Characterization of Choroidal Morphology and Vasculature in the Phenotype of Pachychoroid Diseases by Swept-Source OCT and OCTA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Determination of the Cutoff Value Range of the Pachychoroid Diseases
2.3. Eye Examination
2.4. Examination by Swept-Source Optical Coherence Tomography
2.4.1. OCT B-Scan Images Acquisition
2.4.2. Measurement of Choroidal Thickness
2.5. Images Acquisition of SS-OCTA and En Face SS-OCT
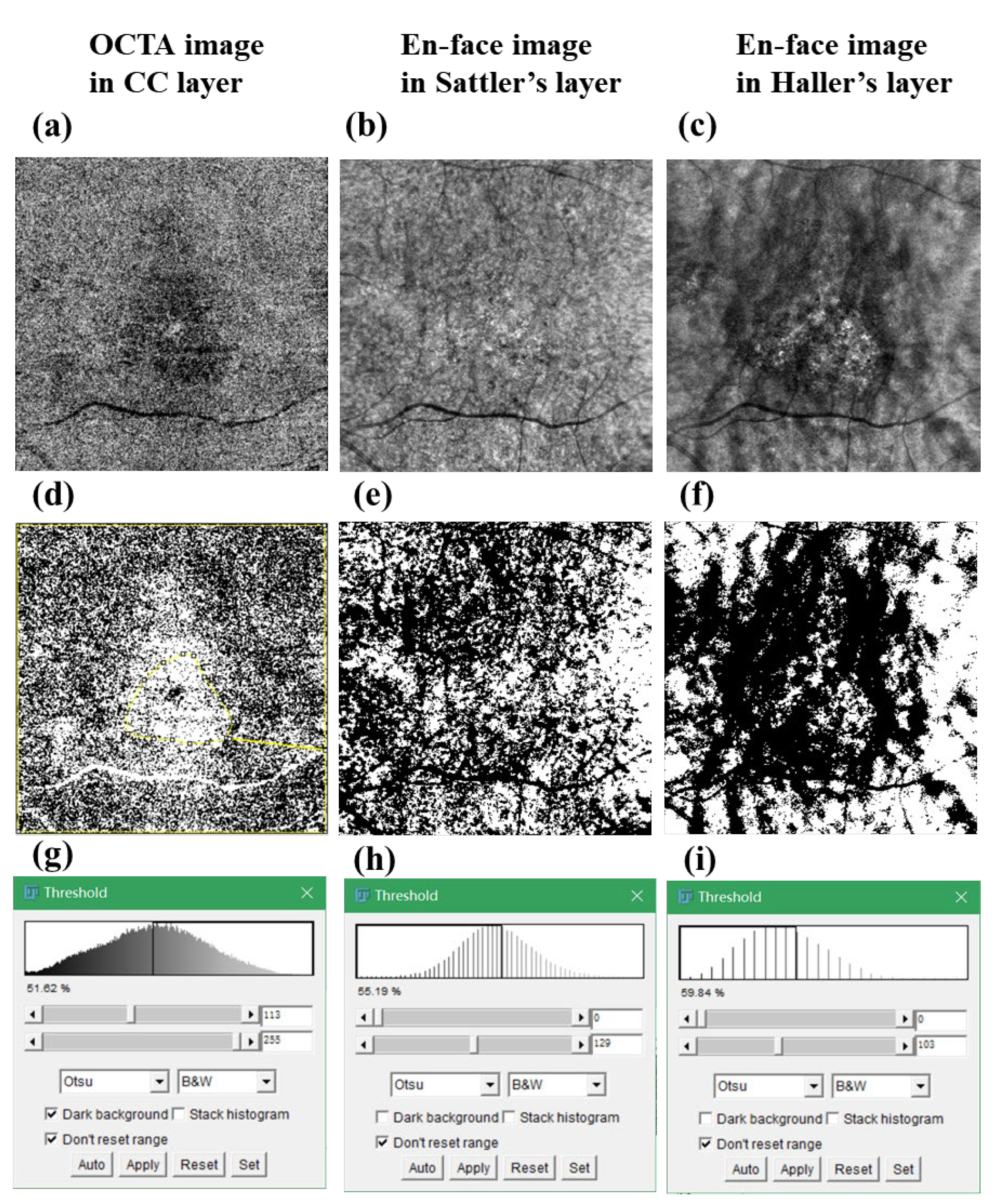
2.5.1. Vascular Density in Choriocapillaris and CVD in Sattler’s and Haller’s Layers
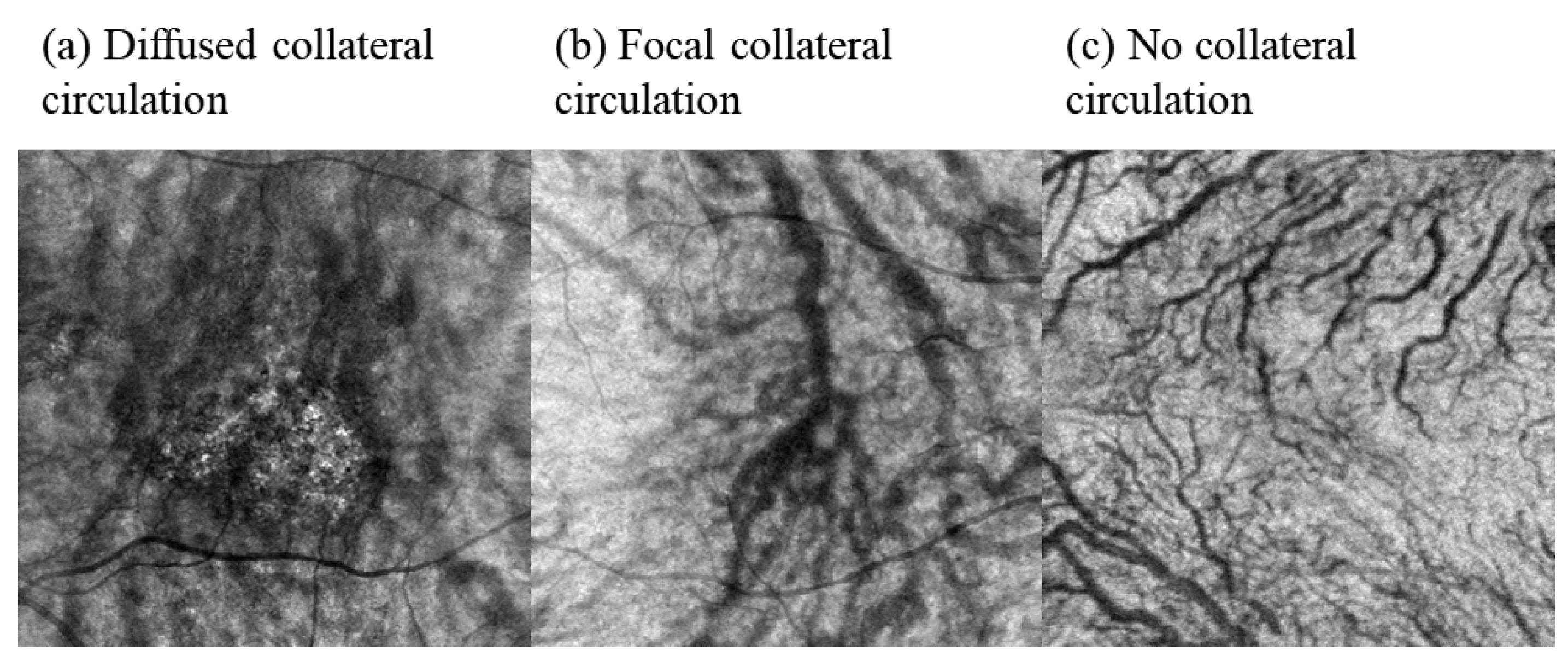
2.5.2. Semi-Quantification of the Collateral Circulation
2.6. Sample Size Determination
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Baseline Demographics of the Study Subjects
3.2. Comparison of SFCT in Normal Control, PCV, nAMD, and CSC
3.3. Comparison of Blood Flow or Vessel Density in Choroidal Layers between the PCV, nAMD, CSC, and Normal Control Groups
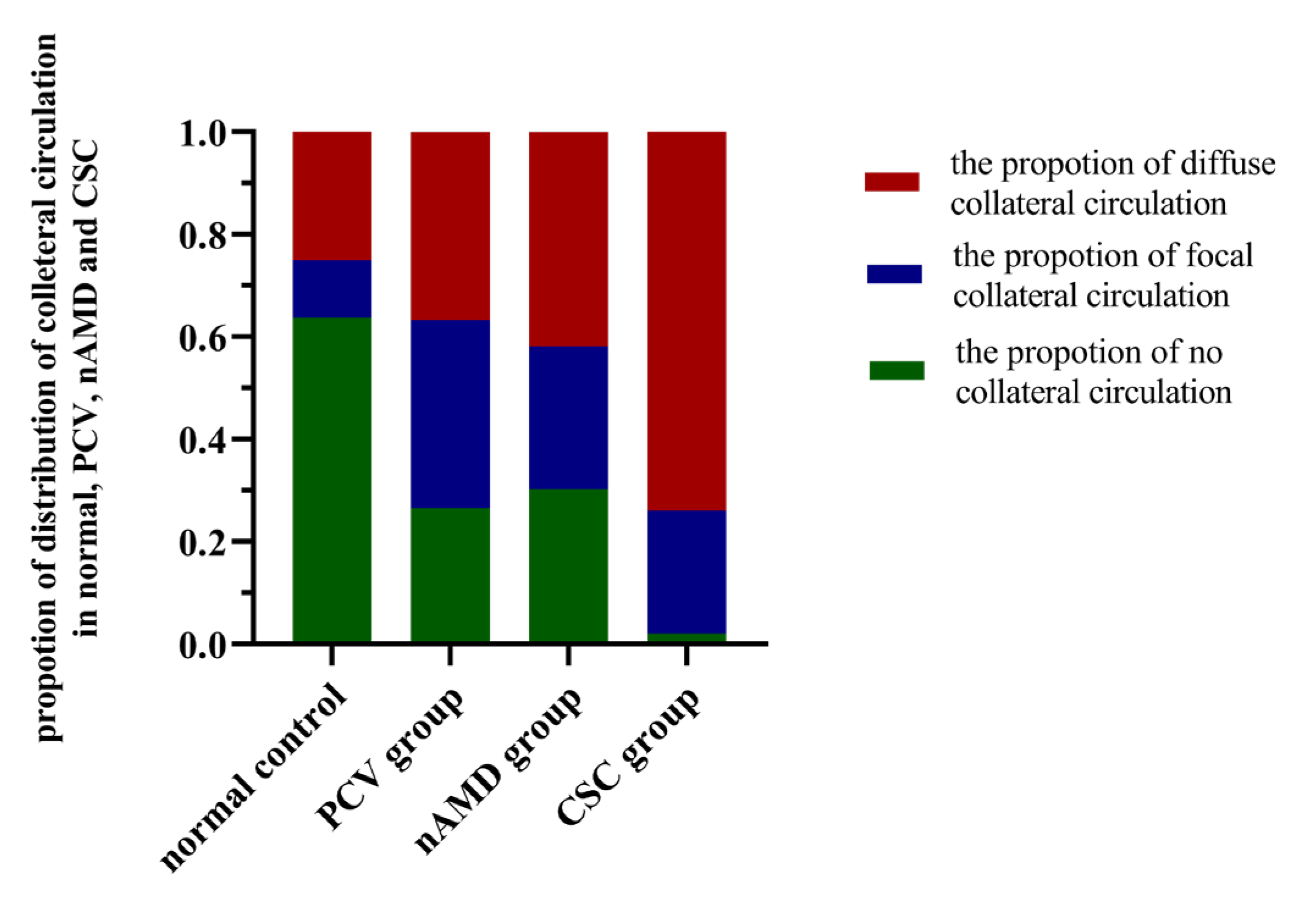
3.4. Comparison of the Proportion of Collateral Circulation between the PCV, nAMD, CSC, and Normal Control Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Warrow, D.J.; Hoang, Q.V.; Freund, K.B. Pachychoroid Pigment Epitheliopathy. Retina 2013, 33, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.T.; Yannuzzi, L.A.; Freund, K.B. Type 1 (sub-retinal Pigment Epithelial) Neovascularization in Central Serous Chorioretinopathy Masquerading As Neovascular Age-related Macular Degeneration. Retina 2012, 32, 1829–1837. [Google Scholar] [CrossRef]
- Baek, J.; Lee, J.H.; Jung, B.J.; Kook, L.; Lee, W.K. Morphologic Features of Large Choroidal Vessel Layer: Age-related Macular Degeneration, Polypoidal Choroidal Vasculopathy, and Central Serous Chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2309–2317. [Google Scholar] [CrossRef]
- Gupta, P.; Ting, D.S.W.; Thakku, S.G.; Wong, T.Y.; Cheng, C.Y.; Wong, E.; Mathur, R.; Wong, D.; Yeo, I.; Gemmy Cheung, C.M. Detailed Characterization of Choroidal Morphologic and Vascular Features in Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. Retina 2017, 37, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coscas, G.; Yamashiro, K.; Coscas, F.; De Benedetto, U.; Tsujikawa, A.; Miyake, M.; Gemmy Cheung, C.M.; Wong, T.Y.; Yoshimura, N. Comparison of Exudative Age-related Macular Degeneration Subtypes in Japanese and French Patients: Multicenter Diagnosis with Multimodal Imaging. Am. J. Ophthalmol. 2014, 158, 309–318.e302. [Google Scholar] [CrossRef]
- Weiter, J.J.; Ernest, J.T. Anatomy of the Choroidal Vasculature. Am. J. Ophthalmol. 1974, 78, 583–590. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kishi, S.; Mukai, R.; Akiyama, H. Remodeling of Macular Vortex Veins in Pachychoroid Neovasculopathy. Sci. Rep. 2019, 9, 14689. [Google Scholar] [CrossRef]
- Waldstein, S.M.; Faatz, H.; Szimacsek, M.; Glodan, A.M.; Podkowinski, D.; Montuoro, A.; Simader, C.; Gerendas, B.S.; Schmidt-Erfurth, U. Comparison of Penetration Depth in Choroidal Imaging Using Swept Source Vs Spectral Domain Optical Coherence Tomography. Eye 2015, 29, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Dansingani, K.K.; Balaratnasingam, C.; Naysan, J.; Freund, K.B. En Face Imaging of Pachychoroid Spectrum Disorders with Swept-Source Optical Coherence Tomography. Retina 2016, 36, 499–516. [Google Scholar] [CrossRef]
- Tan, C.S.; Ngo, W.K.; Chen, J.P.; Tan, N.W.; Lim, T.H.; Group, E.S. Everest Study Report 2: Imaging and Grading Protocol, and Baseline Characteristics of a Randomised Controlled Trial of Polypoidal Choroidal Vasculopathy. Br. J. Ophthalmol. 2015, 99, 624–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.M.G.; Laude, A.; Wong, W.; Mathur, R.; Chan, C.M.; Wong, E.; Wong, D.; Wong, T.Y.; Lim, T.H. Improved Specificity of Polypoidal Choroidal Vasculopathy Diagnosis Using a Modified Everest Criteria. Retina 2015, 35, 1375–1380. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lai, T.Y.Y.; Teo, K.; Ruamviboonsuk, P.; Chen, S.J.; Kim, J.E.; Gomi, F.; Koh, A.H.; Kokame, G.; Jordan-Yu, J.M.; et al. Polypoidal Choroidal Vasculopathy: Consensus Nomenclature and Non-Indocyanine Green Angiograph Diagnostic Criteria from the Asia-Pacific Ocular Imaging Society PCV Workgroup. Ophthalmology 2021, 128, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bartol-Puyal, F.A.; Isanta, C.; Calvo, P.; Ruiz-Moreno, Ó.; Pablo, L. Relationship Between Vascular Densities of Choriocapillaris and the Whole Choroid Using OCTA. Int. Ophthalmol. 2020, 40, 3135–3143. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.X.; Zhang, Q.; Wei, W.B.; Xu, L.; Jonas, J.B. Macular Choroidal Small-Vessel Layer, Sattler’s Layer and Haller’s Layer Thicknesses: The Beijing Eye Study. Sci. Rep. 2018, 8, 4411. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.C.; Laíns, I.; Silverman, R.F.; Sobrin, L.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Visualization of Choriocapillaris and Choroidal Vasculature in Healthy Eyes With En Face Swept-Source Optical Coherence Tomography Versus Angiography. Transl. Vis. Sci. Technol. 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolo, M.; Rosa, R.; Musetti, D.; Musolino, M.; Saccheggiani, M.; Traverso, C.E. Choroidal Vascular Flow Area in Central Serous Chorioretinopathy Using Swept-Source Optical Coherence Tomography Angiography. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2002–2010. [Google Scholar] [CrossRef] [Green Version]
- Qiu, B.; Zhao, L.; Zhang, X.; Wang, Y.; Wang, Q.; Nie, Y.; Chen, X.; Cheung, C.Y.L. Associations Between Diabetic Retinal Microvasculopathy and Neuronal Degeneration Assessed by Swept-Source OCT and OCT Angiography. Front. Med. 2021, 8, 778283. [Google Scholar] [CrossRef]
- Teussink, M.M.; Breukink, M.B.; van Grinsven, M.J.; Hoyng, C.B.; Klevering, B.J.; Boon, C.J.; de Jong, E.K.; Theelen, T. OCT Angiography Compared to Fluorescein and Indocyanine Green Angiography in Chronic Central Serous Chorioretinopathy. Invest. Ophthalmol. Vis. Sci. 2015, 56, 5229–5237. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.E.; Chilian, W.M.; Deindl, E.; van Royen, N.; Simons, M. A Brief Etymology of the Collateral Circulation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1854–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryczkowski, A.W. Anatomical and Functional Choroidal Lobuli. Int. Ophthalmol. 1994, 18, 131–141. [Google Scholar] [CrossRef]
- Faber, J.E.; Zhang, H.; Lassance-Soares, R.M.; Prabhakar, P.; Najafi, A.H.; Burnett, M.S.; Epstein, S.E. Aging Causes Collateral Rarefaction and Increased Severity of Ischemic Injury in Multiple Tissues. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1748–1756. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Sivaprasad, S. Drusen and Pachydrusen: The Definition, Pathogenesis, and Clinical Significance. Eye 2020, 35, 121–133. [Google Scholar] [CrossRef]
- Kawamura, A.; Yuzawa, M.; Mori, R.; Haruyama, M.; Tanaka, K. Indocyanine Green Angiographic and Optical Coherence Tomographic Findings Support Classification of Polypoidal Choroidal Vasculopathy into Two Types. Acta Ophthalmol. 2013, 91, e474–e481. [Google Scholar] [CrossRef]
- Tan, C.S.; Lim, L.W.; Ngo, W.K.; Lim, T.H. EVEREST Report 5: Clinical Outcomes and Treatment Response of Polypoidal Choroidal Vasculopathy Subtypes in a Multicenter, Randomized Controlled Trial. Invest. Ophthalmol. Vis. Sci. 2018, 59, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokame, G.T. Prospective Evaluation of Subretinal Vessel Location in Polypoidal Choroidal Vasculopathy (PCV) and Response of Hemorrhagic and Exudative PCV to High-dose Antiangiogenic Therapy (an American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2014, 112, 74–93. [Google Scholar]
- Dansingani, K.K.; Gal-Or, O.; Sadda, S.R.; Yannuzzi, L.A.; Freund, K.B. Understanding Aneurysmal Type 1 Neovascularization (polypoidal Choroidal Vasculopathy): A Lesson in the Taxonomy of ‘expanded Spectra’—A Review. Clin. Exp. Ophthalmol. 2018, 46, 189–200. [Google Scholar] [CrossRef]
- Lehmann, M.; Bousquet, E.; Beydoun, T.; Behar-Cohen, F. Pachychoroid: An Inherited Condition? Retina 2015, 35, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Yamashiro, K.; Hosoda, Y.; Miyake, M.; Ooto, S.; Tsujikawa, A. Characteristics of Pachychoroid Diseases and Age-Related Macular Degeneration: Multimodal Imaging and Genetic Backgrounds. J. Clin. Med. 2020, 9, 2034. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Qiu, B.; Chhablani, J.; Zhang, X. Evaluation of Choroidal Thickness Using Optical Coherent Tomography: A Review. Front. Med. 2021, 8, 783519. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.; Chen, C.L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Opt. 2016, 21, 66008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Kim, S.S.; Koh, H.J.; Lee, S.C. Choroidal Thickness, Age, and Refractive Error in Healthy Korean Subjects. Optom. Vis. Sci. 2014, 91, 491–496. [Google Scholar] [CrossRef]
- Ikuno, Y.; Kawaguchi, K.; Nouchi, T.; Yasuno, Y. Choroidal Thickness in Healthy Japanese Subjects. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2173–2176. [Google Scholar] [CrossRef] [PubMed]

| Healthy Control Subjects (Eyes) 43(80) | PCV Subjects (Eyes) 49(49) | nAMD Subjects (Eyes) 36(43) | CSC Subjects (Eyes) 45(50) | F/χ2 | p Value | |
|---|---|---|---|---|---|---|
| Age, y (mean ± SD) | 53.60 ± 15.97 | 67.44 ± 9.47 | 67.83 ± 7.85 | 48.53 ± 9.74 | 60.48 a | <0.001 * |
| Gender, male/female (N) | 31/12 | 29/14 | 24/12 | 31/14 | 0.33 b | 0.954 |
| Refractive, D (eyes) | −0.58 ± 1.53 | −0.22 ± 1.44 | −0.21 ± 1.60 | −0.33 ± 0.99 | 6.67 a | 0.083 |
| Control (N = 43) | PCV (N = 43) | nAMD (N = 36) | CSC (N = 45) | F/χ2 | p Value | |
|---|---|---|---|---|---|---|
| SFCT, μm (mean ± SD) | 270.58 ± 99.56 | 285.73 ± 119.28 | 243.51 ± 102.12 | 399.92 ± 117.15 | 43.60 b | <0.001 * |
| VD in CC layer, % (mean ± SD) | 49.14 ± 1.37 | 49.60 ± 2.33 | 49.37 ± 2.47 | 49.91 ± 1.47 | 6.18 b | 0.103 |
| VD in Sattler’s layer, % (mean ± SD) | 53.47 ± 3.51 | 52.04 ± 3.63 | 52.02 ± 5.52 | 52.91 ± 3.06 | 5.30 b | 0.151 |
| VD in Haller’s layer, % (mean ± SD) | 50.61 ± 5.12 | 50.56 ± 7.05 | 50.35 ± 5.85 | 55.60 ± 6.60 | 9.13 a | < 0.001 * |
| SFCT | VD in CC Layer | VD in Sattler’s Layer | VD in Haller’s Layer | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models/Factors | OR | 95% CI | p Value | Factors | OR | 95% CI | p Value | Factors | OR | 95% CI | p Value | Factors | OR | 95% CI | p Value |
| PCV vs. Control | |||||||||||||||
| Gender (Ref: male) | 0.94 | 0.40–2.21 | 0.885 | Gender (Ref: male) | 1.04 | 0.42–2.57 | 0.926 | Gender (Ref: male) | 0.69 | 0.27–1.75 | 0.435 | Gender (Ref: male) | 0.99 | 0.40–2.44 | 0.984 |
| Age | 1.09 | 1.05–1.14 | <0.001 * | Age | 1.10 | 1.06–1.15 | <0.001 * | Age | 1.09 | 1.04–1.13 | <0.001 * | Age | 1.08 | 1.04–1.12 | <0.001 * |
| Refractive | 0.99 | 0.74–1.34 | 0.959 | Refractive | 1.06 | 0.79–1.42 | 0.716 | Refractive | 1.16 | 0.81–1.66 | 0.418 | Refractive | 1.21 | 0.87–1.68 | 0.269 |
| SFCT (per 100μm) | 1.52 | 1.01–2.30 | 0.045 * | VD in CC layer | 1.47 | 1.13–1.91 | 0.004 * | VD in Sattler’s layer | 0.86 | 0.76–0.97 | 0.014 * | VD in Haller’s layer | 1.03 | 0.96–1.11 | 0.418 |
| nAMD vs. Control | |||||||||||||||
| Gender (Ref: male) | 0.74 | 0.27–2.03 | 0.561 | Gender (Ref: male) | 1.15 | 0.42–3.14 | 0.781 | Gender (Ref: male) | 1.16 | 0.44–3.07 | 0.771 | Gender (Ref: male) | 0.97 | 0.38–2.51 | 0.950 |
| Age | 1.07 | 1.03–1.11 | 0.001 * | Age | 1.11 | 1.06–1.16 | <0.001 * | Age | 1.08 | 1.03–1.13 | 0.001 * | Age | 1.08 | 1.03–1.12 | <0.001 * |
| Refractive | 1.11 | 0.81–1.51 | 0.527 | Refractive | 1.11 | 0.80–1.54 | 0.535 | Refractive | 1.03 | 0.74–1.44 | 0.866 | Refractive | 1.14 | 0.83–1.58 | 0.423 |
| SFCT (per 100μm) | 0.79 | 0.48–1.31 | 0.360 | VD in CC layer | 1.61 | 1.16–2.3 | 0.005 * | VD in Sattler’s layer | 0.94 | 0.82–1.07 | 0.322 | VD in Haller’s layer | 1.00 | 0.92–1.09 | 0.998 |
| CSC vs. Control | |||||||||||||||
| Gender (Ref: male) | 1.25 | 0.45–3.46 | 0.673 | Gender (Ref: male) | 0.88 | 0.36–2.11 | 0.767 | Gender (Ref: male) | 0.90 | 0.37–2.18 | 0.812 | Gender (Ref: male) | 1.37 | 0.53–3.55 | 0.521 |
| Age | 0.95 | 0.91–0.99 | 0.013 * | Age | 0.88 | 0.91–0.98 | 0.002 * | Age | 0.94 | 0.91–0.97 | <0.001 * | Age | 0.94 | 0.91–0.98 | 0.002 * |
| Refractive | 1.10 | 0.73–1.66 | 0.637 | Refractive | 1.30 | 0.92–1.82 | 0.132 | Refractive | 1.32 | 0.94–1.87 | 0.111 | Refractive | 1.24 | 0.85–1.81 | 0.265 |
| SFCT (per 100μm) | 3.48 | 2.01–6.02 | <0.001 * | VD in CC layer | 1.19 | 0.89–1.59 | 0.243 | VD in Sattler’s layer | 0.98 | 0.87–1.11 | 0.780 | VD in Haller’s layer | 1.15 | 1.06–1.25 | 0.001 * |
| nAMD vs. PCV | |||||||||||||||
| Gender (Ref: male) | 0.87 | 0.31–2.42 | 0.784 | Gender (Ref: male) | 1.68 | 0.58–4.86 | 0.342 | Gender (Ref: male) | 1.63 | 0.57–4.68 | 0.360 | Gender (Ref: male) | 1.96 | 0.61–6.36 | 0.262 |
| Age | 0.97 | 0.92–1.02 | 0.258 | Age | 0.97 | 0.91–1.03 | 0.324 | Age | 0.99 | 0.94–1.05 | 0.814 | Age | 0.97 | 0.91–1.04 | 0.377 |
| Refractive | 1.15 | 0.83–1.59 | 0.409 | Refractive | 0.85 | 0.60–1.20 | 0.353 | Refractive | 0.91 | 0.62–1.32 | 0.602 | Refractive | 0.77 | 0.50–1.19 | 0.235 |
| SFCT (per 100μm) | 0.53 | 0.32–0.87 | 0.013 * | VD in CC layer, % | 1.04 | 0.83–1.30 | 0.738 | VD in Sattler’s layer | 1.05 | 0.92–1.20 | 0.494 | VD in Haller’s layer | 0.99 | 0.91–1.08 | 0.819 |
| CSC vs. PCV | |||||||||||||||
| Gender (Ref: male) | 1.48 | 0.31–7.14 | 0.623 | Gender (Ref: male) | 1.11 | 0.22–5.53 | 0.903 | Gender (Ref: male) | 2.43 | 0.38–15.67 | 0.351 | Gender (Ref: male) | 2.09 | 0.33–13.22 | 0.432 |
| Age | 0.77 | 0.68–0.86 | <0.001 * | Age | 0.76 | 0.67–0.85 | <0.001 * | Age | 0.72 | 0.62–0.84 | <0.001 * | Age | 0.75 | 0.66–0.85 | <0.001 * |
| Refractive | 0.82 | 0.44–1.53 | 0.525 | Refractive | 0.78 | 0.41–1.4 | 0.447 | Refractive | 0.63 | 0.21–1.87 | 0.400 | Refractive | 0.56 | 0.24–1.27 | 0.163 |
| SFCT (per 100μm) | 1.37 | 0.68–2.77 | 0.385 | VD in CC layer | 0.82 | 0.52–1.29 | 0.380 | VD in Sattler’s layer | 1.27 | 0.99–1.63 | 0.064 | VD in Haller’s layer | 1.11 | 0.97–1.27 | 0.126 |
| CSC vs. nAMD | |||||||||||||||
| Gender (Ref: male) | 57.32 | 1.51–2170.33 | 0.029 * | Gender (Ref: male) | 4.67 | 0.48–45.50 | 0.185 | Gender (Ref: male) | 4.45 | 0.47–41.90 | 0.192 | Gender (Ref: male) | 54.25 | 1.08–2730.27 | 0.046 * |
| Age | 0.64 | 0.46–0.88 | 0.007 * | Age | 0.70 | 0.58–0.85 | <0.001 * | Age | 0.67 | 0.54–0.84 | <0.001 * | Age | 0.53 | 0.33–0.86 | 0.010 * |
| Refractive | 0.72 | 0.38–1.36 | 0.308 | Refractive | 1.04 | 0.54–2.03 | 0.904 | Refractive | 0.96 | 0.51–1.79 | 0.888 | Refractive | 0.76 | 0.35–1.63 | 0.479 |
| SFCT (per 100μm) | 8.16 | 1.30–51.14 | 0.025 * | VD in CC layer | 0.92 | 0.51–1.66 | 0.775 | VD in Sattler’s layer | 1.17 | 0.86–1.59 | 0.310 | VD in Haller’s layer | 1.34 | 1.06–1.70 | 0.015 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, B.; Zhang, X.; Li, Z.; Chhablani, J.; Fan, H.; Wang, Y.; Xie, R. Characterization of Choroidal Morphology and Vasculature in the Phenotype of Pachychoroid Diseases by Swept-Source OCT and OCTA. J. Clin. Med. 2022, 11, 3243. https://doi.org/10.3390/jcm11113243
Qiu B, Zhang X, Li Z, Chhablani J, Fan H, Wang Y, Xie R. Characterization of Choroidal Morphology and Vasculature in the Phenotype of Pachychoroid Diseases by Swept-Source OCT and OCTA. Journal of Clinical Medicine. 2022; 11(11):3243. https://doi.org/10.3390/jcm11113243
Chicago/Turabian StyleQiu, Bingjie, Xinyuan Zhang, Zhiqing Li, Jay Chhablani, Hao Fan, Yanhong Wang, and Rui Xie. 2022. "Characterization of Choroidal Morphology and Vasculature in the Phenotype of Pachychoroid Diseases by Swept-Source OCT and OCTA" Journal of Clinical Medicine 11, no. 11: 3243. https://doi.org/10.3390/jcm11113243
APA StyleQiu, B., Zhang, X., Li, Z., Chhablani, J., Fan, H., Wang, Y., & Xie, R. (2022). Characterization of Choroidal Morphology and Vasculature in the Phenotype of Pachychoroid Diseases by Swept-Source OCT and OCTA. Journal of Clinical Medicine, 11(11), 3243. https://doi.org/10.3390/jcm11113243








