High-Load and Low-Load Resistance Exercise in Patients with Coronary Artery Disease: Feasibility and Safety of a Randomized Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
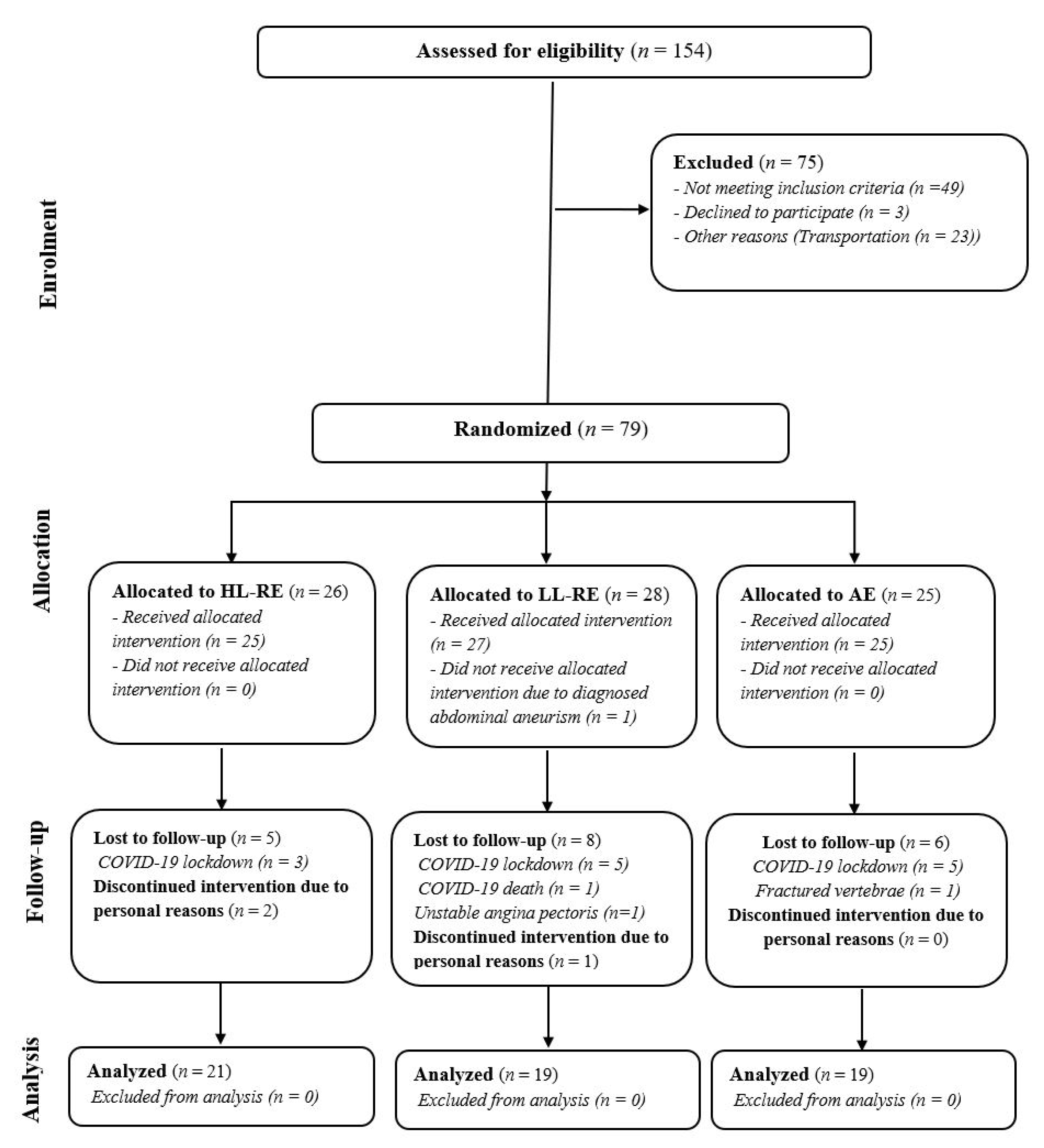
2.2. Participants
2.3. The Training Intervention
2.4. Measurements
2.4.1. Monitoring of Exercise-Related Adverse Cardiovascular and Musculoskeletal Events
2.4.2. Adherence to the Training Intervention, Exercise Tolerance, Workload Data Collection and Analysis
2.4.3. Maximal Leg Press Strength Measurement
2.4.4. Heart Rate Response to Low-Load and High-Load Resistance Exercise
2.5. Statistical Analysis
3. Results
3.1. Safety of the Training Intervention
3.2. Adherence to Aerobic and Resistance Exercise Training
3.3. Workload during Aerobic and Resistance Exercise Training
3.4. Training Loading, Heart Rate and Rating of Perceived Exertion during Resistance Exercise
3.5. Maximal Lower Limb Strength
3.6. Hemodynamic Response and Adaptations to Resistance Exercise
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.E.; Schmid, J.-P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2020, 28, 460–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, D.; Abreu, A.; Ambrosetti, M.; Cornelissen, V.; Gevaert, A.; Kemps, H.; Laukkanen, J.A.; Pedretti, R.; Simonenko, M.; Wilhelm, M.; et al. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: Why and how: A position statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 29, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Balady, G.J.; Williams, M.A.; Ades, P.A.; Bittner, V.; Comoss, P.; Foody, J.M.; Franklin, B.; Sanderson, B.; Southard, D. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2007, 115, 2675–2682. [Google Scholar] [PubMed]
- Price, K.J.; Gordon, B.A.; Bird, S.R.; Benson, A.C. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur. J. Prev. Cardiol. 2016, 23, 1715–1733. [Google Scholar] [CrossRef] [PubMed]
- Xanthos, P.D.; Gordon, B.A.; Kingsley, M.I. Implementing resistance training in the rehabilitation of coronary heart disease: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 230, 493–508. [Google Scholar] [CrossRef]
- Hollings, M.; Mavros, Y.; Freeston, J.; Singh, M.F. The effect of progressive resistance training on aerobic fitness and strength in adults with coronary heart disease: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Prev. Cardiol. 2017, 24, 1242–1259. [Google Scholar] [CrossRef]
- Bjarnason-Wehrens, B.; Mayer-Berger, W.; Meister, E.; Baum, K.; Hambrecht, R.; Gielen, S. Recommendations for resistance exercise in cardiac rehabilitation. Recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 352–361. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Hackett, D.A.; Chow, C.-M. The Valsalva Maneuver: Its Effect on Intra-abdominal Pressure and Safety Issues During Resistance Exercise. J. Strength Cond. Res. 2013, 27, 2338–2345. Available online: https://journals.lww.com/nsca-jscr/Fulltext/2013/08000/The_Valsalva_Maneuver___Its_Effect_on.39.aspx (accessed on 31 March 2022). [CrossRef]
- Saeidifard, F.; Medina-Inojosa, J.R.; West, C.P.; Olson, T.P.; Somers, V.K.; Bonikowske, A.R.; Prokop, L.J.; Vinciguerra, M.; Lopez-Jimenez, F. The association of resistance training with mortality: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 1647–1665. [Google Scholar] [CrossRef]
- American College of Sports Medicine. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sport Exerc. 2009, 41, 687–708. Available online: https://journals.lww.com/acsm-msse/Fulltext/2009/03000/Progression_Models_in_Resistance_Training_for.26.aspx (accessed on 31 March 2022). [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. Available online: https://journals.lww.com/nsca-jscr/Fulltext/2019/08000/Resistance_Training_for_Older_Adults__Position.1.aspx (accessed on 31 March 2022). [CrossRef]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and hypertrophy adaptations between low- versus high-load resistance training. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef]
- Raymond, M.J.; Bramley-Tzerefos, R.E.; Jeffs, K.J.; Winter, A.; Holland, A. Systematic Review of High-Intensity Progressive Resistance Strength Training of the Lower Limb Compared with Other Intensities of Strength Training in Older Adults. Arch. Phys. Med. Rehabil. 2013, 94, 1458–1472. [Google Scholar] [CrossRef]
- Wise, F.M.; Patrick, J.M. Resistance exercise in cardiac rehabilitation. Clin. Rehabil. 2011, 25, 1059–1065. [Google Scholar] [CrossRef]
- Gjøvaag, T.F.; Mirtaheri, P.; Simon, K.; Berdal, G.; Tuchel, I.; Westlie, T.; Bruusgaard, K.A.; Nilsson, B.B.; Hisdal, J. Hemodynamic Responses to Resistance Exercise in Patients with Coronary Artery Disease. Med. Sci. Sports Exerc. 2016, 48, 581–588. [Google Scholar] [CrossRef]
- LaMotte, M.; Niset, G.; Van De Borne, P. The Effect of Different Intensity Modalities of Resistance Training on Beat-to-Beat Blood Pressure in Cardiac Patients. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 12–17. [Google Scholar] [CrossRef]
- LaMotte, M.; Fleury, F.; Pirard, M.; Jamon, A.; Van De Borne, P. Acute cardiovascular response to resistance training during cardiac rehabilitation: Effect of repetition speed and rest periods. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 17, 329–336. [Google Scholar] [CrossRef]
- Kambic, T.; Hadžić, V.; Lainscak, M. Hemodynamic Response to High- and Low-Load Resistance Exercise in Patients with Coronary Artery Disease: A Randomized, Crossover Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 3905. [Google Scholar] [CrossRef]
- Hansen, D.; Abreu, A.; Doherty, P.; Völler, H. Dynamic strength training intensity in cardiovascular rehabilitation: Is it time to reconsider clinical practice? A systematic review. Eur. J. Prev. Cardiol. 2019, 26, 1483–1492. [Google Scholar] [CrossRef]
- Kambic, T.; Šarabon, N.; Hadžić, V.; Lainscak, M. Effects of high-load and low-load resistance training in patients with coronary artery disease: Rationale and design of a randomised controlled clinical trial. BMJ Open 2021, 11, e051325. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. Available online: http://www.bmj.com/content/340/bmj.c332.abstract (accessed on 31 March 2022). [CrossRef]
- Williams, M.A.; Haskell, W.L.; Ades, P.A.; Amsterdam, E.A.; Bittner, V.; Franklin, B.A.; Gulanick, M.; Laing, S.T.; Laing, K.J. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: A scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007, 116, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.; Bethell, H.; Turner, S.; Yadegarfar, G. Characteristics of Patients Entering Cardiac Rehabilitation in the United Kingdom 1993-2006: IMPLICATIONS FOR THE FUTURE. J. Cardiopulm. Rehabil. Prev. 2011, 31, 181–187. Available online: https://journals.lww.com/jcrjournal/Fulltext/2011/05000/Characteristics_of_Patients_Entering_Cardiac.6.aspx (accessed on 31 March 2022). [CrossRef]
- Schwaab, B.; Bjarnason-Wehrens, B.; Meng, K.; Albus, C.; Salzwedel, A.; Schmid, J.-P.; Benzer, W.; Metz, M.; Jensen, K.; Rauch, B.; et al. Cardiac Rehabilitation in German Speaking Countries of Europe—Evidence-Based Guidelines from Germany, Austria and Switzerland LLKardReha-DACH—Part 2. J. Clin. Med. 2021, 10, 3071. [Google Scholar] [CrossRef]
- Gayda, M.; Choquet, D.; Ahmaidi, S. Effects of exercise training modality on skeletal muscle fatigue in men with coronary heart disease. J. Electromyogr. Kinesiol. 2009, 19, e32–e39. [Google Scholar] [CrossRef]
- Marzolini, S.; Thomas, S.G.; Goodman, J.M. Aerobic and resistance training in coronary disease: Single versus multiple sets. Med. Sci. Sport Exerc. 2008, 40, 1557–1564. [Google Scholar] [CrossRef]
- Baechle, T.R.; Earle, R.W.; Wathen, D. Resistance Training. In Essentials of Strength and Conditioning Researchq, 3rd ed.; Baechle, T.R., Earle, R.W., Eds.; Human Kinetics: Champagne, IL, USA, 2008; pp. 381–412. [Google Scholar]
- Brzycki, M. Strength Testing—Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Educ. Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-Intensity Interval Training for Patients with Cardiovascular Disease—Is It Safe? A Systematic Review. J. Am. Heart Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.; Hartling, L.; VanderMeer, B.; McAlister, F.A. Meta-Analysis: Secondary Prevention Programs for Patients with Coronary Artery Disease. Ann. Intern. Med. 2005, 143, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Janssen, V.; De Gucht, V.; Dusseldorp, E.; Maes, S. Lifestyle modification programmes for patients with coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Prev. Cardiol. 2012, 20, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Ruano-Ravina, A.; Pena-Gil, C.; Abu-Assi, E.; Raposeiras, S.; van’t Hof, A.; Meindersma, E.; Prescott, E.I.B.; González-Juanatey, J.R. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int. J. Cardiol. 2016, 223, 436–443. Available online: https://www.sciencedirect.com/science/article/pii/S0167527316318290 (accessed on 31 March 2022). [CrossRef] [PubMed]
- Benzer, W.; Rauch, B.; Schmid, J.-P.; Zwisler, A.D.; Dendale, P.; Davos, C.; Koudi, E.; Simon, A.; Abreu, A.; Pogosova, N.; et al. Exercise-based cardiac rehabilitation in twelve European countries results of the European cardiac rehabilitation registry. Int. J. Cardiol. 2017, 228, 58–67. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Vincent, K.R.; Braith, R.W.; Feldman, R.A.; Magyari, P.M.; Cutler, R.B.; Persin, S.A.; Lennon, S.L.; Gabr, A.H.; Lowenthal, D.T. Resistance Exercise and Physical Performance in Adults Aged 60 to 83. J. Am. Geriatr. Soc. 2002, 50, 1100–1107. [Google Scholar] [CrossRef] [Green Version]
- Harris, C.; DeBeliso, M.A.; Spitzer-Gibson, T.A.; Adams, K.J. The Effect of Resistance-Training Intensity on Strength-Gain Response in the Older Adult. J. Strength Cond. Res. 2004, 18, 833–838. [Google Scholar] [CrossRef]
- Beniamini, Y.; Rubenstein, J.J.; Faigenbaum, A.D.; Lichtenstein, A.H.; Crim, M.C. High-Intensity Strength Training of Patients Enrolled in an Outpatient Cardiac Rehabilitation Program. J. Cardiopulm. Rehabil. 1999, 19, 8–17. Available online: https://journals.lww.com/jcrjournal/Fulltext/1999/01000/High_Intensity_Strength_Training_of_Patients.1.aspx (accessed on 31 March 2022). [CrossRef]
- Arthur, H.; Gunn, E.; Thorpe, K.; Ginis, K.; Mataseje, L.; McCartney, N.; McKelvie, R. Effect of aerobic vs. combined aerobic-strength training on 1-year, post-cardiac rehabilitation outcomes in women after a cardiac event. Acta Derm. Venereol. 2007, 39, 730–735. [Google Scholar] [CrossRef] [Green Version]
- Kambic, T.; Šarabon, N.; Hadžić, V.; Lainscak, M. Effects of high- and low-load resistance training in patients with coronary artery disease: A randomized controlled clinical trial. Eur. J. Prev. Cardiol. 2022. [Google Scholar] [CrossRef]
- Lotze, M.; Braun, C.; Birbaumer, N.; Anders, S.; Cohen, L.G. Motor learning elicited by voluntary drive. Brain 2003, 126, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Berent, R.; von Duvillard, S.P.; Crouse, S.F.; Sinzinger, H.; Green, J.S.; Schmid, P. Resistance Training Dose Response in Combined Endurance-Resistance Training in Patients With Cardiovascular Disease: A Randomized Trial. Arch. Phys. Med. Rehabil. 2011, 92, 1527–1533. [Google Scholar] [CrossRef]
- Currie, K.D.; Bailey, K.J.; Jung, M.E.; McKelvie, R.S.; MacDonald, M.J. Effects of resistance training combined with moderate-intensity endurance or low-volume high-intensity interval exercise on cardiovascular risk factors in patients with coronary artery disease. J. Sci. Med. Sport 2015, 18, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Grafe, K.; Bendick, P.; Burr, M.; Boura, J.; Franklin, B.A. Effects of Resistance Training on Vascular and Hemodynamic Responses in Patients with Coronary Artery Disease. Res. Q. Exerc. Sport 2018, 89, 457–464. [Google Scholar] [CrossRef]
- Ghilarducci, L.E.C.; Holly, R.G.; Amsterdam, E.A. Effects of high resistance training in coronary artery disease. Am. J. Cardiol. 1989, 64, 866–870. [Google Scholar] [CrossRef]
- Morishita, S.; Tsubaki, A.; Takabayashi, T.; Fu, J.B. Relationship Between the Rating of Perceived Exertion Scale and the Load Intensity of Resistance Training. Strength Cond. J. 2018, 40, 94–109. [Google Scholar] [CrossRef]
- Sardeli, A.V.; Santos, L.D.C.; Ferreira, M.L.V.; Gáspari, A.F.; Rodrigues, B.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Cardiovascular Responses to Different Resistance Exercise Protocols in Elderly. Int. J. Sports Med. 2017, 38, 928–936. [Google Scholar] [CrossRef]
- Conlon, J.A.; Haff, G.G.; Tufano, J.J.; Newton, R.U. Application of Session Rating of Perceived Exertion Among Different Models of Resistance Training in Older Adults. J. Strength Cond. Res. 2015, 29, 3439–3446. [Google Scholar] [CrossRef]


| Study Visit | Measurement or Training Session |
|---|---|
| 1. visit | Diagnostic screening (7–14 days prior to enrolment) |
| 2. visit | Familiarization with leg press exercise (3–10 days prior to enrolment) |
| 1. training session | 1-RM measurement followed by AE: AE: interval cycling (5 min workload/2 min active recovery) at 50% of peak power output |
| 2.–3. training session | Haemodynamic response to HL-RE (80% of 1-RM) and LL-RE (40% of 1-RM) followed by AE: interval cycling (5 min workload/2 min active recovery) at 50% of peak power output |
| 4.–11. training session (1. mesocycle) | HL-RE: 3 sets, 6–11 reps/sets, 70% of 1-RM LL-RE: 3 sets, 12–22 reps/sets, 35% of 1-RM AE: interval cycling (5 min workload/2 min active recovery) at 50–56% of peak power output |
| 12.–16. training session (2. mesocycle) | HL-RE: 3 sets, 8–10 reps/sets, 75% of 1-RM LL-RE: 3 sets, 16–20 reps/sets, 37.5% of 1-RM AE: interval cycling (5 min workload/2 min active recovery) at 58–62% of peak power output |
| 17.–20. training session (3. mesocycle) | HL-RE: 3 sets, 6–8 reps/sets, 80% of 1-RM LL-RE: 3 sets, 12–16 reps/sets, 40% of 1-RM AE: interval cycling (4–5 min workload/2 min active recovery) at 62–68% of peak power output |
| 22. training session | 1-RM measurement followed by AE: interval cycling (4 min workload/2 min active recovery) at 68% of peak power output |
| 23.–24. training session (4. mesocycle) | HL-RE: 3 sets, 11 reps/sets, 70% of 1-RM LL-RE: 3 sets, 22 reps/sets, 35% of 1-RM AE: interval cycling (4 min workload/2 min active recovery) at 68–70% of peak power output |
| 25.–28. training session (5. mesocycle) | HL-RE: 3 sets, 9–10 reps/sets, 75% of 1-RM LL-RE: 3 sets, 18–20 reps/sets, 37.5% of 1-RM AE: interval cycling (4 min workload/2 min active recovery) at 72–74% of peak power output |
| 29.–33. training session (6. mesocycle) | HL-RE: 3 sets, 6–8 reps/sets, 80% of 1-RM LL-RE: 3 sets, 12–16 reps/sets, 40% of 1-RM AE: interval cycling (3–4 min workload/2 min active recovery) at 74–80% of peak power output |
| 34.–35. training session | Haemodynamic response to HL-RE (80% of 1-RM) and LL-RE (40% of 1-RM) followed by AE: AE: interval cycling (3 min workload/2 min active recovery) at 80% of peak power output |
| 36. training session | 1-RM measurement followed by AE: AE: interval cycling (3 min workload/2 min active recovery) at 80% of peak power output |
| Variable | Sample (n = 59) | AE Group (n = 19) | LL-RE Group (n = 19) | HL-RE Group (n = 21) | p (ANOVA) |
|---|---|---|---|---|---|
| Sex (females, (%)) | 14 (25) | 5 (16) | 4 (21) | 6 (29) | 0.931 |
| Age (years) | 61 (8) | 61 (9) | 61 (7) | 62 (8) | 0.910 |
| Anthropometrics | |||||
| Height (cm) | 172.1 (8.4) | 170.4 (8.8) | 172.8 (8.6) | 172.9 (7.9) | 0.582 |
| Weight (kg) | 85.47 (15.43) | 90.94 (19.04) | 81.46 (13.37) | 84.15 (12.56) | 0.148 |
| Body mass index (kg/m2) | 28.81 (4.47) | 31.25 (5.71) | 27.13 (3.04) | 28.81 (3.39) | 0.010 |
| Clinical data | |||||
| LVEF (%) | 53 (9) | 50 (45, 60) | 55 (50, 60) | 50 (45, 58) | 0.454 |
| Time from clinical event to inclusion to CR (months) | 2.0 (1.5, 3.0) | 2.0 (2.0, 2.5) | 2.5 (1.5, 3.0) | 2.0 (1.5, 2.8) | 0.832 |
| Myocardial infarction, f (%) | |||||
| NSTEMI | 25 (42.37) | 9 (47.4) | 8 (42.1) | 8 (38.1) | 0.947 |
| STEMI | 24 (40.68) | 7 (36.8) | 7 (36.8) | 10 (47.6) | |
| Unstable AP/PCI | 10 (16.95) | 3 (15.8) | 4 (21.1) | 3 (14.3) | |
| Comorbidities and risk factors, f (%) | |||||
| Arterial hypertension | 41 (69.49) | 15 (78.9) | 11 (57.9) | 15 (71.4) | 0.383 |
| Hyperlipidemia | 49 (83.10) | 16 (84.2) | 14 (73.7) | 19 (90.5) | 0.384 |
| Diabetes | 9 (15.25) | 4 (21.1) | 3 (15.8) | 2 (9.5) | 0.602 |
| Atrial fibrillation | 5 (8.48) | 4 (21.1) | 1 (5.3) | 0 (0.0) | 0.038 |
| Thyroid disease | 5 (8.48) | 2 (10.5) | 2 (10.5) | 1 (4.8) | 0.727 |
| Renal disease | 4 (6.78) | 0 (0.0) | 2 (10.5) | 2 (9.5) | 0.534 |
| Smoking, f (%) | |||||
| Non-smoker | 14 (23.73) | 3 (15.8) | 3 (15.8) | 8 (38.1) | 0.346 |
| Ex-smoker | 35 (59.32) | 13 (68.4) | 11 (57.9) | 11 (52.4) | |
| Smoker | 10 (16.95) | 3 (15.8) | 5 (26.3) | 2 (9.5) | |
| Pharmacological therapy, f (%) | |||||
| Aspirin | 57 (96.60) | 17 (89.5) | 19 (100.0) | 21 (100.0) | 0.200 |
| Beta blocker | 59 (100.00) | 19 (100.0) | 19 (100.0) | 21 (100.0) | 1.000 |
| ACE inhibitor/ARB | 58 (98.30) | 19 (100.0) | 18 (94.7) | 21 (100.0) | 0.644 |
| Statin | 59 (100.00) | 19 (100.0) | 19 (100.0) | 21 (100.0) | 1.000 |
| Antiplatelet drug | 58 (98.30) | 18 (94.7) | 19 (100.0) | 21 (100.0) | 0.644 |
| Anticoagulation drug | 5 (8.48) | 3 (15.8) | 1 (5.3) | 1 (4.8) | 0.509 |
| Diuretic | 5 (8.48) | 4 (21.1) | 0 (0.0) | 1 (4.8) | 0.071 |
| Variable | Group | Mean (SD) | Test Statistics |
|---|---|---|---|
| Mean AE workload (kJ) | AE | 117 (37) | F = 1.658 p = 0.200 |
| LL-RE | 113 (35) | ||
| HL-RE | 98 (34) | ||
| Cumulative AE workload (kJ) | AE | 4175 (1352) | F = 2.056 p = 0.138 |
| LL-RE | 3987 (1242) | ||
| HL-RE | 3388 (1274) | ||
| Mean RE workload (kg) | LL-RE | 3091 (856) | t = 0.703 p = 0.487 |
| HL-RE | 3016 (797) | ||
| Cumulative RE workload (kg) | LL-RE | 89,505 (24,949) | t = 0.285 p = 0.777 |
| HL-RE | 84,092 (23,765) |
| Variable | Group | 1. Part of This Study (Mesocycles) | 2. Part of This Study (Mesocycles) | Time Effect | Time × Group | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | ||||
| Cumulative RE workload (kg) | LL-RE (n =19) | 22,372 (6472) | 15,514 (4410) | 12,991 (3711) | 8014 (2176) | 15,221 (4152) | 15,394 (4201) | p < 0.001 η2 = 0.915 | p = 0.188 η2 = 0.047 |
| HL-RE (n = 19) | 21,013 (5871) | 14,494 (4048) | 12,187 (3423) | 8022 (2161) | 15,207 (4171) | 15,391 (4149) | |||
| Δ HR during RE vs. pre-exercise (%) | LL-RE (n =19) | 29 (10) | 30 (13) | 28 (13) | 35 (15) | 36 (14) | 32 (13) | p < 0.001 η2 = 0.322 | p = 0.555 η2 = 0.020 |
| HL-RE (n = 19) | 26 (8) | 26 (9) | 23 (8) | 32 (12) | 29 (11) | 27 (9) | |||
| RPE during RE (point) | LL-RE (n =19) | 5 (2) | 5 (2) | 4 (2) | 5 (2) | 5 (2) | 4 (2) | p = 0.001 η2 = 0.172 | p < 0.001 η2 = 0.214 |
| HL-RE (n = 19) | 6 (1) | 5 (1) | 5 (1) | 6 (1) | 6 (1) | 6 (1) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kambic, T.; Šarabon, N.; Hadžić, V.; Lainscak, M. High-Load and Low-Load Resistance Exercise in Patients with Coronary Artery Disease: Feasibility and Safety of a Randomized Controlled Clinical Trial. J. Clin. Med. 2022, 11, 3567. https://doi.org/10.3390/jcm11133567
Kambic T, Šarabon N, Hadžić V, Lainscak M. High-Load and Low-Load Resistance Exercise in Patients with Coronary Artery Disease: Feasibility and Safety of a Randomized Controlled Clinical Trial. Journal of Clinical Medicine. 2022; 11(13):3567. https://doi.org/10.3390/jcm11133567
Chicago/Turabian StyleKambic, Tim, Nejc Šarabon, Vedran Hadžić, and Mitja Lainscak. 2022. "High-Load and Low-Load Resistance Exercise in Patients with Coronary Artery Disease: Feasibility and Safety of a Randomized Controlled Clinical Trial" Journal of Clinical Medicine 11, no. 13: 3567. https://doi.org/10.3390/jcm11133567
APA StyleKambic, T., Šarabon, N., Hadžić, V., & Lainscak, M. (2022). High-Load and Low-Load Resistance Exercise in Patients with Coronary Artery Disease: Feasibility and Safety of a Randomized Controlled Clinical Trial. Journal of Clinical Medicine, 11(13), 3567. https://doi.org/10.3390/jcm11133567








