI-R: Echelles Différentielles D’Efficiences Intellectuelle; Editions et applications psychologiques: Paris, France, 1996. [Google Scholar]
- Tourrette, C. Evaluer les Enfants avec des Déficiences ou Troubles du Développement. [To Assess Children with Disabilities or Developmental Disorders]; Dunod: Paris, France, 2006. [Google Scholar]
- Schopler, E.; Reichler, R.J.; DeVellis, R.F.; Daly, K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. 1980, 10, 91–103. [Google Scholar] [CrossRef]
- Bourreau, Y.; Roux, S.; Gomot, M.; Bonnet-Brilhault, F.; Barthélémy, C. Validation of the repetitive and restricted behaviour scale in autism spectrum disorders. Eur. Child Adolesc. Psychiatry 2009, 18, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Assaf, M.; Jagannathan, K.; Calhoun, V.D.; Miller, L.; Stevens, M.C.; Sahl, R.; O’Boyle, J.G.; Schultz, R.T.; Pearlson, G.D. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage 2010, 53, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Mann, C.A.; Lubar, J.F.; Zimmerman, A.W.; Miller, C.A.; Muenchen, R.A. Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: Controlled study with clinical implications. Pediatric Neurol. 1992, 8, 30–36. [Google Scholar] [CrossRef]
- Wang, J.; Barstein, J.; Ethridge, L.E.; Mosconi, M.W.; Takarae, Y.; Sweeney, J.A. Resting state EEG abnormalities in autism spectrum disorders. J. Neurodev. Disord. 2013, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coben, R.; Clarke, A.R.; Hudspeth, W.; Barry, R.J. EEG power and coherence in autistic spectrum disorder. Clin. Neurophysiol. 2008, 119, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.M.; Wallace, M.T. Dysfunction of sensory oscillations in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2016, 68, 848–861. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. Age and sex effects in the EEG: Development of the normal child. Clin. Neurophysiol. 2001, 112, 806–814. [Google Scholar] [CrossRef]
- Dykman, R.A.; Holcomb, P.J.; Oglesby, D.M.; Ackerman, P.T. Electrocortical frequencies in hyperactive, learning-disabled, mixed, and normal children. Biol. Psychiatry 1982, 17, 675–685. [Google Scholar] [PubMed]
- Katada, A.; Ozaki, H.; Suzuki, H.; Suhara, K. Developmental characteristics of normal and mentally retarded children’s EEGs. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 192–201. [Google Scholar] [CrossRef]
- Stroganova, T.A.; Nygren, G.; Tsetlin, M.M.; Posikera, I.N.; Gillberg, C.; Elam, M.; Orekhova, E.V. Abnormal EEG lateralization in boys with autism. Clin. Neurophysiol. 2007, 118, 1842–1854. [Google Scholar] [CrossRef]
- Spaak, E.; de Lange, F.P.; Jensen, O. Local entrainment of alpha oscillations by visual stimuli causes cyclic modulation of perception. J. Neurosci. 2014, 34, 3536–3544. [Google Scholar] [CrossRef]
- Murphy, J.W.; Foxe, J.J.; Peters, J.B.; Molholm, S. Susceptibility to distraction in autism spectrum disorder: Probing the integrity of oscillatory alpha-band suppression mechanisms. Autism Res. 2014, 7, 442–458. [Google Scholar] [CrossRef] [Green Version]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Mathewson, K.E.; Lleras, A.; Beck, D.M.; Fabiani, M.; Ro, T.; Gratton, G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front. Psychol. 2011, 2, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.S.; Leung, W.W.M. Differentiating autistic children with quantitative encephalography: A 3-month longitudinal study. J. Child Neurol. 2006, 21, 391–399. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.J.; Brumback, A.C. Rethinking Stereotypies in Autism. Semin. Pediatr. Neurol. 2021, 38, 100897. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, E.V.; Stroganova, T.A.; Nygren, G.; Tsetlin, M.M.; Posikera, I.N.; Gillberg, C.; Elam, M. Excess of high frequency electroencephalogram oscillations in boys with autism. Biol. Psychiatry 2007, 62, 1022–1029. [Google Scholar] [CrossRef]
- Sutton, S.K.; Burnette, C.P.; Mundy, P.C.; Meyer, J.; Vaughan, A.; Sanders, C.; Yale, M. Resting cortical brain activity and social behavior in higher functioning children with autism. J. Child Psychol. Psychiatry 2005, 46, 211–222. [Google Scholar] [CrossRef]
- Burnette, C.P.; Henderson, H.A.; Inge, A.P.; Zahka, N.E.; Schwartz, C.B.; Mundy, P.C. Anterior EEG asymmetry and the modifier model of autism. J. Autism Dev. Disord. 2011, 41, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Brugger, P.; Monsch, A.U.; Johnson, S.A. Repetitive behavior and repetition avoidance: The role of the right hemisphere. J. Psychiatry Neurosci. 1996, 21, 53. [Google Scholar]
- Milovanovic, M.; Grujicic, R. Electroencephalography in Assessment of Autism Spectrum Disorders: A Review. Front. Psychiatry 2021, 1637. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG frequency bands in psychiatric disorders: A review of resting state studies. Front. Hum. Neurosci. 2019, 12, 521. [Google Scholar] [CrossRef]
- Neuhaus, E.; Lowry, S.J.; Santhosh, M.; Kresse, A.; Edwards, L.A.; Keller, J.; Libsack, E.J.; Kang, V.Y.; Naples, A.; Jack, A. Resting state EEG in youth with ASD: Age, sex, and relation to phenotype. J. Neurodev. Disord. 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Casanova, M.F.; Buxhoeveden, D.P.; Switala, A.E.; Roy, E. Minicolumnar pathology in autism. Neurology 2002, 58, 428–432. [Google Scholar] [CrossRef]
- Zafeiriou, D.I.; Ververi, A.; Dafoulis, V.; Kalyva, E.; Vargiami, E. Autism spectrum disorders: The quest for genetic syndromes. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2013, 162, 327–366. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Kessler, R.; Gaughan, T.; Buckley, A.W. Electroencephalogram coherence patterns in autism: An updated review. Pediatric Neurol. 2017, 67, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; Shigihara, Y. Age-and gender-specific characteristics of the resting-state brain activity: A magnetoencephalography study. Aging 2020, 12, 21613. [Google Scholar] [CrossRef] [PubMed]
| n (%) | |
|---|---|
| Male | 67 (82.72%) |
| Female | 14 (17.28%) |
| Diagnosis | n (%) |
| F84.0 | 45 (55.56%) |
| F84.1 | 8 (9.88%) |
| F84.5 | 3 (3.7%) |
| F84.8 | 12 (14.81%) |
| F84.9 | 13 (16.05%) |
| IQ | n (%) |
| >70 | 42 (51.85%) |
| 69–50 | 22 (27.16%) |
| 49–35 | 12 (14.82%) |
| 34–20 | 5 (6.17%) |
| Wavelet Frequency Range | Approximate EEG Labels |
|---|---|
| 3–7 Hz | Delta–Thêta (δθ) |
| 10–15 Hz | Alpha (α) |
| 17–25 Hz | Beta (β) |
| PD δθ/α+ N./Mean/(sd) | PD − N./Mean/(sd) | PD δθ+ N./Mean/(sd) | PD δθ/α/β + N./Mean/(sd) | ANOVA p-Value | Post hoc Analysis | |
|---|---|---|---|---|---|---|
| AGE (MONTHS) | 32/110.6/(41.5) | 25/108.4/(42.7) | 14/93.9/(42.2) | 10/118.9/(51.3) | 0.509 | / |
| VIQ | 23/75.0/(33.3) | 21/59.9/(35.6) | 13/61.3/(33.9) | 8/59.0/(41.6) | 0.334 | / |
| NVIQ | 22/83.9/(28.7) | 21/73.9/(36.7) | 13/81.8/(24.9) | 8/65.7/(23.4) | 0.380 | / |
| CARS | 29/29.1/(7.1) | 22/31.1/(6.1) | 13/29.2/(5.3) | 9/27.5/(3.8) | 0.401 | / |
| RRB-F1 | 28/6.3/(6.5) | 21/6.2/(6.0) | 14/9.7/(9.4) | 10/5.4/(5.6) | 0.682 | / |
| RRB-F2 | 28/2.4/(2.8) | 21/2.6/(3.5) | 14/3.5/(4.9) | 10/1.8/(2.0) | 0.972 | / |
| RRB-F3 | 28/4.6/(3.5) | 21/5.4/(5.2) | 14/7.4/(5.7) | 10/2.2/(1.9) | 0.044 | PD δθ+ > PD δθ/α/β + |
| RRB-F4 | 28/4.1/(3.4) | 21/3.9/(2.8) | 14/5.2/(3.1) | 10/4.9/(2.5) | 0.450 | / |
| DSM5-SCI | 25/1.5/(0.7) | 17/1.6/(0.7) | 11/1.4/(0.5) | 8/1.0/(0.0) | 0.147 | / |
| DSM5-RRB | 25/1.4/(0.6) | 17/1.7/(0.8) | 11/1.9/(0.8) | 8/1.4/(0.5) | 0.194 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetri, L.; Maniscalco, L.; Diana, P.; Guidotti, M.; Matranga, D.; Bonnet-Brilhault, F.; Tripi, G. A Preliminary Study on Photic Driving in the Electroencephalogram of Children with Autism across a Wide Cognitive and Behavioral Range. J. Clin. Med. 2022, 11, 3568. https://doi.org/10.3390/jcm11133568
Vetri L, Maniscalco L, Diana P, Guidotti M, Matranga D, Bonnet-Brilhault F, Tripi G. A Preliminary Study on Photic Driving in the Electroencephalogram of Children with Autism across a Wide Cognitive and Behavioral Range. Journal of Clinical Medicine. 2022; 11(13):3568. https://doi.org/10.3390/jcm11133568
Chicago/Turabian StyleVetri, Luigi, Laura Maniscalco, Paola Diana, Marco Guidotti, Domenica Matranga, Frédérique Bonnet-Brilhault, and Gabriele Tripi. 2022. "A Preliminary Study on Photic Driving in the Electroencephalogram of Children with Autism across a Wide Cognitive and Behavioral Range" Journal of Clinical Medicine 11, no. 13: 3568. https://doi.org/10.3390/jcm11133568
APA StyleVetri, L., Maniscalco, L., Diana, P., Guidotti, M., Matranga, D., Bonnet-Brilhault, F., & Tripi, G. (2022). A Preliminary Study on Photic Driving in the Electroencephalogram of Children with Autism across a Wide Cognitive and Behavioral Range. Journal of Clinical Medicine, 11(13), 3568. https://doi.org/10.3390/jcm11133568








