Systematic Cardiovascular Screening in Olympic Athletes before and after SARS-CoV-2 Infection
Abstract
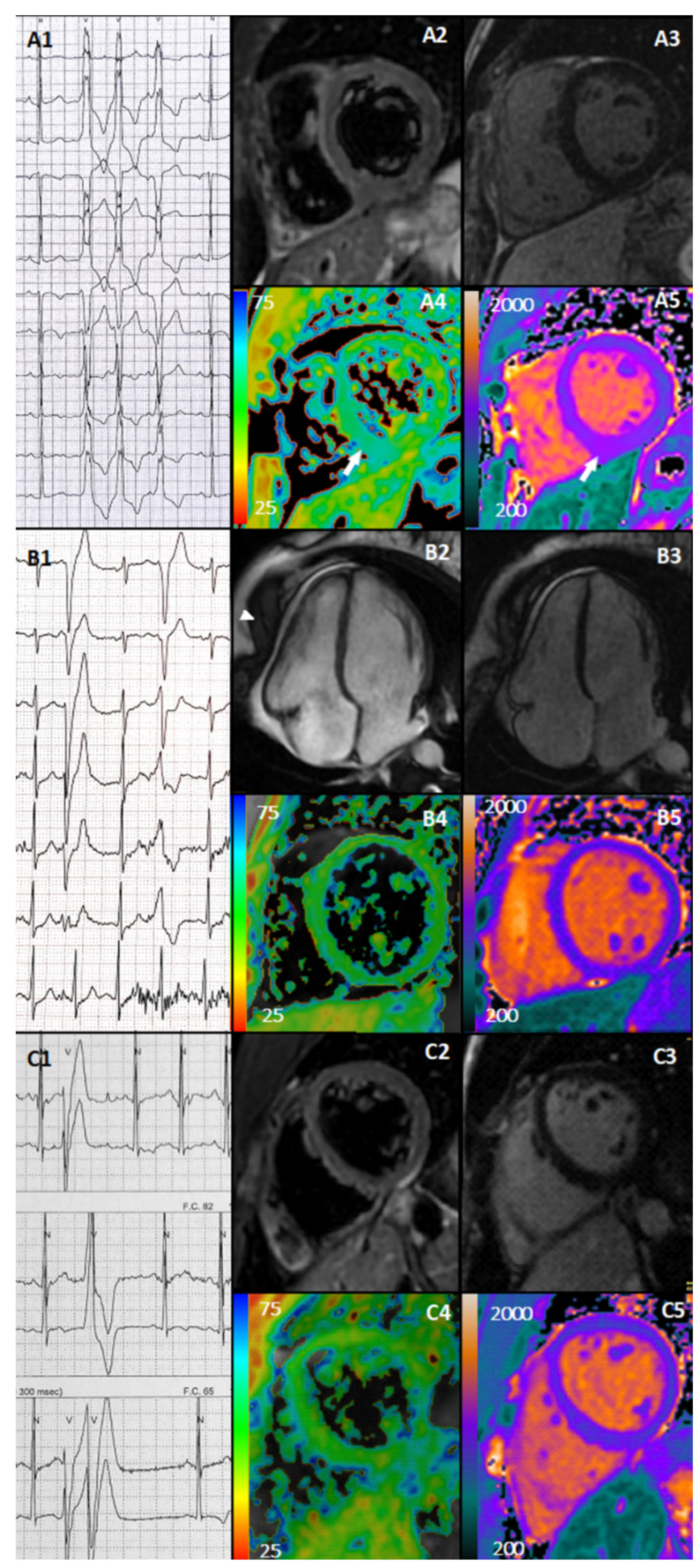
:1. Introduction
2. Methods
2.1. Study Population
- -
- Physical evaluation and medical history: specifically, the duration of infection (time from the first positive NPS to the first negative), the time from the negative NPS to evaluation, the presence of symptoms related to the infection [16], and eventually any medications were collected.
- -
- Blood tests: the list of the blood tests is listed in the Supplementary Materials.
- -
- Pulmonary function tests (PFTs): parameters measured are listed in the Supplementary Materials.
- -
- 12-lead resting electrocardiogram (ECG): ECG patterns were analyzed according to the international criteria [17]. In case of abnormalities, ECGs post-COVID-19 were compared to the ones recorded previously.
- -
- Transthoracic echocardiogram (TTE): left and right ventricle (LV and RV) dimension, wall thickness, global and regional systolic function, indexes of diastolic function, as well as the presence of pericardial effusion were evaluated. Imaging interpretation was based off the international recommendation [18,19].
- -
- Cardiopulmonary exercise test (CPET): cardiopulmonary performance parameters were collected, and are listed in the Supplementary Materials [20]. Exercise-induced ventricular arrhythmias were evaluated as well.
- -
- 24-h ECG monitoring: the occurrence of rhythm and conduction abnormalities, and supra-ventricular and ventricular arrhythmias were investigated. The burden of premature ventricular contractions (PVCs) was arbitrarily classified as <50, 50–500, >500 PVCs/24 h.
2.2. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.; Meester, S.; Yanamala, N.; Patel, H.B.; Balcik, B.J.; Casaclang-Verzosa, G.; Seetharam, K.; Riveros, D.; Beto, R.J., 2nd; Balla, S.; et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc. Imaging 2021, 14, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; DiFiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Sills, A.K.; et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021, 6, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Malek, L.A.; Marczak, M.; Milosz-Wieczorek, B.; Konopka, M.; Braksator, W.; Drygas, W.; Krzywanski, J. Cardiac involvement in consecutive elite athletes recovered from COVID-19: A magnetic resonance study. J. Magn. Reson. Imaging JMRI 2021, 53, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Vago, H.; Szabo, L.; Dohy, Z.; Merkely, B. Cardiac magnetic resonance findings in patients recovered from COVID-19: Initial experiences in elite athletes. JACC Cardiovasc. Imaging 2021, 14, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Starekova, J.; Bluemke, D.A.; Bradham, W.S.; Eckhardt, L.L.; Grist, T.M.; Kusmirek, J.E.; Purtell, C.S.; Schiebler, M.L.; Reeder, S.B. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021, 6, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E.; Parikh, A.; Dendy, J.M.; Diamond, A.B.; George-Durrett, K.; Fish, F.A.; Slaughter, J.C.; Fitch, W.; Hughes, S.G.; Soslow, J.H. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (compete cmr). Circulation 2021, 143, 609–612. [Google Scholar] [CrossRef]
- Moulson, N.; Petek, B.J.; Drezner, J.A.; Harmon, K.G.; Kliethermes, S.A.; Patel, M.R.; Baggish, A.L. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation 2021, 144, 256–266. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: Results from the big ten COVID-19 cardiac registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef]
- Hendrickson, B.S.; Stephens, R.E.; Chang, J.V.; Amburn, J.M.; Pierotti, L.L.; Johnson, J.L.; Hyden, J.C.; Johnson, J.N.; Philip, R.R. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: Results of an algorithm-guided screening. Circulation 2021, 143, 1926–1928. [Google Scholar] [CrossRef]
- Szabó, L.; Juhász, V.; Dohy, Z.; Fogarasi, C.; Kovács, A.; Lakatos, B.K.; Kiss, O.; Sydó, N.; Csulak, E.; Suhai, F.I.; et al. Is cardiac involvement prevalent in highly trained athletes after SARS-CoV-2 infection? A cardiac magnetic resonance study using sex-matched and age-matched controls. Br. J. Sports Med. 2021, 56, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.T.; Marwaha, S.; Malhotra, A.; Iqbal, Z.; Hughes, C.; Börjesson, M.; Niebauer, J.; Pelliccia, A.; Schmied, C.; Serratosa, L.; et al. Exercise in the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) era: A question and answer session with the experts endorsed by the section of sports cardiology & exercise of the european association of preventive cardiology (eapc). Eur. J. Prev. Cardiol. 2020, 27, 1242–1251. [Google Scholar] [PubMed]
- Phelan, D.; Kim, J.H.; Elliott, M.D.; Wasfy, M.M.; Cremer, P.; Johri, A.M.; Emery, M.S.; Sengupta, P.P.; Sharma, S.; Martinez, M.W.; et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: An expert consensus statement. JACC Cardiovasc. Imaging 2020, 13, 2635–2652. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fmsi.it/images/pdf/archivio_news/CS_Raccomandazioni_FMSI_2020.04.04-2.pdf (accessed on 15 October 2020).
- Wilson, M.G.; Hull, J.H.; Rogers, J.; Pollock, N.; Dodd, M.; Haines, J.; Harris, S.; Loosemore, M.; Malhotra, A.; Pieles, G.; et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020, 54, 1157–1161. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Lynch, J.B.; Del Rio, C. Mild or moderate COVID-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. Internal reviewers for E, Eacvi. European association of preventive cardiology (eapc) and european association of cardiovascular imaging (eacvi) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. European Association for Cardiovascular P, Rehabilitation, American Heart A. Eacpr/aha scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Heidbüchel, H.; Corrado, D.; Biffi, A.; Hoffmann, E.; Panhuyzen-Goedkoop, N.; Hoogsteen, J.; Delise, P.; Hoff, P.I.; Pelliccia, A.; Saenen, J.; et al. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part 2: Ventricular arrhythmias, channelopathies, and implantable defibrillators. Eur. J. Prev. Cardiol. 2021, 23, 147–148. [Google Scholar] [CrossRef]
- Kelle, S.; Bucciarelli-Ducci, C.; Judd, R.M.; Kwong, R.Y.; Simonetti, O.; Plein, S.; Raimondi, F.; Weinsaft, J.W.; Wong, T.C.; Carr, J. Society for cardiovascular magnetic resonance (scmr) recommended cmr protocols for scanning patients with active or convalescent phase COVID-19 infection. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of t1, t2, t2* and extracellular volume: A consensus statement by the society for cardiovascular magnetic resonance (scmr) endorsed by the european association for cardiovascular imaging (eacvi). J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation expert recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Modica, G.; Bianco, M.; Sollazzo, F.; Di Murro, E.; Monti, R.; Cammarano, M.; Morra, L.; Nifosì, F.M.; Gervasi, S.F.; Manes Gravina, E.; et al. Myocarditis in Athletes Recovering from COVID-19: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4279. [Google Scholar] [CrossRef]
- Gervasi, S.F.; Pengue, L.; Damato, L.; Monti, R.; Pradella, S.; Pirronti, T.; Bartoloni, A.; Epifani, F.; Saggese, A.; Cuccaro, F.; et al. Is extensive cardiopulmonary screening useful in athletes with previous asymptomatic or mild SARS-CoV-2 infection? Br. J. Sports Med. 2021, 55, 54–61. [Google Scholar] [CrossRef]
- Cavigli, L.; Frascaro, F.; Turchini, F.; Mochi, N.; Sarto, P.; Bianchi, S.; Parri, A.; Carraro, N.; Valente, S.; Focardi, M.; et al. A prospective study on the consequences of SARS-CoV-2 infection on the heart of young adult competitive athletes: Implications for a safe return-to-play. Int. J. Cardiol. 2021, 336, 130–136. [Google Scholar] [CrossRef]
- Cavigli, L.; Cillis, M.; Mochi, V.; Frascaro, F.; Mochi, N.; Hajdarevic, A.; Roselli, A.; Capitani, M.; Alvino, F.; Giovani, S.; et al. SARS-CoV-2 infection and return to play in junior competitive athletes: Is systematic cardiac screening needed? Br. J. Sports Med. 2022, 56, 264–270. [Google Scholar] [CrossRef]
| Parameter | Athletes (n = 47) |
|---|---|
| Age, y.o. | 26 ± 4 |
| Male sex, n (%) | 32 (68) |
| Caucasian, n (%)Afro-Caribbean, n (%) | 45 (96)2 (4) |
| Years of training, y | 16 (10–20) * |
| Hours of training/week, h/week | 22 (18–26) * |
| Weight, kg | 78 ± 15 |
| Height, cm | 180 ± 11 |
| BSA, m2 | 1.9 ± 0.23 |
| BMI, kg/m2 | 24 ± 3 |
| SBP, mmHg | 112 ± 11 |
| DBP, mmHg | 71 ± 9 |
| HR, bpm | 57 ± 12 |
| Symptoms, n (%) | 41 (87) |
| Ageusia, n (%) | 19 (40) |
| Anosmia, n (%) | 15 (32) |
| Fever, n (%) | 17 (36) |
| Dyspnea, n (%) | 3 (6) |
| Diarrhea, n (%) | 3 (6) |
| Chest pain, n (%) | 1 (2) |
| Faintness, n (%) | 12 (26) |
| Headache, n (%) | 20 (43) |
| Myalgia, n (%) | 13 (28) |
| Cold, n (%) | 12 (26) |
| Palpitations, n (%) | 2 (4) |
| Parameter | Athletes (n = 47) | |
|---|---|---|
| 12-lead ECG | ||
| TWI, n (%) | 2 (4) | |
| LAD, n (%) | 3 (6) | |
| Newly detected abnormal ECG, n (%) | 0 (0) | |
| 24-h ECG Holter monitoring (12 lead) | ||
| SVPCs, n (%) | 40 (85) | |
| SVPCs >500/h, n (%) | 3 (6) | |
| PVCs, n (%) | 32 (68) | |
| PVCs | <50/24 h, n (%) | 26 (55) |
| 50–500/24 h, n (%) | 2 (4) | |
| >500/24 h, n (%) | 4 (9) | |
| Cardiopulmonary test (CPET) | ||
| HR max, bpm | 169 ± 9 | |
| SBP max, mmHg | 174 ± 17 | |
| DBP max, mmHg | 75 ± 8 | |
| Watt max, W | 304 ± 94 | |
| VO2 max, L/min | 3223 ± 781 | |
| VO2/Kg max, mL/kg/min | 42 ± 6 | |
| VO2/Kg AT, mL/kg/min | 22 ± 4 | |
| VE max, L/min | 111 ± 34 | |
| VE/VCO2 slope | 25 ± 5 | |
| VO2/HR, L/min/bpm | 19 ± 5 | |
| RER max | 1.2 ± 0.1 | |
| Exercise-induced VA, n (%) | 6 (13) | |
| Complex exercise-induced VA, n (%) | 3 (6) | |
| Newly diagnosed exercise-induced VA, n (%) | 3 (6) | |
| Parameter | Athletes (n = 47) |
|---|---|
| Cardiac magnetic resonance | |
| LV EDVi, mL/m2 | 111 ± 18 |
| LV ESVi, mL/m2 | 49 ± 10 |
| LV SVi, mL/m2 | 62 ± 10 |
| LV EF, % | 56 ± 4 |
| RV EDVi, mL/m2 | 107 ± 17 |
| RV ESVi, mL/m2 | 46 ± 10 |
| RV SVi, mL/m2 | 61 ± 10 |
| RV EF, % | 57 ± 4 |
| LAAi, cm2/m2 | 13 ± 2 |
| RAAi, cm2/m2 | 13 ± 2 |
| IVST, mm | 9 ± 1.2 |
| PWT, mm | 8 ± 1.2 |
| Pathological LGE, n (%) | 0 (0) |
| RV insertion point LGE, n (%) | 4 (8.5) |
| Pericardial effusion, n (%) | 1 (2) |
| Parametric Mapping | |
| T1 Blood, ms | 1499 ± 105 |
| T1 Myo, ms | 950 ± 36 |
| T2 Myo, ms | 50 ± 2 |
| ECV, % | 0.27 ± 0.02 |
| Positive Lake Louise Criteria, n (%) | 1 (2) |
| Echocardiography | |
| E/A | 1.7 ± 0.5 |
| E, cm/s | 77 ± 16 |
| A, cm/s | 46 ± 10 |
| e’, cm/s | 11 ± 3 |
| E/e’ | 10 ± 3 |
| TAPSE, mm | 26 ± 4 |
| sPAP, mmHg | 25 ± 3 |
| Parameter | Before SARS-CoV-2 n = 18 | After SARS-CoV-2 n = 18 | p |
|---|---|---|---|
| LV EDVi, mL/m2 | 118 ± 19 | 119 ± 19 | 0.493 |
| LV ESVi, mL/m2 | 51 ± 13 | 53 ± 11 | 0.069 |
| LV SVi, mL/m2 | 67 ± 9 | 66 ± 9 | 0.516 |
| LV EF, % | 57 ± 5 | 56 ± 4 | 0.081 |
| RV EDVi, mL/m2 | 117 ± 17 | 116 ± 17 | 0.494 |
| RV ESVi, mL/m2 | 55 ± 21 | 51 ± 10 | 0.333 |
| RV SVi, mL/m2 | 66 ± 9 | 65 ± 10 | 0.648 |
| RV EF, (%) | 56 ± 4 | 56 ± 4 | 0.957 |
| T1 Mapping Blood, ms | 1438 ± 73 | 1466 ± 98 | 0.26 |
| T1 Mapping Myo, ms | 935 ± 29 | 939 ± 48 | 0.746 |
| T2 Mapping Myo, ms | 51 ± 3 | 48 ± 2 | 0.099 |
| RV insertion point LGE, n (%) | NA | 2 (11) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestrini, V.; Filomena, D.; Birtolo, L.I.; Serdoz, A.; Fiore, R.; Tatangelo, M.; Lemme, E.; Squeo, M.R.; Mango, R.; Di Gioia, G.; et al. Systematic Cardiovascular Screening in Olympic Athletes before and after SARS-CoV-2 Infection. J. Clin. Med. 2022, 11, 3499. https://doi.org/10.3390/jcm11123499
Maestrini V, Filomena D, Birtolo LI, Serdoz A, Fiore R, Tatangelo M, Lemme E, Squeo MR, Mango R, Di Gioia G, et al. Systematic Cardiovascular Screening in Olympic Athletes before and after SARS-CoV-2 Infection. Journal of Clinical Medicine. 2022; 11(12):3499. https://doi.org/10.3390/jcm11123499
Chicago/Turabian StyleMaestrini, Viviana, Domenico Filomena, Lucia Ilaria Birtolo, Andrea Serdoz, Roberto Fiore, Mario Tatangelo, Erika Lemme, Maria Rosaria Squeo, Ruggiero Mango, Giuseppe Di Gioia, and et al. 2022. "Systematic Cardiovascular Screening in Olympic Athletes before and after SARS-CoV-2 Infection" Journal of Clinical Medicine 11, no. 12: 3499. https://doi.org/10.3390/jcm11123499
APA StyleMaestrini, V., Filomena, D., Birtolo, L. I., Serdoz, A., Fiore, R., Tatangelo, M., Lemme, E., Squeo, M. R., Mango, R., Di Gioia, G., Fedele, F., Gualdi, G., Spataro, A., Pelliccia, A., & Di Giacinto, B. (2022). Systematic Cardiovascular Screening in Olympic Athletes before and after SARS-CoV-2 Infection. Journal of Clinical Medicine, 11(12), 3499. https://doi.org/10.3390/jcm11123499











