Sarcopenia as Manifested by L3SMI Is Associated with Increased Long-Term Mortality amongst Internal Medicine Patients—A Prospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Variable Definition
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Frailty Measurements
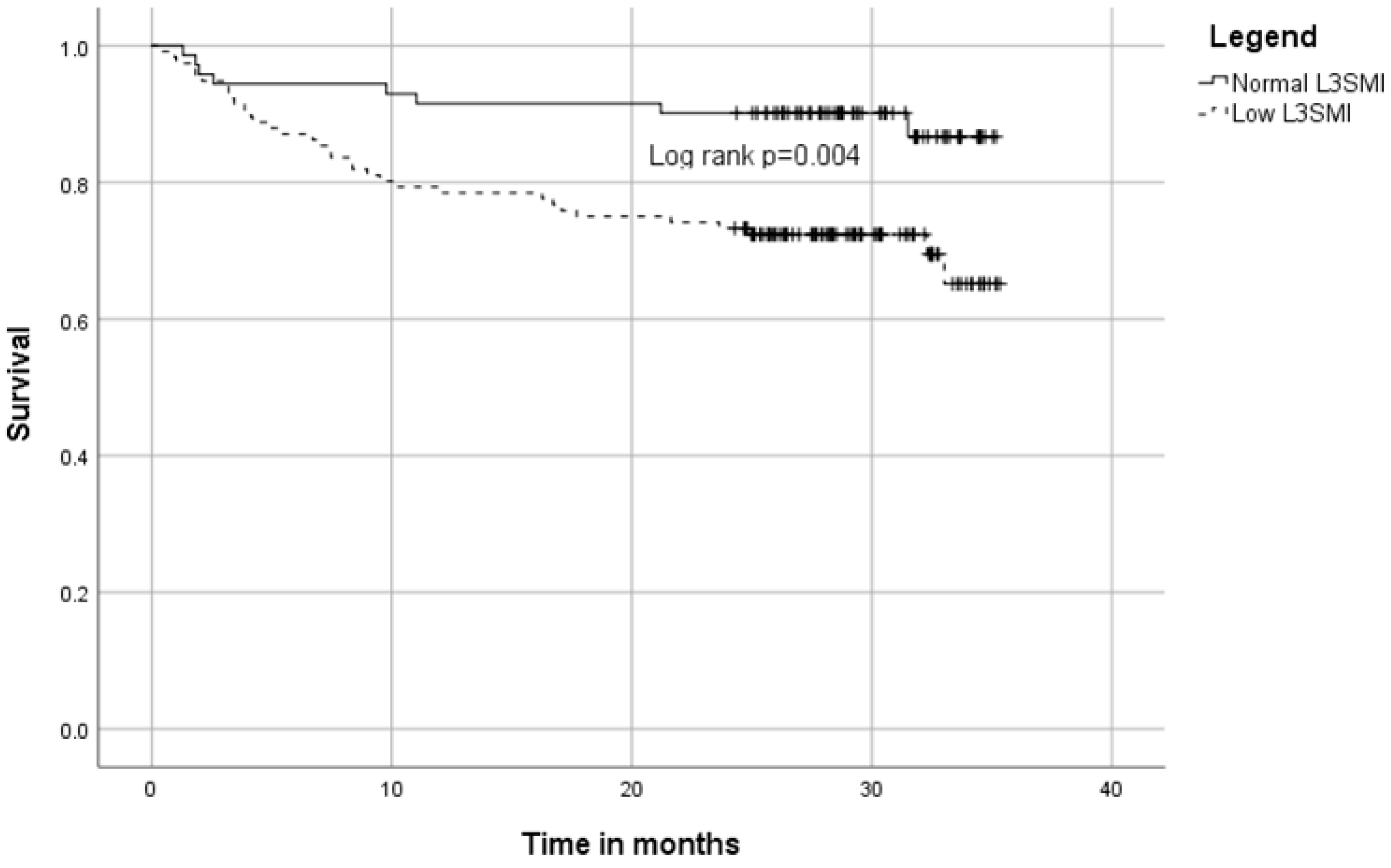
3.3. Long Term Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Punyanitya, M.; Wang, Z.M.; Gallagher, D.; St.-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nishide, K.; Okuno, S.; Shoji, T.; Emoto, M.; Tsuda, A.; Nakatani, S.; Imanishi, Y.; Ishimura, E.; Yamakawa, T.; et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 2019, 20, 105. [Google Scholar] [CrossRef]
- Segev, A.; Itelman, E.; Avaky, C.; Negru, L.; Shenhav-Saltzman, G.; Grupper, A.; Wasserstrum, Y.; Segal, G. Low ALT Levels Associated with Poor Outcomes in 8700 Hospitalized Heart Failure Patients. J. Clin. Med. 2020, 9, 3185. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Peltz-Sinvani, N.; Klempfner, R.; Ramaty, E.; Sela, B.A.; Goldenberg, I.; Segal, G. Low ALT Levels Independently Associated with 22-Year All-Cause Mortality Among Coronary Heart Disease Patients. J. Gen. Intern. Med. 2016, 31, 209–214. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Cooper, C.; Rizzoli, R.; Kanis, J.A.; Appelboom, G.; Bautmans, I.; Bischoff-Ferrari, H.; Boers, M.; Brandi, M.L.; Bruyere, O.; et al. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin. Exp. Res. 2016, 28, 47–58. [Google Scholar] [CrossRef]
- Han, A.; Bokshan, S.L.; Marcaccio, S.E.; Depasse, J.M.; Daniels, A.H. Diagnostic Criteria and Clinical Outcomes in Sarcopenia Research: A Literature Review. J. Clin. Med. 2018, 7, 70. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, R.; Wan, Q.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med. 2020, 9, 7964–7978. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Seino, S.; Abe, T.; Nofuji, Y.; Yokoyama, Y.; Amano, H.; Nishi, M.; Taniguchi, Y.; Narita, M.; Fujiwara, Y.; et al. Sarcopenia: Prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J. Cachexia Sarcopenia Muscle 2021, 12, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, Epidemiology, and Pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Portal, D.; Hofstetter, L.; Eshed, I.; Dan-Lantsman, C.; Sella, T.; Urban, D.; Onn, A.; Bar, J.; Segal, G. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag. Res. 2019, 11, 2579–2588. [Google Scholar] [CrossRef]
- Morley, J.E. Treatment of sarcopenia: The road to the future. J. Cachexia Sarcopenia Muscle 2018, 9, 1196–1199. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- TOol 3H: Morse Fall Scale for Identifying Fall Risk Factors. Available online: https://scholar.google.com/scholar?lookup=0&q=TOol+3H+:+morse+fall+scale+for+identifying+fall+risk+factors&hl=en&as_sdt=0,5#d=gs_cit&u=%2Fscholar%3Fq%3Dinfo%3AsqFPoatiGhgJ%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Den (accessed on 24 March 2022).
- Exemplary Research for Nursing and Midwifery—Google Books. Available online: https://books.google.co.il/books?hl=en&lr=&id=-aiBAgAAQBAJ&oi=fnd&pg=PA69&dq=an+investigation+of+geriatric+nursing+problems+in+hospital&ots=9wiWRoV0fp&sig=53FDphwrUqXy5ntYGqCJqKimlA8&redir_esc=y#v=onepage&q=an%20investigation%20of%20geriatric%20nursing%20problems%20in%20hospital&f=false (accessed on 24 March 2022).
- Cruz-Jentoft, A.J.; Landi, F. Sarcopenia. Clin. Med. 2014, 14, 183–186. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Meza-Junco, J.; Baracos, V.E.; Prado, C.M.M.; Ma, M.; Meeberg, G.; Beaumont, C.; Tandon, P.; Esfandiari, N.; Sawyer, M.B.; et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014, 20, 640–648. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef]
- Baracos, V.; Kazemi-Bajestani, S.M.R. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int. J. Biochem. Cell Biol. 2013, 45, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torralvo, F.J.; Ruiz-García, I.; Contreras-Bolívar, V.; González-Almendros, I.; Ruiz-Vico, M.; Abuín-Fernández, J.; Barrios, M.; Alba, E.; Olveira, G. Ct-determined sarcopenia in glim-defined malnutrition and prediction of 6-month mortality in cancer inpatients. Nutrients 2021, 13, 2647. [Google Scholar] [CrossRef] [PubMed]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E.; Bulger, E.; Kozar, R.A.; for the Nutrition and Rehabilitation Investigators Consortium (NUTRIC). Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care 2013, 17, R206. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients | Sarcopenic (n = 116) | Non-Sarcopenic (n = 71) | p Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 70.4 ± 9.2 | 72.0 ± 8.9 | 67.7 ± 9.1 | 0.002 |
| Male, n (%) | 111 (59.4) | 79 (68.1) | 32 (45.1) | 0.002 |
| BMI, median (IQR) | 25.8 (23.1–28.7) | 25.0 (22.4–27.5) | 27.7 (25.0–32.0) | <0.001 |
| Comorbidity, n (%) | ||||
| CHF | 17 (9.1) | 8 (6.9) | 9 (52.9) | 0.182 |
| COPD | 38 (20.3) | 26 (22.4) | 12 (16.9) | 0.363 |
| CRF | 32 (17.1) | 23 (19.8) | 9 (12.7) | 0.208 |
| DM | 82 (43.9) | 52 (44.8) | 30 (42.3) | 0.731 |
| HTN | 108 (57.8) | 66 (56.9) | 42 (59.2) | 0.762 |
| IHD | 55 (29.4) | 35 (30.2) | 20 (28.2) | 0.770 |
| Solid malignancy | 33 (17.6) | 22 (19.0) | 11 (15.5) | 0.545 |
| Cardiac cause of admission, n (%) | 30 (16) | 19 (16.4) | 11 (15.5) | 0.873 |
| Infectious cause of admission, n (%) | 24 (12.8) | 13 (11.2) | 11 (15.5) | 0.395 |
| MAMC, median (IQR) | 26.9 (25.0–29.5) | 26.2 (24.6–28.9) | 28.1 (26.2–30.7) | <0.001 |
| ALT below 12 | 38 (20.5) | 31 (26.7) | 7 (10.1) | 0.007 |
| FRAIL score 3+ | 115 (61.5) | 75 (64.7) | 40 (56.3) | 0.257 |
| One Year Survival | All Time Survival | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Low L3SMI | 2.60 (1.06–6.37) | 0.036 | 2.91 (1.35–6.29) | 0.007 |
| Age | 1.06 (1.02–1.11) | 0.008 | 1.06 (1.02–1.10) | 0.003 |
| Female sex | 0.72 (0.34–1.54) | 0.401 | 0.70 (0.37–1.33) | 0.278 |
| BMI | 0.98 (0.91–1.06) | 0.688 | 0.97 (0.91–1.08) | 0.382 |
| Comorbidity | ||||
| IHD | 1.05 (0.48–2.30) | 0.901 | 0.76 (0.37–1.54) | 0.441 |
| CHF | 3.05 (1.25–7.47) | 0.015 | 2.06 (0.87–4.89) | 0.102 |
| HTN | 1.11 (0.54–2.31) | 0.775 | 0.98 (0.53–1.80) | 0.934 |
| CRF | 2.20 (1.01–4.80) | 0.048 | 1.88 (0.95–3.75) | 0.072 |
| DM | 1.75 (0.85–3.61) | 0.127 | 1.99 (1.08–3.69) | 0.028 |
| COPD | 1.77 (0.81–3.86) | 0.152 | 1.75 (0.90–3.43) | 0.102 |
| Solid malignancy | 0.90 (0.34–2.34) | 0.821 | 0.95 (0.42–2.14) | 0.898 |
| Marital Status | 0.099 | 0.107 | ||
| Married | REF | |||
| Divorced | 1.05 (0.35–3.13) | 0.90 (0.33–2.26) | ||
| Widowed | 2.74 (1.22–6.15) | 2.31 (1.14–4.69) | ||
| Other | -- | -- | ||
| Albumin | 0.29 (0.15–0.57) | <0.001 | 0.41 (0.23–0.72) | 0.002 |
| Hemoglobin | 0.85 (0.73–0.98) | 0.030 | 0.80 (0.70–0.91) | <0.001 |
| MAMC | 0.96 (0.87–1.07) | 0.503 | 0.94 (0.86–1.03) | 0.182 |
| ALT above 12 | 0.69 (0.31–1.55) | 0.370 | 0.67 (0.34–1.34) | 0.255 |
| FRAIL score 3+ | 1.79 (0.80–4.01) | 0.160 | 1.24 (0.66–2.33) | 0.508 |
| One Year Survival | All Time Survival | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Low L3SMI | 2.07 (0.81–5.27) | 0.127 | 2.38 (1.07–5.29) | 0.034 |
| Age | 1.05 (1.01–1.10) | 0.026 | 1.05 (1.01–1.09) | 0.016 |
| Female sex | 0.91 (0.42–1.99) | 0.814 | 0.91 (0.47–1.76) | 0.777 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portal, D.; Melamed, G.; Segal, G.; Itelman, E. Sarcopenia as Manifested by L3SMI Is Associated with Increased Long-Term Mortality amongst Internal Medicine Patients—A Prospective Cohort Study. J. Clin. Med. 2022, 11, 3500. https://doi.org/10.3390/jcm11123500
Portal D, Melamed G, Segal G, Itelman E. Sarcopenia as Manifested by L3SMI Is Associated with Increased Long-Term Mortality amongst Internal Medicine Patients—A Prospective Cohort Study. Journal of Clinical Medicine. 2022; 11(12):3500. https://doi.org/10.3390/jcm11123500
Chicago/Turabian StylePortal, Doron, Guy Melamed, Gad Segal, and Edward Itelman. 2022. "Sarcopenia as Manifested by L3SMI Is Associated with Increased Long-Term Mortality amongst Internal Medicine Patients—A Prospective Cohort Study" Journal of Clinical Medicine 11, no. 12: 3500. https://doi.org/10.3390/jcm11123500
APA StylePortal, D., Melamed, G., Segal, G., & Itelman, E. (2022). Sarcopenia as Manifested by L3SMI Is Associated with Increased Long-Term Mortality amongst Internal Medicine Patients—A Prospective Cohort Study. Journal of Clinical Medicine, 11(12), 3500. https://doi.org/10.3390/jcm11123500






