Effects of Intensive Vibratory Treatment with a Robotic System on the Recovery of Sensation and Function in Patients with Subacute and Chronic Stroke: A Non-Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
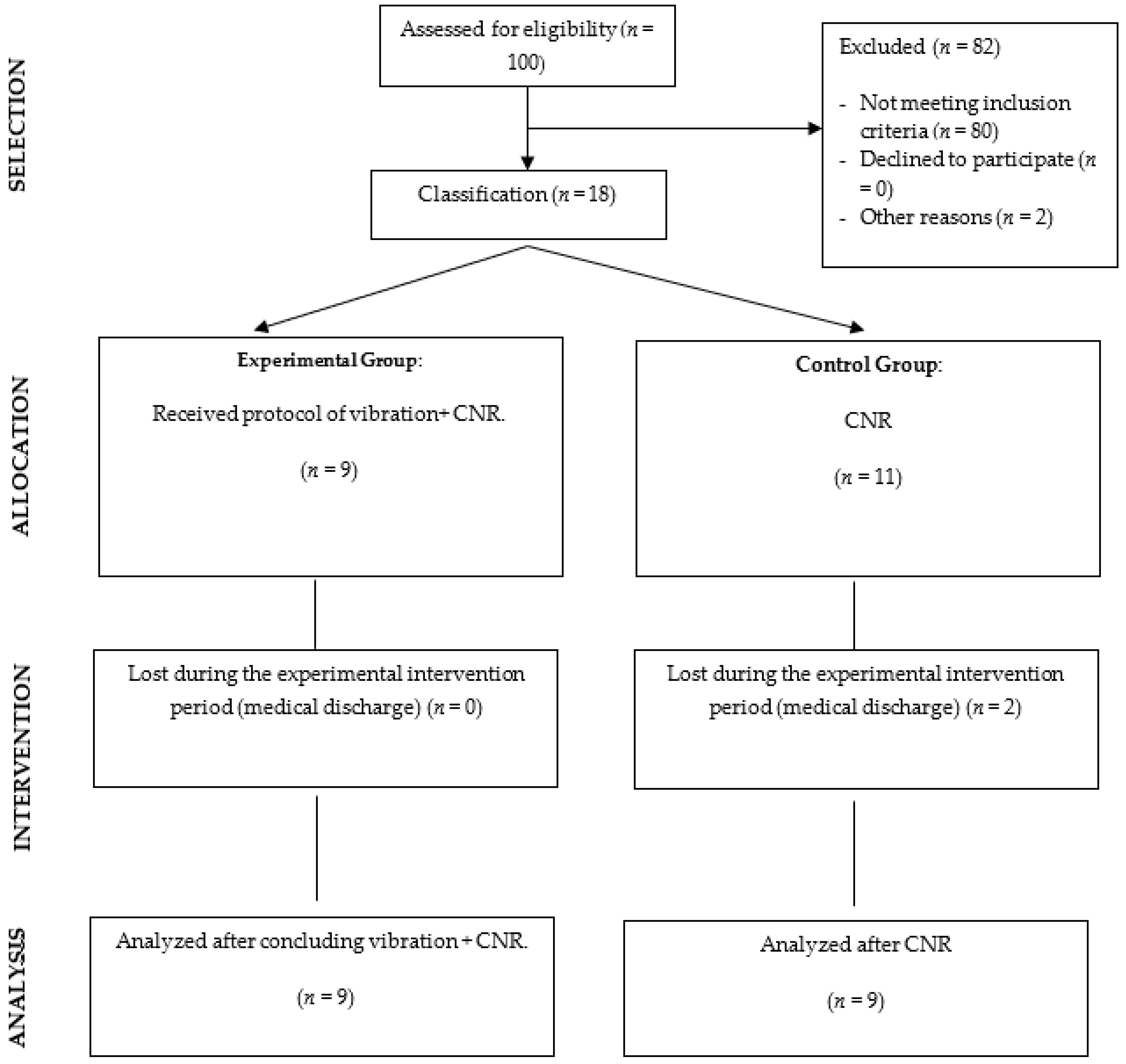
2.2. Participants
2.3. Intervention
2.4. Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Krishnamurthi, R.V.; Theadom, A.M.; Abajobir, A.A.; Mishra, S.R.; Ahmed, M.B.; Abate, K.H.; Mengistie, M.A.; Wakayo, T.; Abd-Allah, F.; et al. Global, Regional, and National Burden of Neurological Disorders during 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.A.; Resteghini, C.; Feys, P.; Lamers, I. An Overview of Systematic Reviews on Upper Extremity Outcome Measures after Stroke. BMC Neurol. 2015, 15, 29. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Huang, P.C.; Chen, Y.T.; Lin, K.C.; Yang, H.W. Effects of Mirror Therapy on Motor and Sensory Recovery in Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2013, 94, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, M.Y.; Lee, J.Y.; Jeon, Y.J.; Kim, S.; Lee, S.; Seo, B.; Choi, Y. Effects of Virtual Reality-Based Rehabilitation on Distal Upper Extremity Function and Health-Related Quality of Life: A Single-Blinded, Randomized Controlled Trial. J. Neuroeng. Rehabil. 2016, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Levin, M.F.; Weiss, P.L.; Keshner, E.A. Emergence of Virtual Reality as a Tool for Upper Limb Rehabilitation: Incorporation of Motor Control and Motor Learning Principles. Phys. Ther. 2015, 95, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, M.; Negrini, S.; Carda, S.; Lanzotti, L.; Cisari, C.; Baricich, A. The value of adding mirror therapy for upper limb motor recovery of subacute stroke patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2013, 49, 311–317. [Google Scholar]
- Yu, C.; Wang, W.; Zhang, Y.; Wang, Y.; Hou, W.; Liu, S.; Gao, C.; Wang, C.; Mo, L.; Wu, J. The Effects of Modified Constraint-Induced Movement Therapy in Acute Subcortical Cerebral Infarction. Front. Hum. Neurosci. 2017, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Da-Silva, R.H.; Moore, S.A.; Rodgers, H.; Shaw, L.; Sutcliffe, L.; van Wijck, F.; Price, C.I. Wristband Accelerometers to MotiVate Arm Exercises after Stroke (WAVES): A Pilot Randomized Controlled Trial. Clin. Rehabil. 2019, 33, 1391–1403. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Taveggia, G.; Galeri, S.; Bissolotti, L.; Mullè, C.; Imperio, G.; Valdes, K.; Borboni, A.; Negrini, S. Efficacy of Short-Term Robot-Assisted Rehabilitation in Patients with Hand Paralysis After Stroke: A Randomized Clinical Trial. Hand 2018, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Orihuela-Espina, F.; Roldán, G.F.; Sánchez-Villavicencio, I.; Palafox, L.; Leder, R.; Sucar, L.E.; Hernández-Franco, J. Robot Training for Hand Motor Recovery in Subacute Stroke Patients: A Randomized Controlled Trial. J. Hand Ther. 2016, 29, 51–57. [Google Scholar] [CrossRef]
- Yue, Z.; Zhang, X.; Wang, J. Hand Rehabilitation Robotics on Poststroke Motor Recovery. Behav. Neurol. 2017, 2017, 3908135. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, M.; Polonio-López, B.; Corregidor-Sánchez, A.I.; Martín-Conty, J.L.; Mohedano-Moriano, A.; Criado-Álvarez, J.J. Effects of Specific Virtual Reality-Based Therapy for the Rehabilitation of the Upper Limb Motor Function Post-Ictus: Randomized Controlled Trial. Brain Sci. 2021, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Mekbib, D.B.; Han, J.; Zhang, L.; Fang, S.; Jiang, H.; Zhu, J.; Roe, A.W.; Xu, D. Virtual Reality Therapy for Upper Limb Rehabilitation in Patients with Stroke: A Meta-Analysis of Randomized Clinical Trials. Brain Inj. 2020, 34, 456–465. [Google Scholar] [CrossRef]
- AMADEO®: The Pioneer in Finger-Hand-Rehabilitation| Tyrotherapy. Available online: https://tyromotion.com/en/products/amadeo/ (accessed on 30 May 2022).
- Stein, J.; Bishop, L.; Gillen, G.; Helbok, R. Robot-Assisted Exercise for Hand Weakness after Stroke: A Pilot Study. Am. J. Phys. Med. Rehabil. Assoc. Acad. Physiatr. 2011, 90, 887–894. [Google Scholar] [CrossRef]
- Sale, P.; Mazzoleni, S.; Lombardi, V.; Galafate, D.; Massimiani, M.P.; Posteraro, F.; Damiani, C.; Franceschini, M. Recovery of Hand Function with Robot-Assisted Therapy in Acute Stroke Patients: A Randomized-Controlled Trial. Int. J. Rehabil. Res. 2014, 37, 236–242. [Google Scholar] [CrossRef]
- Kiran, S. What Is the Nature of Poststroke Language Recovery and Reorganization? ISRN Neurol. 2012, 2012, 786872. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Flor, H. Cortical Reorganisation and Chronic Pain: Implications for Rehabilitation. J. Rehabil. Med. 2003, 35, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Lakshminarayanan, K.; Lauer, A.W.; Ramakrishnan, V.; Webster, J.G.; Seo, N.J. Application of Vibration to Wrist and Hand Skin Affects Fingertip Tactile Sensation. Physiol. Rep. 2015, 3, e12465. [Google Scholar] [CrossRef]
- VA/DoD Clinical Practice Guidelines Home. Available online: https://www.healthquality.va.gov/ (accessed on 30 May 2022).
- Pendleton, H.M.; Schultz-Krohn, W. Pedretti’s Occupational Therapy: Practice Skills for Physical Dysfunction; Elsevier Health Sciences: St. Louis, MO, USA, 2013; pp. 10–28. [Google Scholar]
- Weinstein, S. Fifty Years of Somatosensory Research: From the Semmes-Weinstein Monofilaments to the Weinstein Enhanced Sensory Test. J. Hand Ther. 1993, 6, 11–22. [Google Scholar] [CrossRef]
- Suda, M.; Kawakami, M.; Okuyama, K.; Ishii, R.; Oshima, O.; Hijikata, N.; Nakamura, T.; Oka, A.; Kondo, K.; Liu, M. Validity and Reliability of the Semmes-Weinstein Monofilament Test and the Thumb Localizing Test in Patients with Stroke. Front. Neurol. 2021, 11, 625917. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K.; Fukutake, T.; Kawamura, M. “Thumb Localizing Test” for Detecting a Lesion in the Posterior Column-Medial Lemniscal System. J. Neurol. Sci. 1999, 167, 45–49. [Google Scholar] [CrossRef]
- Raji, P.; Ansari, N.N.; Naghdi, S.; Forogh, B.; Hasson, S. Relationship between Semmes-Weinstein Monofilaments Perception Test and Sensory Nerve Conduction Studies in Carpal Tunnel Syndrome. NeuroRehabilitation 2014, 35, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.; Arya, K.N. Stroke-Related Motor Outcome Measures: Do They Quantify the Neurophysiological Aspects of Upper Extremity Recovery? J. Bodyw. Mov. Ther. 2014, 18, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Umeji, A.; Uchita, A.; Hashimoto, Y.; Takebayashi, T.; Takahashi, K.; Uchiyama, Y.; Domen, K. Clinimetric Properties of the Fugl-Meyer Assessment with Adapted Guidelines for the Assessment of Arm Function in Hemiparetic Patients after Stroke. Top. Stroke Rehabil. 2018, 25, 500–508. [Google Scholar] [CrossRef]
- Sullivan, K.J.; Tilson, J.K.; Cen, S.Y.; Rose, D.K.; Hershberg, J.; Correa, A.; Gallichio, J.; McLeod, M.; Moore, C.; Wu, S.S.; et al. Fugl-Meyer Assessment of Sensorimotor Function after Stroke: Standardized Training Procedure for Clinical Practice and Clinical Trials. Stroke 2011, 42, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Singer, B.; Garcia-Vega, J. The Fugl-Meyer Upper Extremity Scale. J. Physiother. 2017, 63, 53. [Google Scholar] [CrossRef] [Green Version]
- van der Lee, J.H.; Beckerman, H.; Knol, D.L.; de Vet, H.C.W.; Bouter, L.M. Clinimetric Properties of the Motor Activity Log for the Assessment of Arm Use in Hemiparetic Patients. Stroke 2004, 35, 1410–1414. [Google Scholar] [CrossRef] [Green Version]
- Uswatte, G.; Taub, E.; Morris, D.; Light, K.; Thompson, P.A. The Motor Activity Log-28: Assessing Daily Use of the Hemiparetic Arm after Stroke. Neurology 2006, 67, 1189–1194. [Google Scholar] [CrossRef]
- Contribución de La Adaptación y Validación de La Escala Sis-16 (Stroke Impact Scale) En El Manejo de La Rehabilitación de Pacientes Con Ictus—E-Prints Complutense. Available online: https://eprints.ucm.es/id/eprint/11558/ (accessed on 31 May 2022).
- Duncan, P.W.; Bode, R.K.; Lai, S.M.; Perera, S. Rasch Analysis of a New Stroke-Specific Outcome Scale: The Stroke Impact Scale. Arch. Phys. Med. Rehabil. 2003, 84, 950–963. [Google Scholar] [CrossRef]
- Collins, D.F.; Refshauge, K.M.; Todd, G.; Gandevia, S.C. Cutaneous Receptors Contribute to Kinesthesia at the Index Finger, Elbow, and Knee. J. Neurophysiol. 2005, 94, 1699–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, R. Meta-Analytic Procedures for Social Research; Sage: Newbury Park, CA, USA, 1991. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Hagbarth, K.E.; Homma, I.; Wallin, U. Excitation from Skin Receptors Contributing to the Tonic Vibration Reflex in Man. Brain Res. 1978, 150, 194–197. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Functional Brain Imaging Based on ERD/ERS. Vis. Res. 2001, 41, 1257–1260. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Liu, T.; Szarkowski, R.; Rios, C.; Ashe, J.; He, B. Negative Covariation between Task-Related Responses in Alpha/Beta-Band Activity and BOLD in Human Sensorimotor Cortex: An EEG and FMRI Study of Motor Imagery and Movements. Neuroimage 2010, 49, 2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, C.; Rong, W.; Li, W.; Xie, Y.; Hu, X.; Zheng, Y. The Effects of Upper-Limb Training Assisted with an Electromyography-Driven Neuromuscular Electrical Stimulation Robotic Hand on Chronic Stroke. Front. Neurol. 2017, 8, 679. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, C.; Xiang, Y.; Ji, L.; Hu, H.; Liu, Y. Study of the Activation in Sensorimotor Cortex and Topological Properties of Functional Brain Network Following Focal Vibration on Healthy Subjects and Subacute Stroke Patients: An EEG Study. Brain Res. 2019, 1722, 146338. [Google Scholar] [CrossRef] [PubMed]
- Feys, H.M.; de Weerdt, W.J.; Selz, B.E.; Cox Steck, G.A.; Spichiger, R.; Vereeck, L.E.; Putman, K.D.; van Hoydonck, G.A. Effect of a Therapeutic Intervention for the Hemiplegic Upper Limb in the Acute Phase after Stroke: A Single-Blind, Randomized, Controlled Multicenter Trial. Stroke 1998, 29, 785–792. [Google Scholar] [CrossRef]
- Macerollo, A.; Palmer, C.; Foltynie, T.; Korlipara, P.; Limousin, P.; Edwards, M.; Kilner, J.M. High-Frequency Peripheral Vibration Decreases Completion Time on a Number of Motor Tasks. Eur. J. Neurosci. 2018, 48, 1789–1802. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, R.S.; Accorinti, M.; Porcari, B.; Carioti, L.; Ciatto, L.; Billeri, L.; Andronaco, V.A.; Galletti, F.; Filoni, S.; Naro, A. Does Hand Robotic Rehabilitation Improve Motor Function by Rebalancing Interhemispheric Connectivity after Chronic Stroke? Encouraging Data from a Randomised-Clinical-Trial. Clin. Neurophysiol. 2019, 130, 767–780. [Google Scholar] [CrossRef]
- Costantino, C.; Galuppo, L.; Romiti, D. Short-Term Effect of Local Muscle Vibration Treatment versus Sham Therapy on Upper Limb in Chronic Post-Stroke Patients: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 32–40. [Google Scholar] [CrossRef]
- Scalha, T.B.; Miyasaki, E.; Lima, N.M.F.V.; Borges, G. Correlations between Motor and Sensory Functions in Upper Limb Chronic Hemiparetics after Stroke. Arq. Neuro-Psiquiatr. 2011, 69, 624–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grefkes, C.; Fink, G.R. Reorganization of Cerebral Networks after Stroke: New Insights from Neuroimaging with Connectivity Approaches. Brain 2011, 134, 1264–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| CNR | Vibration + CNR | Total | |
|---|---|---|---|
| n = 9 | n = 9 | n = 18 | |
| Age M (SD) | 72.89 (10.203) | 66.56 (9.876) | 69.72 (10.272) |
| Range of age (years) | 54–85 | 45–77 | 45–85 |
| Sex [N (%)] | |||
| 4 (44%) | 8 (89%) | 12 (67%) |
| 5 (56%) | 1 (11%) | 6 (33%) |
| Disease duration (months) M (SD) | 8.44 (4.639) | 9.222 (3.382) | 8.83 (3.959) |
| Range of disease duration (months) | 3–14 | 4–12 | 3–14 |
| Variable | Control Group | Experimental Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | p-Value | Rosenthal’s r | Median (IQR) | p-Value | Rosenthal’s r | |||
| SEMMES–WEINSTEIN MONOFILAMENT TEST | Hand | Pre | 6.65 (3.39) | 0.593 | 0.12 | 5.57 (4.19) | 0.012 * | 0.60 |
| Post | 5.98 (3.39) | 4.47 (4.07) | ||||||
| Forearm | Pre | 5.65 (3.39) | 1.000 | 0.00 | 4.56 (3.39) | 0.161 | 0.33 | |
| Post | 6.15 (3.39) | 4.56 (13.75) | ||||||
| Arm | Pre | 6.65 (3.39) | 0.276 | 0.25 | 4.65 (3.39) | 0.018 * | 0.56 | |
| Post | 6.65 (3.39) | 4.43 (4.19) | ||||||
| Shoulder | Pre | 6.65 (2.69) | 0.180 | 0.31 | 4.56 (3.39) | 0.027 * | 0.53 | |
| Post | 6.65 (3.39) | 4.31 (4.19) | ||||||
| FMA-EU | Sensation | Pre | 4.00 (10.00) | 0.180 | 0.31 | 6.00 (6.00) | 0.172 | 0.32 |
| Post | 6.00 (10.00) | 6.00 (10.00) | ||||||
| Joint pain | Pre | 20.00 (22.00) | 0.221 | 0.28 | 20.00 (7.00) | 0.106 | 0.38 | |
| Post | 20.00 (12.00) | 20.00 (7.00) | ||||||
| Variable | Control Group | Experimental Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | p-Value | Rosenthal’s r | Median (IQR) | p-Value | Rosenthal’s r | |||
| FMA-EU | Motor function | Pre | 33.00 (57.00) | 0.249 | 0.27 | 14.00 (54.00) | 0.028 * | 0.52 |
| Post | 33.00 (55.00) | 18.00 (56.00) | ||||||
| Passive joint motion | Pre | 20.00 (22.00) | 0.167 | 0.32 | 20.00 (19.00) | 0.461 | 0.17 | |
| Post | 21.00 (22.00) | 19.00 (11.00) | ||||||
| MAL-14 | Amount scale | Pre | 0.00 (54.00) | 0.144 | 0.34 | 15.00 (38.00) | 0.011 * | 0.60 |
| Post | 2.00 (62.00) | 21.00 (51.00) | ||||||
| How well scale | Pre | 0.00 (55.00) | 0.109 | 0.37 | 14.00 (29.00) | 0.008 * | 0.63 | |
| Post | 2.00 (62.00) | 18.00 (46.00) | ||||||
| SIS-16 | Pre | 53.00 (49.00) | 1.000 | 0.00 | 46.00 (42.00) | 0.008 * | 0.62 | |
| Post | 53.00 (49.00) | 55.00 (46.00) | ||||||
| Variable | Median (Interquartile Range) | p-Value | Rosenthal’ r | |||
|---|---|---|---|---|---|---|
| Control | Experimental | |||||
| Group | Group | |||||
| Post | Semmes–Weinstein Monofilament Test | Hand | 5.98 (3.39) | 4.47 (4.07) | 0.076 | 0.41 |
| Forearm | 6.15 (3.39) | 4.56 (13.75) | 0.231 | 0.28 | ||
| Arm | 6.65 (3.39) | 4.43 (4.19) | 0.060 | 0.44 | ||
| Shoulder | 6.65 (3.39) | 4.31 (4.19) | 0.077 | 0.43 | ||
| FMA-EU | Sensation | 6.00 (10.00) | 6.00 (10.00) | 0.449 | 0.17 | |
| Joint Pain | 20.00 (12.00) | 20.00 (7.00) | 0.655 | 0.10 | ||
| Motor Function | 33.00 (55.00) | 18.00 (56.00) | 0.894 | 0.03 | ||
| Passive joint motion | 21.00 (22.00) | 19.00 (11.00) | 0.304 | 0.24 | ||
| MAL-14 | Amount scale | 2.00 (62.00) | 18.00 (46.00) | 0.021 * | 0.55 | |
| How well scale | 2.00 (62.00) | 18.00 (46.00) | 0.037 * | 0.50 | ||
| SIS-16 | 53.00 (49.00) | 55.00 (46.00) | 0.546 | 0.14 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pérez, M.P.; Sánchez-Herrera-Baeza, P.; Cano-de-la-Cuerda, R.; Camacho-Montaño, L.R.; Serrada-Tejeda, S.; Pérez-de-Heredia-Torres, M. Effects of Intensive Vibratory Treatment with a Robotic System on the Recovery of Sensation and Function in Patients with Subacute and Chronic Stroke: A Non-Randomized Clinical Trial. J. Clin. Med. 2022, 11, 3572. https://doi.org/10.3390/jcm11133572
Rodríguez-Pérez MP, Sánchez-Herrera-Baeza P, Cano-de-la-Cuerda R, Camacho-Montaño LR, Serrada-Tejeda S, Pérez-de-Heredia-Torres M. Effects of Intensive Vibratory Treatment with a Robotic System on the Recovery of Sensation and Function in Patients with Subacute and Chronic Stroke: A Non-Randomized Clinical Trial. Journal of Clinical Medicine. 2022; 11(13):3572. https://doi.org/10.3390/jcm11133572
Chicago/Turabian StyleRodríguez-Pérez, Mª Pilar, Patricia Sánchez-Herrera-Baeza, Roberto Cano-de-la-Cuerda, Lucía Rocío Camacho-Montaño, Sergio Serrada-Tejeda, and Marta Pérez-de-Heredia-Torres. 2022. "Effects of Intensive Vibratory Treatment with a Robotic System on the Recovery of Sensation and Function in Patients with Subacute and Chronic Stroke: A Non-Randomized Clinical Trial" Journal of Clinical Medicine 11, no. 13: 3572. https://doi.org/10.3390/jcm11133572
APA StyleRodríguez-Pérez, M. P., Sánchez-Herrera-Baeza, P., Cano-de-la-Cuerda, R., Camacho-Montaño, L. R., Serrada-Tejeda, S., & Pérez-de-Heredia-Torres, M. (2022). Effects of Intensive Vibratory Treatment with a Robotic System on the Recovery of Sensation and Function in Patients with Subacute and Chronic Stroke: A Non-Randomized Clinical Trial. Journal of Clinical Medicine, 11(13), 3572. https://doi.org/10.3390/jcm11133572








