Predictive Scale for Amyloid PET Positivity Based on Clinical and MRI Variables in Patients with Amnestic Mild Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Tests
2.3. Axial Visual Rating Scale of Medial Temporal Lobe Atrophy
2.4. MRI and PET Imaging Acquisition
2.5. Validation of the Predictive Scale
2.6. Statistical Analyses
3. Results
3.1. Participants’ Characteristics
3.2. Development of a New Predictive Scale for Amyloid PET Positivity
- Model 1: included age, both aMCI.
- Model 2: included age, both aMCI, MTA aVRS.
- Model 3: included age, both aMCI, APOE genotype.
- Model 4: included age, both aMCI, MTA aVRS, APOE genotype.
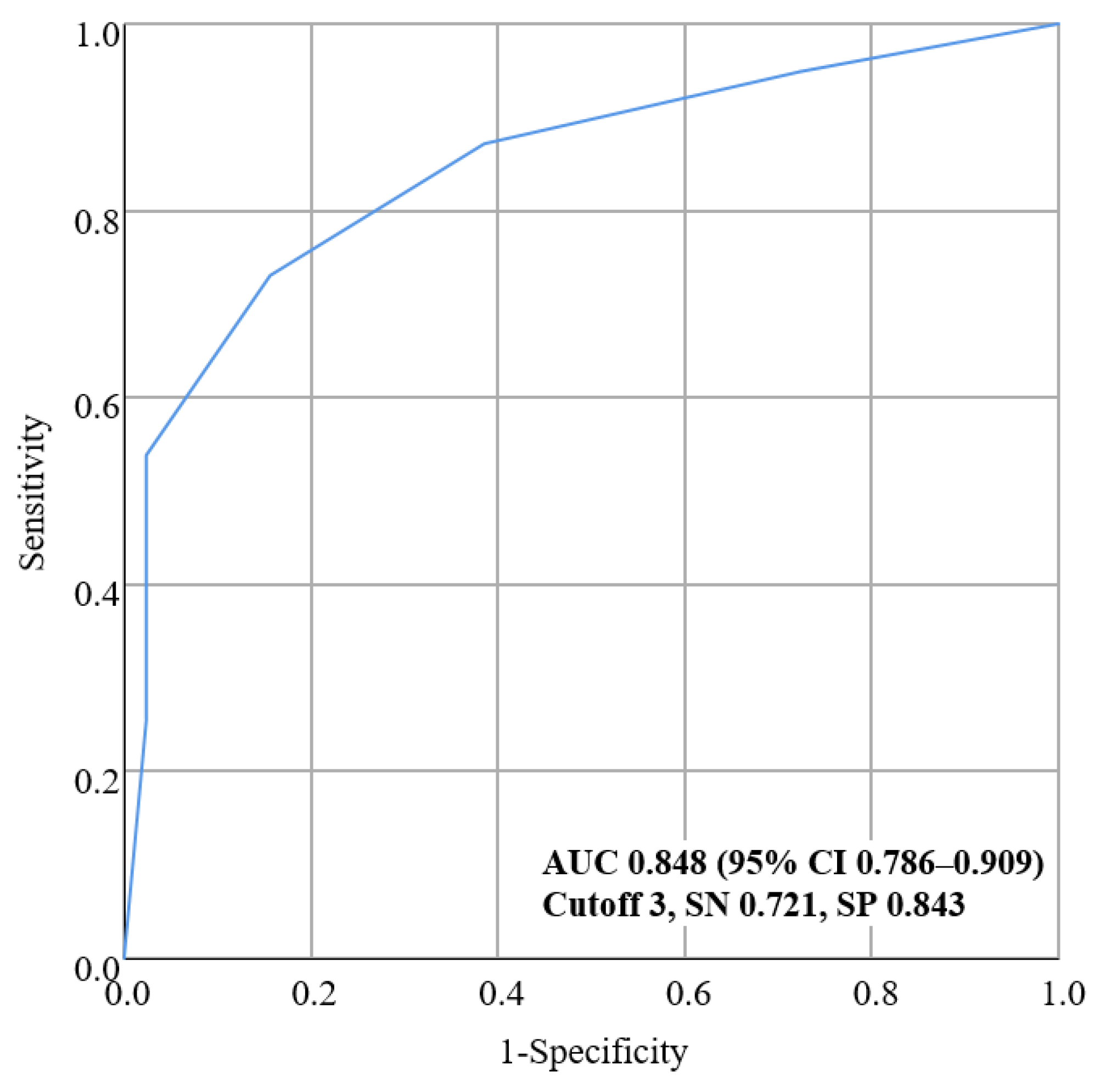
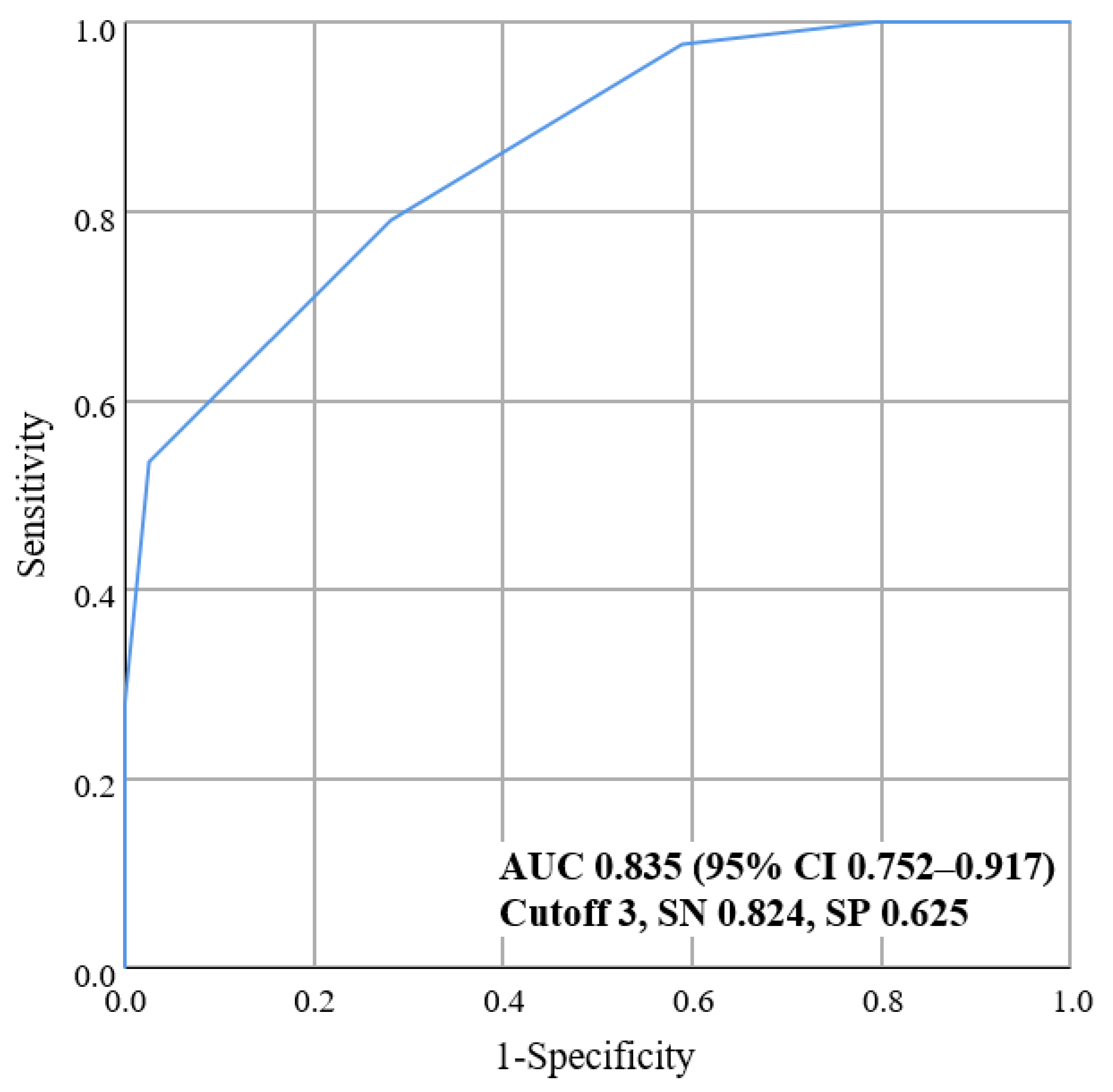
3.3. Validation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- GBD Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, M.; Berr, C.; De Rotrou, J.; Fabrigoule, C.; Pasquier, F.; Legrain, S.; Michel, B.; Puel, M.; Volteau, M.; Touchon, J.; et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: A longitudinal study. Neurology 2007, 69, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Palmqvist, S.; Zetterberg, H.; Mattsson, N.; Johansson, P.; Minthon, L.; Blennow, K.; Olsson, M.; Hansson, O.; Initiative, A.s.D.N.; Group, S.B.S. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 2015, 85, 1240–1249. [Google Scholar] [CrossRef]
- Johnson, K.A.; Minoshima, S.; Bohnen, N.I.; Donohoe, K.J.; Foster, N.L.; Herscovitch, P.; Karlawish, J.H.; Rowe, C.C.; Carrillo, M.C.; Hartley, D.M.; et al. Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J. Nucl. Med. 2013, 54, 476–490. [Google Scholar] [CrossRef] [Green Version]
- Teipel, S.; Drzezga, A.; Grothe, M.J.; Barthel, H.; Chételat, G.; Schuff, N.; Skudlarski, P.; Cavedo, E.; Frisoni, G.B.; Hoffmann, W. Multimodal imaging in Alzheimer’s disease: Validity and usefulness for early detection. Lancet Neurol. 2015, 14, 1037–1053. [Google Scholar] [CrossRef]
- Daly, E.; Zaitchik, D.; Copeland, M.; Schmahmann, J.; Gunther, J.; Albert, M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch. Neurol. 2000, 57, 675–680. [Google Scholar] [CrossRef] [Green Version]
- De Simone, M.S.; Perri, R.; Fadda, L.; Caltagirone, C.; Carlesimo, G.A. Predicting progression to Alzheimer’s disease in subjects with amnestic mild cognitive impairment using performance on recall and recognition tests. J. Neurol 2019, 266, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Yoon, B.; Shim, Y.S.; Kim, S.O.; Kim, H.J.; Choi, S.H.; Jeong, J.H.; Yoon, S.J.; Yang, D.W.; Lee, J.H. Predictors of Clinical Progression of Subjective Memory Impairment in Elderly Subjects: Data from the Clinical Research Centers for Dementia of South Korea (CREDOS). Dement. Geriatr. Cogn. Disord. 2015, 40, 158–165. [Google Scholar] [CrossRef]
- Tabert, M.H.; Manly, J.J.; Liu, X.; Pelton, G.H.; Rosenblum, S.; Jacobs, M.; Zamora, D.; Goodkind, M.; Bell, K.; Stern, Y.; et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch. Gen. Psychiatry 2006, 63, 916–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, E.; Pepe, A.; Gaser, C.; Huttunen, H.; Tohka, J.; Alzheimer’s Disease Neuroimaging Initiative. Machine learning framework for early MRI-based Alzheimer’s conversion prediction in MCI subjects. Neuroimage 2015, 104, 398–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Kate, M.; Redolfi, A.; Peira, E.; Bos, I.; Vos, S.J.; Vandenberghe, R.; Gabel, S.; Schaeverbeke, J.; Scheltens, P.; Blin, O.; et al. MRI predictors of amyloid pathology: Results from the EMIF-AD Multimodal Biomarker Discovery study. Alzheimer’s Res. Ther. 2018, 10, 100. [Google Scholar] [CrossRef]
- Ye, B.S.; Seo, S.W.; Kim, C.H.; Jeon, S.; Kim, G.H.; Noh, Y.; Cho, H.; Yoon, C.W.; Kim, H.J.; Jang, E.Y.; et al. Hippocampal and cortical atrophy in amyloid-negative mild cognitive impairments: Comparison with amyloid-positive mild cognitive impairment. Neurobiol. Aging 2014, 35, 291–300. [Google Scholar] [CrossRef]
- Haghighi, M.; Smith, A.; Morgan, D.; Small, B.; Huang, S. Identifying cost-effective predictive rules of amyloid-beta level by integrating neuropsychological tests and plasma-based markers. J. Alzheimer’s Dis. 2015, 43, 1261–1270. [Google Scholar] [CrossRef]
- Palmqvist, S.; Insel, P.S.; Zetterberg, H.; Blennow, K.; Brix, B.; Stomrud, E.; Alzheimer’s Disease Neuroimaging Initiative; Swedish BioFINDER Study; Mattsson, N.; Hansson, O. Accurate risk estimation of beta-amyloid positivity to identify prodromal Alzheimer’s disease: Cross-validation study of practical algorithms. Alzheimer’s Dement. 2019, 15, 194–204. [Google Scholar] [CrossRef]
- Alladi, S.; Arnold, R.; Mitchell, J.; Nestor, P.J.; Hodges, J.R. Mild cognitive impairment: Applicability of research criteria in a memory clinic and characterization of cognitive profile. Psychol. Med. 2006, 36, 507–515. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Villemagne, V.; Ong, K.; Chetelat, G.; Lamb, F.; Reininger, C.B.; Woodward, M.; Rowe, C.C. Prediction of amyloid-beta pathology in amnestic mild cognitive impairment with neuropsychological tests. J. Alzheimer’s Dis. 2013, 33, 451–462. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Maruff, P.; Pietrzak, R.H.; Ames, D.; Ellis, K.A.; Harrington, K.; Lautenschlager, N.T.; Szoeke, C.; Martins, R.N.; Masters, C.L.; et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 2014, 137, 221–231. [Google Scholar] [CrossRef]
- Summers, M.J.; Saunders, N.L. Neuropsychological measures predict decline to Alzheimer’s dementia from mild cognitive impairment. Neuropsychology 2012, 26, 498–508. [Google Scholar] [CrossRef]
- Clerx, L.; van Rossum, I.A.; Burns, L.; Knol, D.L.; Scheltens, P.; Verhey, F.; Aalten, P.; Lapuerta, P.; van de Pol, L.; van Schijndel, R.; et al. Measurements of medial temporal lobe atrophy for prediction of Alzheimer’s disease in subjects with mild cognitive impairment. Neurobiol. Aging 2013, 34, 2003–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCarli, C.; Frisoni, G.B.; Clark, C.M.; Harvey, D.; Grundman, M.; Petersen, R.C.; Thal, L.J.; Jin, S.; Jack, C.R., Jr.; Scheltens, P.; et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch. Neurol. 2007, 64, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Risacher, S.L.; Saykin, A.J.; West, J.D.; Shen, L.; Firpi, H.A.; McDonald, B.C.; Alzheimer’s Disease Neuroimaging Initiative. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr. Alzheimer Res. 2009, 6, 347–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, P.J.; Verhey, F.R.; Hofman, P.A.; Scheltens, P.; Jolles, J. Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2002, 72, 491–497. [Google Scholar]
- Petersen, R.C.; Smith, G.E.; Ivnik, R.J.; Tangalos, E.G.; Schaid, D.J.; Thibodeau, S.N.; Kokmen, E.; Waring, S.C.; Kurland, L.T. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA 1995, 273, 1274–1278. [Google Scholar] [CrossRef]
- Risacher, S.L.; Kim, S.; Shen, L.; Nho, K.; Foroud, T.; Green, R.C.; Petersen, R.C.; Jack, C.R., Jr.; Aisen, P.S.; Koeppe, R.A.; et al. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI). Front. Aging Neurosci. 2013, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.J.; Chin, J.; Park, A.; Lee, B.H.; Suh, M.K.; Seo, S.W.; Na, D.L. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci 2010, 25, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kim, J.E.; Choi, K.G.; Lim, S.M.; Lee, J.M.; Na, D.L.; Jeong, J.H. T1-weighted axial visual rating scale for an assessment of medial temporal atrophy in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 41, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Scheltens, P.; Leys, D.; Barkhof, F.; Huglo, D.; Weinstein, H.C.; Vermersch, P.; Kuiper, M.; Steinling, M.; Wolters, E.C.; Valk, J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 1992, 55, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.S.; Kim, G.H.; Choi, J.H.; Hwang, J.; Kwon, E.; Lee, S.A.; Kong, K.A.; Kang, H.J.; Yoon, B.; Kim, B.C.; et al. Age-Specific Cutoff Scores on a T1-Weighted Axial Medial Temporal-Lobe Atrophy Visual Rating Scale in Alzheimer’s Disease Using Clinical Research Center for Dementia of South Korea Data. J. Clin. Neurol. 2018, 14, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Barkhof, F.; Fox, N.C.; Schott, J.M. Using visual rating to diagnose dementia: A critical evaluation of MRI atrophy scales. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Cavallin, L.; Spulber, G.; Aguilar, C.; Mecocci, P.; Vellas, B.; Tsolaki, M.; Kloszewska, I.; Soininen, H.; Spenger, C.; et al. Influence of age, disease onset and ApoE4 on visual medial temporal lobe atrophy cut-offs. J. Intern. Med. 2014, 275, 317–330. [Google Scholar] [CrossRef]
- Hwang, J.; Jeong, J.H.; Yoon, S.J.; Park, K.W.; Kim, E.J.; Yoon, B.; Jang, J.W.; Kim, H.J.; Hong, J.Y.; Lee, J.M.; et al. Clinical and Biomarker Characteristics According to Clinical Spectrum of Alzheimer’s Disease (AD) in the Validation Cohort of Korean Brain Aging Study for the Early Diagnosis and Prediction of AD. J. Clin. Med. 2019, 8, 341. [Google Scholar] [CrossRef] [Green Version]
- Gupta, Y.; Lama, R.K.; Kwon, G.R.; Alzheimer’s Disease Neuroimaging Initiative. Prediction and Classification of Alzheimer’s Disease Based on Combined Features From Apolipoprotein-E Genotype, Cerebrospinal Fluid, MR, and FDG-PET Imaging Biomarkers. Front. Comput. Neurosci. 2019, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Long, X.; Chen, L.; Jiang, C.; Zhang, L.; Alzheimer’s Disease Neuroimaging Initiative. Prediction and classification of Alzheimer disease based on quantification of MRI deformation. PLoS ONE 2017, 12, e0173372. [Google Scholar] [CrossRef]
- Tosun, D.; Joshi, S.; Weiner, M.W.; Alzheimer’s Disease Neuroimaging Initiative. Neuroimaging predictors of brain amyloidosis in mild cognitive impairment. Ann. Neurol. 2013, 74, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, L.G.; Hwang, K.S.; Avila, D.; Elashoff, D.; Kohannim, O.; Teng, E.; Sokolow, S.; Jack, C.R.; Jagust, W.J.; Shaw, L.; et al. Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology 2015, 84, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Dore, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Kim, S.E.; Woo, S.; Kim, S.W.; Chin, J.; Kim, H.J.; Lee, B.I.; Park, J.; Park, K.W.; Kang, D.Y.; Noh, Y.; et al. A Nomogram for Predicting Amyloid PET Positivity in Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2018, 66, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Byun, M.S.; Yi, D.; Sohn, B.K.; Jeon, S.Y.; Lee, Y.; Lee, J.Y.; Kim, Y.K.; Lee, Y.S.; Lee, D.Y. Prediction of Cerebral Amyloid With Common Information Obtained From Memory Clinic Practice. Front. Aging Neurosci. 2018, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, T.; Hall, A.; Ngandu, T.; van Gils, M.; Helisalmi, S.; Hanninen, T.; Kemppainen, N.; Liu, Y.; Lotjonen, J.; Paajanen, T.; et al. Detecting Amyloid Positivity in Elderly With Increased Risk of Cognitive Decline. Front. Aging Neurosci. 2020, 12, 228. [Google Scholar] [CrossRef]
- Landau, S.M.; Mintun, M.A.; Joshi, A.D.; Koeppe, R.A.; Petersen, R.C.; Aisen, P.S.; Weiner, M.W.; Jagust, W.J.; Alzheimer’s Disease Neuroimaging Initiative. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol. 2012, 72, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Aakre, J.A.; Kremers, W.K.; Vassilaki, M.; Knopman, D.S.; Mielke, M.M.; Alhurani, R.; Geda, Y.E.; Machulda, M.M.; Coloma, P.; et al. Prevalence and Outcomes of Amyloid Positivity Among Persons Without Dementia in a Longitudinal, Population-Based Setting. JAMA Neurol. 2018, 75, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Bruscoli, M.; Lovestone, S. Is MCI really just early dementia? A systematic review of conversion studies. Int. Psychogeriatr. 2004, 16, 129–140. [Google Scholar] [CrossRef]
- Jang, H.; Ye, B.S.; Woo, S.; Kim, S.W.; Chin, J.; Choi, S.H.; Jeong, J.H.; Yoon, S.J.; Yoon, B.; Park, K.W.; et al. Prediction Model of Conversion to Dementia Risk in Subjects with Amnestic Mild Cognitive Impairment: A Longitudinal, Multi-Center Clinic-Based Study. J. Alzheimer’s Dis. 2017, 60, 1579–1587. [Google Scholar] [CrossRef]
- Rabin, L.A.; Pare, N.; Saykin, A.J.; Brown, M.J.; Wishart, H.A.; Flashman, L.A.; Santulli, R.B. Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Aging Neuropsychol. Cogn. 2009, 16, 357–376. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Lopresti, B.J.; Campbell, E.M.; Yu, Z.; Anderson, S.J.; Cohen, A.D.; Minhas, D.S.; Snitz, B.E.; Royse, S.K.; Becker, C.R.; Aizenstein, H.J.; et al. Influence of apolipoprotein-E genotype on brain amyloid load and longitudinal trajectories. Neurobiol. Aging 2020, 94, 111–120. [Google Scholar] [CrossRef]
- Mormino, E.C.; Betensky, R.A.; Hedden, T.; Schultz, A.P.; Amariglio, R.E.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014, 71, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Bilgel, M.; An, Y.; Helphrey, J.; Elkins, W.; Gomez, G.; Wong, D.F.; Davatzikos, C.; Ferrucci, L.; Resnick, S.M. Effects of amyloid pathology and neurodegeneration on cognitive change in cognitively normal adults. Brain 2018, 141, 2475–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Lim, J.H.; Jeong, Y.J.; Kang, D.Y.; Park, K.W. The Effect of Clinical Characteristics and Subtypes on Amyloid Positivity in Patients with Amnestic Mild Cognitive Impairment. Dement. Neurocogn. Disord. 2019, 18, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Im, K.; Lee, J.M.; Park, A.; Chin, J.; Kim, G.H.; Kim, J.H.; Roh, J.H.; Seo, S.W.; Na, D.L. Cortical thinning in verbal, visual, and both memory-predominant mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2011, 25, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Oltra-Cucarella, J.; Sanchez-SanSegundo, M.; Lipnicki, D.M.; Crawford, J.D.; Lipton, R.B.; Katz, M.J.; Zammit, A.R.; Scarmeas, N.; Dardiotis, E.; Kosmidis, M.H.; et al. Visual memory tests enhance the identification of amnestic MCI cases at greater risk of Alzheimer’s disease. Int. Psychogeriatr. 2019, 31, 997–1006. [Google Scholar] [CrossRef]
- van de Pol, L.A.; Hensel, A.; Barkhof, F.; Gertz, H.J.; Scheltens, P.; van der Flier, W.M. Hippocampal atrophy in Alzheimer disease: Age matters. Neurology 2006, 66, 236–238. [Google Scholar] [CrossRef]
- Barkhof, F.; Polvikoski, T.M.; van Straaten, E.C.; Kalaria, R.N.; Sulkava, R.; Aronen, H.J.; Niinisto, L.; Rastas, S.; Oinas, M.; Scheltens, P.; et al. The significance of medial temporal lobe atrophy: A postmortem MRI study in the very old. Neurology 2007, 69, 1521–1527. [Google Scholar] [CrossRef]
- Marks, S.M.; Lockhart, S.N.; Baker, S.L.; Jagust, W.J. Tau and beta-Amyloid Are Associated with Medial Temporal Lobe Structure, Function, and Memory Encoding in Normal Aging. J. Neurosci. 2017, 37, 3192–3201. [Google Scholar] [CrossRef]
- De Souza, L.C.; Chupin, M.; Bertoux, M.; Lehéricy, S.; Dubois, B.; Lamari, F.; Le Ber, I.; Bottlaender, M.; Colliot, O.; Sarazin, M. Is hippocampal volume a good marker to differentiate Alzheimer’s disease from frontotemporal dementia? J. Alzheimer’s Dis. 2013, 36, 57–66. [Google Scholar] [CrossRef]
- Burton, E.J.; Barber, R.; Mukaetova-Ladinska, E.B.; Robson, J.; Perry, R.H.; Jaros, E.; Kalaria, R.N.; O’Brien, J.T. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: A prospective study with pathological verification of diagnosis. Brain 2009, 132, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Harper, L.; Fumagalli, G.G.; Barkhof, F.; Scheltens, P.; O’Brien, J.T.; Bouwman, F.; Burton, E.J.; Rohrer, J.D.; Fox, N.C.; Ridgway, G.R.; et al. MRI visual rating scales in the diagnosis of dementia: Evaluation in 184 post-mortem confirmed cases. Brain 2016, 139, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Varon, D.; Barker, W.; Loewenstein, D.; Greig, M.; Bohorquez, A.; Santos, I.; Shen, Q.; Harper, M.; Vallejo-Luces, T.; Duara, R.; et al. Visual rating and volumetric measurement of medial temporal atrophy in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort: Baseline diagnosis and the prediction of MCI outcome. Int. J. Geriatr. Psychiatry 2015, 30, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, L.O.; Julin, P.; Johansson, S.E.; Scheltens, P. Visual rating and volumetry of the medial temporal lobe on magnetic resonance imaging in dementia: A comparative study. J. Neurol. Neurosurg. Psychiatry 2000, 69, 630–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullich, S.; Seibyl, J.; Catafau, A.M.; Jovalekic, A.; Koglin, N.; Barthel, H.; Sabri, O.; De Santi, S. Optimized classification of (18)F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment. NeuroImage Clin. 2017, 15, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Seibyl, J.; Catafau, A.M.; Barthel, H.; Ishii, K.; Rowe, C.C.; Leverenz, J.B.; Ghetti, B.; Ironside, J.W.; Takao, M.; Akatsu, H.; et al. Impact of Training Method on the Robustness of the Visual Assessment of 18F-Florbetaben PET Scans: Results from a Phase-3 Study. J. Nucl. Med. 2016, 57, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Kandel, B.M.; Avants, B.B.; Gee, J.C.; Arnold, S.E.; Wolk, D.A.; Initiative, A.s.D.N. Neuropsychological testing predicts cerebrospinal fluid amyloid-β in mild cognitive impairment. J. Alzheimer’s Dis. 2015, 46, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013, 136, 2697–2706. [Google Scholar] [CrossRef]

| Total (n = 161) | Aβ− (n = 83) | Aβ+ (n = 78) | p-Value | |
|---|---|---|---|---|
| Age (years) | 69.88 ± 7.23 | 68.71 ± 7.57 | 71.12 ± 6.67 | 0.034 |
| Sex (men:women) | 71:90 | 42:41 | 29:49 | 0.086 |
| Education (years) | 11.09 ± 4.36 | 11.23 ± 4.38 | 10.95 ± 4.36 | 0.685 |
| Family history of dementia | 48/153 (31.4%) | 27/81 (33.3%) | 21/72 (29.2%) | 0.579 |
| Hypertension | 74/152 (49.0%) | 40/76 (53.3%) | 34/76 (44.7%) | 0.291 |
| Diabetes mellitus | 33/152 (21.9%) | 20/76 (26.7%) | 13/76 (17.1%) | 0.155 |
| Hyperlipidemia | 63/152 (41.4%) | 34/76 (44.7%) | 29/76 (38.2%) | 0.410 |
| Heart disease | 4/151 (2.6%) | 3/75 (4.0%) | 1/76 (1.3%) | 0.367 |
| Stroke | 2/151 (1.3%) | 2/75 (2.7%) | 0/76 (0.0%) | 0.245 |
| Cancer | 13/137 (9.5%) | 3/65 (4.6%) | 10/72 (13.9%) | 0.064 |
| Total (n = 161) | Aβ− (n = 83) | Aβ+ (n = 78) | p-Value | |
|---|---|---|---|---|
| SVLT-DR z-score | −1.51 ± 1.06 | −1.18 ± 0.96 | −1.86 ± 1.06 | <0.001 |
| RCFT-DR z-score | −1.19 ± 1.02 | −0.78 ± 1.03 | −1.63 ± 0.82 | <0.001 |
| DST-forward z-score | 0.03 ± 1.05 | 0.17 ± 1.12 | −0.12 ± 0.96 | 0.090 |
| K-BNT z-score | −0.39 ± 1.22 | −0.18 ± 1.27 | −0.12 ± 0.96 | 0.029 |
| RCFT-copy | −0.65 ± 1.65 | −0.58 ± 1.35 | −0.72 ± 1.93 | 0.576 |
| COWAT-phonemic z-score | −0.29 ± 0.82 | −0.23 ± 0.84 | −0.35 ± 0.79 | 0.341 |
| K-CWST-CR z-score | −0.41 ± 1.11 | −0.33 ± 1.01 | −0.49 ± 1.22 | 0.366 |
| DSC z-score | 0.09 ± 0.98 | 0.10 ± 1.01 | 0.08 ± 0.96 | 0.930 |
| K-TMT-B z-score | −0.63 ± 1.84 | −0.54 ± 1.78 | −0.75 ± 1.92 | 0.493 |
| Visual aMCI | 38 (23.6%) | 28 (33.7%) | 10 (12.8%) | <0.001 |
| Verbal aMCI | 50 (31.1%) | 36 (43.4%) | 14 (17.9%) | |
| Both aMCI | 73 (45.3%) | 19 (22.9%) | 54 (69.2%) | |
| K-MMSE | 25.80 ± 2.79 | 26.88 ± 2.20 | 24.64 ± 2.90 | <0.001 |
| Global CDR | 0.42 ± 0.25 | 0.36 ± 0.24 | 0.48 ± 0.25 | 0.002 |
| CDR-SB | 1.76 ± 1.50 | 1.31 ± 1.24 | 2.22 ± 1.61 | <0.001 |
| MTA aVRS ≥ 2 | 81 (50.3%) | 29 (34.9%) | 52 (66.7%) | <0.001 |
| APOE-ε4 carrier (%) | 70 (43.5%) | 14 (16.9%) | 56 (71.8%) | <0.001 |
| Variables | OR (95% CI) | p-Value |
|---|---|---|
| SVLT-DR (Ref. > −1 SD) * | 3.652 (1.637–8.149) | 0.002 |
| RCFT-DR (Ref. > −1 SD) * | 3.333 (1.616–6.876) | 0.001 |
| Both aMCI (Ref. single aMCI) | 7.579 (3.754–15.299) | <0.001 |
| K-BNT (Ref. > −1 SD) * | 1.684 (0.840–3.377) | 0.142 |
| MTA aVRS (Ref. < 2) * | 3.724 (1.940–7.149) | <0.001 |
| APOE-ε4 carrier (Ref. noncarrier) * | 12.545 (5.884–26.749) | <0.001 |
| Age (Ref. < 70) | 1.916 (1.022–3.594) | 0.043 |
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | Model 4 OR (95% CI) | |
|---|---|---|---|---|
| Both aMCI (Ref. single aMCI) | 7.368 *** (3.633–14.943) | 6.348 *** (3.080–13.083) | 4.114 ** (1.850–9.150) | 3.485 ** (1.530–7.940) |
| MTA aVRS (Ref. < 2) | NI | 2.533 * (1.177–5.449) | NI | 2.668 * (1.106–6.436) |
| APOE-ε4 carrier (Ref. noncarrier) | NI | NI | 9.090 *** (3.988–20.722) | 9.254 *** (3.971–21.566) |
| Age (Ref. < 70) | 1.684 (0.833–3.405) | 1.228 (0.571–2.638) | 2.082 (0.933–4.645) | 1.443 (0.600–3.473) |
| Predictors | β Coefficient | Scoring System | |
|---|---|---|---|
| Age | <70 | 0 | 0 |
| ≥70 | 0.367 | 1 | |
| Memory impairment | Single (verbal or visual) | 0 | 0 |
| Both (verbal and visual) | 1.249 | 1 | |
| MTA aVRS | <2 | 0 | 0 |
| ≥2 | 0.981 | 1 | |
| APOE-ε4 allele | Noncarrier | 0 | 0 |
| Carrier | 2.225 | 2 | |
| Total | 0~5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, M.Y.; Kim, G.H.; Park, H.K.; Yang, D.W.; Kim, S.; Choi, S.H.; Jeong, J.H. Predictive Scale for Amyloid PET Positivity Based on Clinical and MRI Variables in Patients with Amnestic Mild Cognitive Impairment. J. Clin. Med. 2022, 11, 3433. https://doi.org/10.3390/jcm11123433
Chun MY, Kim GH, Park HK, Yang DW, Kim S, Choi SH, Jeong JH. Predictive Scale for Amyloid PET Positivity Based on Clinical and MRI Variables in Patients with Amnestic Mild Cognitive Impairment. Journal of Clinical Medicine. 2022; 11(12):3433. https://doi.org/10.3390/jcm11123433
Chicago/Turabian StyleChun, Min Young, Geon Ha Kim, Hee Kyung Park, Dong Won Yang, SangYun Kim, Seong Hye Choi, and Jee Hyang Jeong. 2022. "Predictive Scale for Amyloid PET Positivity Based on Clinical and MRI Variables in Patients with Amnestic Mild Cognitive Impairment" Journal of Clinical Medicine 11, no. 12: 3433. https://doi.org/10.3390/jcm11123433
APA StyleChun, M. Y., Kim, G. H., Park, H. K., Yang, D. W., Kim, S., Choi, S. H., & Jeong, J. H. (2022). Predictive Scale for Amyloid PET Positivity Based on Clinical and MRI Variables in Patients with Amnestic Mild Cognitive Impairment. Journal of Clinical Medicine, 11(12), 3433. https://doi.org/10.3390/jcm11123433






