Benchmark of Intraoperative Activity in Cardiac Surgery: A Comparison between Pre- and Post-Operative Prognostic Models
Abstract
:1. Introduction
2. Methods
2.1. Ethical Statement
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Data Validity
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study Population
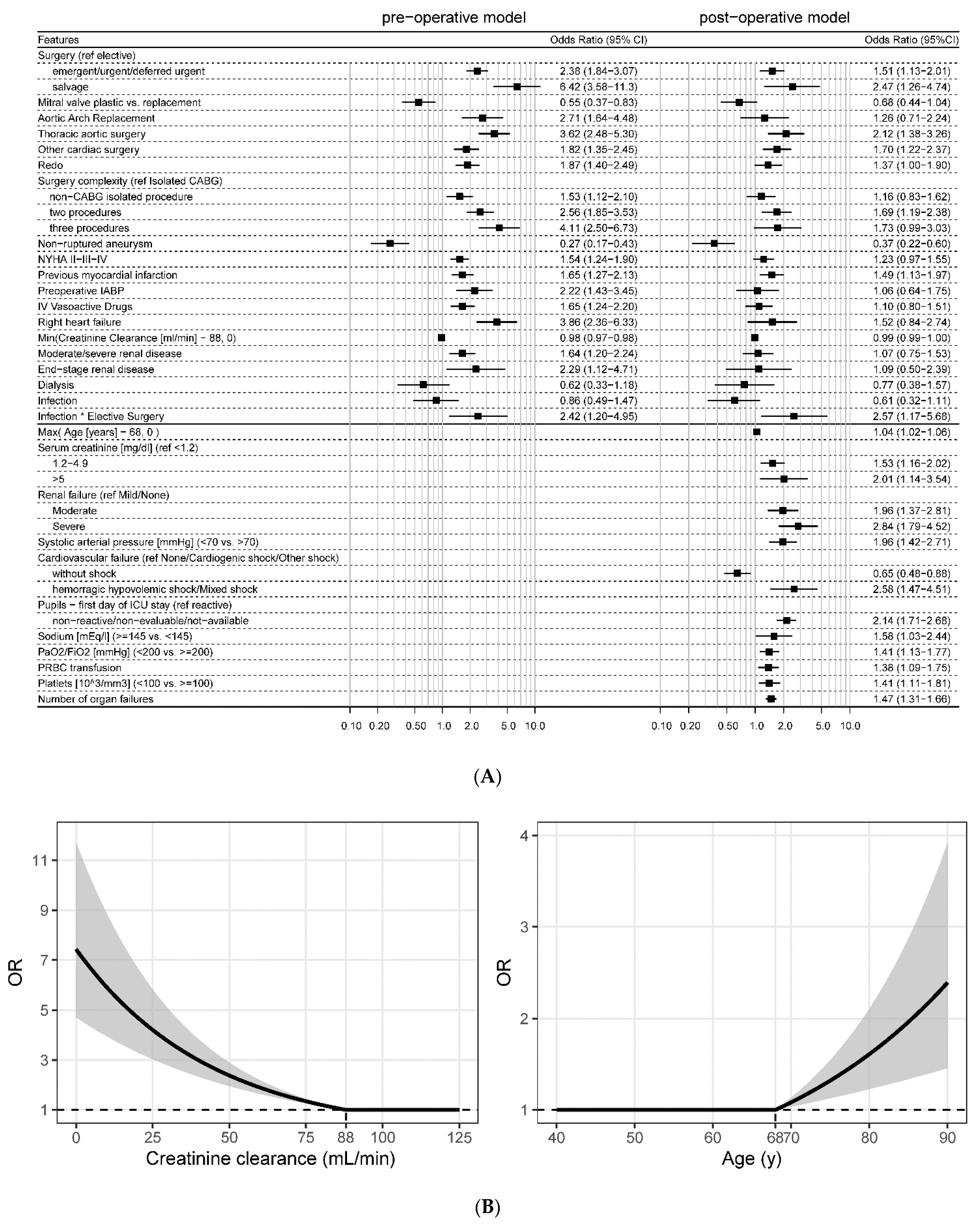
3.2. Patients’ Characteristics and Prognostic Models
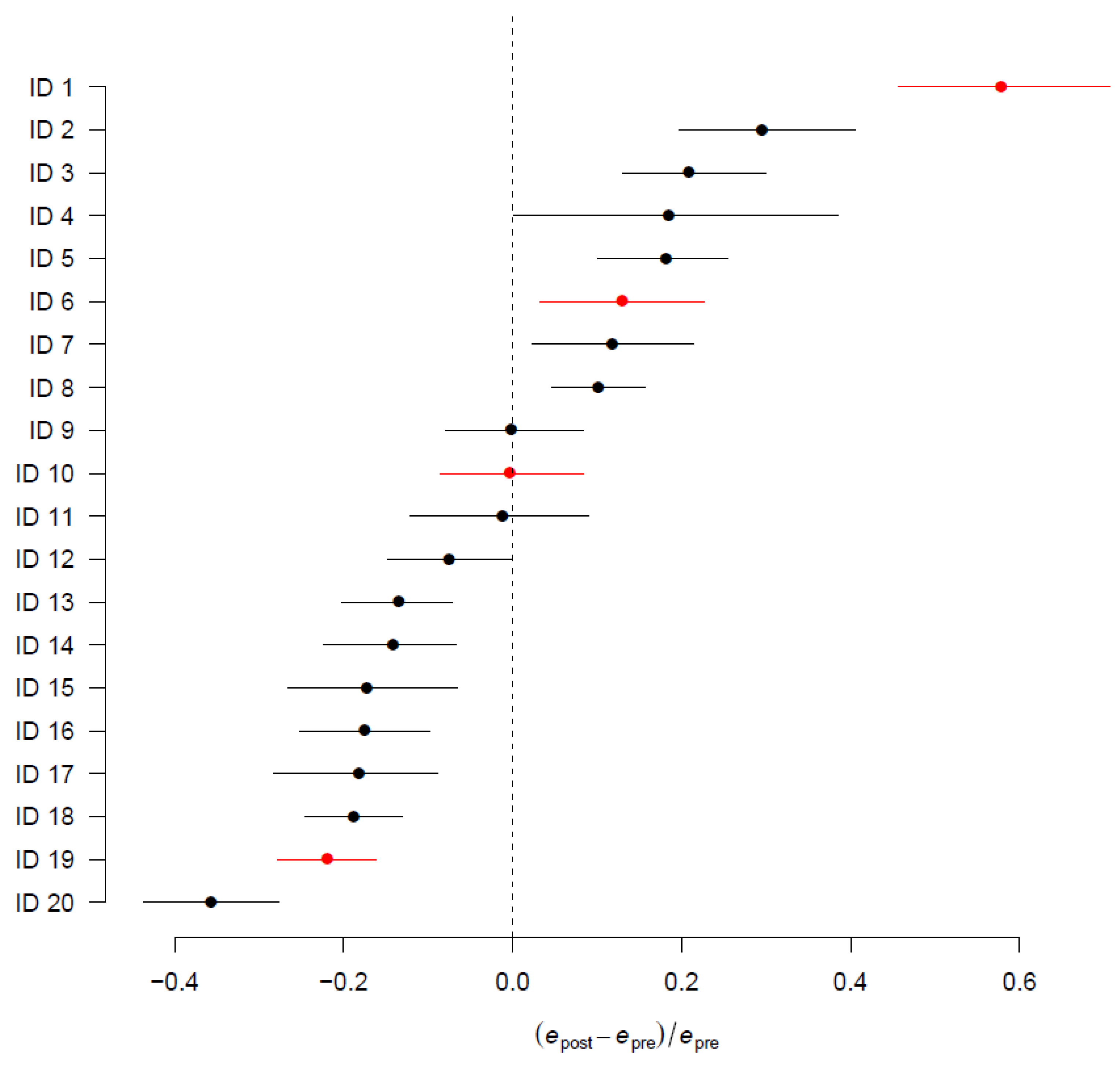
3.3. Difference between Pre- and Post-Operative Mortality
3.4. Subgroup and Centre-Specific Analysis
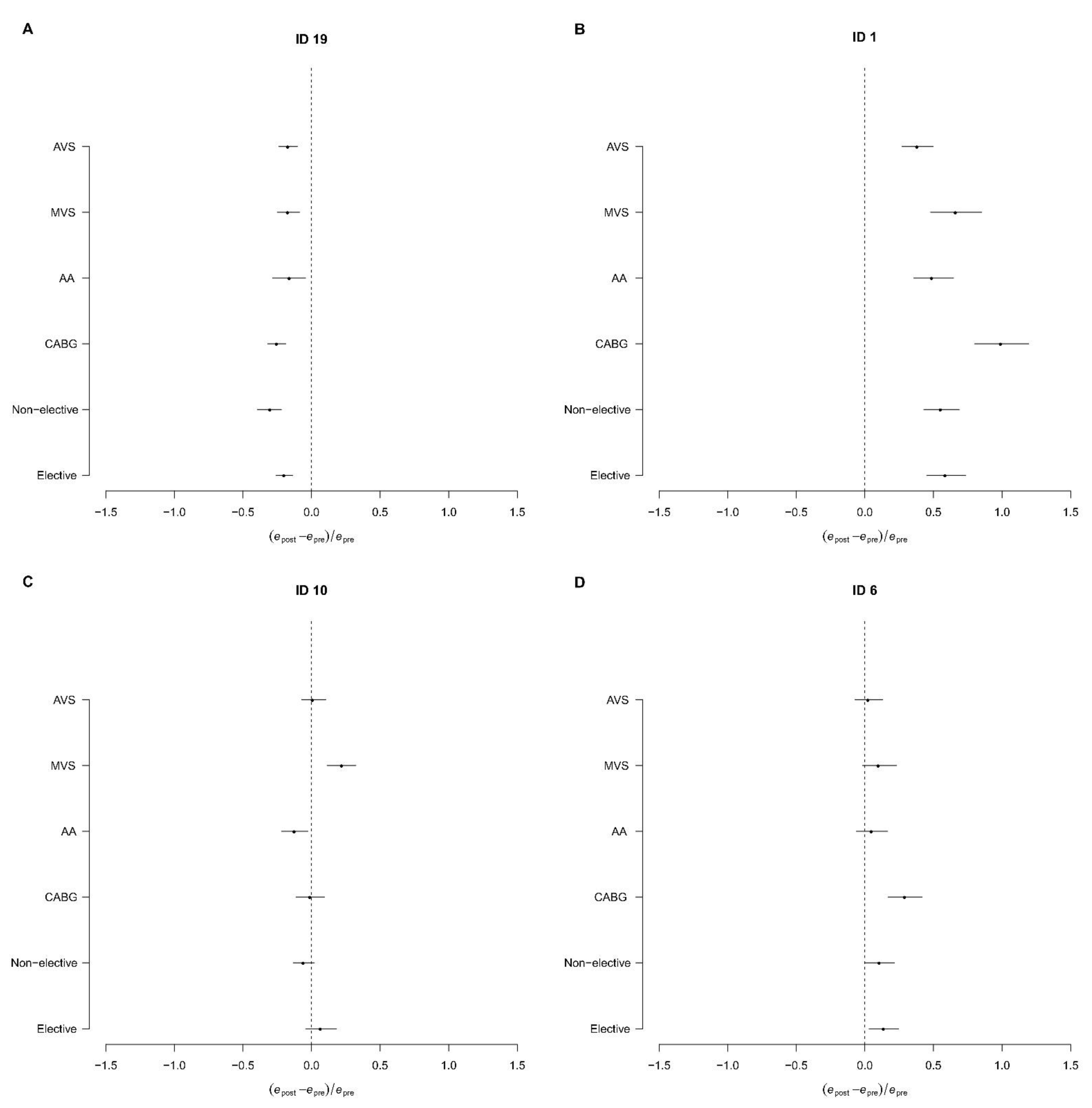
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Perrotti, A.; Ecarnot, F.; Monaco, F.; Dorigo, E.; Monteleone, P.; Besch, G.; Chocron, S. Quality of life 10 years after cardiac surgery in adults: A long-term follow-up study. Health Qual. Life Outcomes 2019, 17, 88. [Google Scholar] [CrossRef] [Green Version]
- Gjeilo, K.H.; Stenseth, R.; Wahba, A.; Lydersen, S.; Klepstad, P. Long-term health-related quality of life and survival after cardiac surgery: A prospective study. J. Thorac. Cardiovasc. Surg. 2018, 156, 2183–2190.e2. [Google Scholar] [CrossRef]
- Ferket, B.S.; Oxman, J.M.; Iribarne, A.; Gelijns, A.C.; Moskowitz, A.J. Cost-effectiveness analysis in cardiac surgery: A review of its concepts and methodologies. J. Thorac. Cardiovasc. Surg. 2017, 155, 1671–1681.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nashef, S.A.M.; Roques, F.; Michel, P.; Gauducheau, E.; Lemeshow, S.; Salamon, R. European system for cardiac operative risk evaluation (EuroSCORE). Eur. J. Cardio-Thorac. Surg. 1999, 16, 9–13. [Google Scholar] [CrossRef]
- Roques, F.; Nashef, S.A.M.; Michel, P.; Gauducheau, E.; De Vincentiis, C.; Baudet, E.; Cortina, J.; David, M.; Faichney, A.; Gavrielle, F.; et al. Risk factors and outcome in European cardiac surgery: Analysis of the EuroSCORE multinational database of 19,030 patients. Eur. J. Cardio-Thorac. Surg. 1999, 15, 816–822; discussion 822–823. [Google Scholar] [CrossRef] [Green Version]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. Euroscore II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef] [Green Version]
- Shahian, D.M.; O’Brien, S.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 1—Coronary Artery Bypass Grafting Surgery. Ann. Thorac. Surg. 2009, 88, S2–S22. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.; Delong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 2—Isolated Valve Surgery. Ann. Thorac. Surg. 2009, 88, S23–S42. [Google Scholar] [CrossRef]
- Shahian, D.M.; O’Brien, S.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 3—Valve Plus Coronary Artery Bypass Grafting Surgery. Ann. Thorac. Surg. 2009, 88, S43–S62. [Google Scholar] [CrossRef]
- Ranucci, M.; Castelvecchio, S.; Conte, M.; Megliola, G.; Speziale, G.; Fiore, F.; Guarracino, F.; Scolletta, S.; Escobar, R.M.; Falco, M.; et al. The easier, the better: Age, creatinine, ejection fraction score for operative mortality risk stratification in a series of 29,659 patients undergoing elective cardiac surgery. J. Thorac. Cardiovasc. Surg. 2011, 142, 581–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranucci, M.; Pistuddi, V.; Scolletta, S.; De Vincentiis, C.; Menicanti, L. The ACEF II Risk Score for cardiac surgery: Updated but still parsimonious. Eur. Heart J. 2017, 39, 2183–2189. [Google Scholar] [CrossRef]
- Lamarche, Y.; Elmi-Sarabi, M.; Ding, L.; Abel, J.G.; Sirounis, D.; Denault, A.Y. A score to estimate 30-day mortality after intensive care admission after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2017, 153, 1118–1125.e4. [Google Scholar] [CrossRef] [Green Version]
- Lemeshow, S.; Hosmer, D.W., Jr. A review of goodness of fit statistics for use in the development of logistic regression models. Am. J. Epidemiol. 1982, 115, 92–106. [Google Scholar] [CrossRef]
- Finazzi, S.; Poole, D.; Luciani, D.; Cogo, P.E.; Bertolini, G. Calibration Belt for Quality-of-Care Assessment Based on Dichotomous Outcomes. PLoS ONE 2011, 6, e16110. [Google Scholar] [CrossRef] [PubMed]
- LaPar, D.J.; Ailawadi, G.; Isbell, J.M.; Crosby, I.K.; Kern, J.A.; Rich, J.B.; Speir, A.M.; Kron, I.L. Mitral valve repair rates correlate with surgeon and institutional experience. J. Thorac. Cardiovasc. Surg. 2014, 148, 995–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.J.; Bojko, M.M.; Duda, M.M.; Iyengar, A.; Kelly, J.J.; Patrick, W.L.; Helmers, M.R.; Atluri, P. Association Among Surgeon Experience, Patient Risk, and Outcomes in Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2021, 111, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cromhout, P.F.; Berg, S.K.; Moons, P.; Damgaard, S.; Nashef, S.; Thygesen, L. Updating EuroSCORE by including emotional, behavioural, social and functional factors to the risk assessment of patients undergoing cardiac surgery: A study protocol. BMJ Open 2019, 9, e026745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cromhout, P.F.; Thygesen, L.C.; Moons, P.; Nashef, S.; Damgaard, S.; Christensen, A.V.; Rasmussen, T.B.; Borregaard, B.; Thrysoee, L.; Thorup, C.B.; et al. Supplementing prediction by EuroSCORE with social and patient-reported measures among patients undergoing cardiac surgery. J. Card. Surg. 2020, 36, 509–521. [Google Scholar] [CrossRef] [PubMed]

| Total (N = 15,533) | Alive (N = 14,971) | Dead (N = 562) | p-Value | |
|---|---|---|---|---|
| Age | <0.001 | |||
| Median (Q1, Q3) | 70 (62, 76) | 70 (62, 76) | 73 (65, 78) | |
| Gender (Male) | 10,506 (67.6%) | 10,143 (67.8%) | 363 (64.6%) | 0.116 |
| BMI | 0.141 | |||
| Underweight | 522 (3.4%) | 506 (3.4%) | 16 (2.9%) | |
| Normal | 6617 (42.8%) | 6351 (42.7%) | 266 (47.6%) | |
| Overweight | 5809 (37.6%) | 5614 (37.7%) | 195 (34.9%) | |
| Obese | 2498 (16.2%) | 2416 (16.2%) | 82 (14.7%) | |
| Missing | 87 | 84 | 3 | |
| Hypertension | 11,364 (73.2%) | 10,951 (73.1%) | 413 (73.5%) | 0.858 |
| NYHA class | <0.001 | |||
| I | 7798 (50.2%) | 7600 (50.8%) | 198 (35.2%) | |
| II–III | 7198 (46.3%) | 6919 (46.2%) | 279 (49.6%) | |
| IV | 537 (3.5%) | 452 (3.0%) | 85 (15.1%) | |
| Previous myocardial infarction | 2577 (16.6%) | 2452 (16.4%) | 125 (22.2%) | <0.001 |
| Arrhythmia | 2376 (15.3%) | 2238 (14.9%) | 138 (24.6%) | <0.001 |
| Diabetes | 0.006 | |||
| None | 12,076 (77.7%) | 11,656 (77.9%) | 420 (74.7%) | |
| Type 1 | 132 (0.8%) | 124 (0.8%) | 8 (1.4%) | |
| Type 2 without insulin treatment | 2258 (14.5%) | 2181 (14.6%) | 77 (13.7%) | |
| Type 2 with insulin treatment | 1067 (6.9%) | 1010 (6.7%) | 57 (10.1%) | |
| Ejection fraction | <0.001 | |||
| <30% | 397 (2.6%) | 357 (2.4%) | 40 (7.1%) | |
| 30–50% | 4611 (29.7%) | 4383 (29.3%) | 228 (40.6%) | |
| >50% | 10,525 (67.8%) | 10,231 (68.3%) | 294 (52.3%) | |
| Serum creatinine (mg/dL) | <0.001 | |||
| Median (Q1, Q3) | 1 (1, 1) | 1 (1, 1) | 1 (1, 2) | |
| Missing | 4 | 4 | 0 | |
| Creatinine clearance (mL/min) (computed with Cokcroft–Gault formula) | <0.001 | |||
| Median (Q1, Q3) | 74 (55, 95) | 74 (56, 95) | 56 (39, 76) | |
| Missing | 4 | 4 | 0 | |
| Urgency of intervention | <0.001 | |||
| Elective | 13,021 (83.8%) | 12,696 (84.8%) | 325 (57.8%) | |
| Deferred urgent | 1329 (8.6%) | 1252 (8.4%) | 77 (13.7%) | |
| Emergent/urgent | 1083 (7.0%) | 960 (6.4%) | 123 (21.9%) | |
| Salvage | 100 (0.6%) | 63 (0.4%) | 37 (6.6%) | |
| Redo | 1064 (6.8%) | 974 (6.5%) | 90 (16.0%) | <0.001 |
| Valve surgery | 8551 (55.1%) | 8252 (55.1%) | 299 (53.2%) | 0.370 |
| Aortic repair | 206 (1.3%) | 199 (1.3%) | 7 (1.2%) | 0.865 |
| Aortic replacement | 5468 (35.2%) | 5264 (35.2%) | 204 (36.3%) | 0.579 |
| Mitral repair | 1732 (11.2%) | 1698 (11.3%) | 34 (6.0%) | <0.001 |
| Mitral replacement | 1809 (11.6%) | 1705 (11.4%) | 104 (18.5%) | <0.001 |
| Tricuspid repair | 504 (3.2%) | 471 (3.1%) | 33 (5.9%) | <0.001 |
| Tricuspid replacement | 34 (0.2%) | 29 (0.2%) | 5 (0.9%) | <0.001 |
| CABG | 7454 (48.0%) | 7227 (48.3%) | 227 (40.4%) | <0.001 |
| Thoracic aorta surgery | 1748 (11.3%) | 1617 (10.8%) | 131 (23.3%) | <0.001 |
| Other cardiac surgery | 1023 (6.6%) | 927 (6.2%) | 96 (17.1%) | <0.001 |
| length of ICU stay | <0.001 | |||
| Median (Q1, Q3) | 1 (1, 2) | 1 (1, 2) | 4 (1, 12) | |
| Hospital length of stay (copy) | <0.001 | |||
| Median (Q1, Q3) | 11 (8, 17) | 11 (8, 17) | 16 (7, 32) | |
| Missing | 7 | 0 | 7 | |
| ICU outcome | 315 (2.0%) | 0 (0.0%) | 315 (56.0%) | <0.001 |
| Hospital outcome | 562 (3.6%) | 0 (0.0%) | 562 (100.0%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamperoni, A.; Carrara, G.; Greco, M.; Rossi, C.; Garbero, E.; Nattino, G.; Minniti, G.; Del Sarto, P.; Bertolini, G.; Finazzi, S.; et al. Benchmark of Intraoperative Activity in Cardiac Surgery: A Comparison between Pre- and Post-Operative Prognostic Models. J. Clin. Med. 2022, 11, 3231. https://doi.org/10.3390/jcm11113231
Zamperoni A, Carrara G, Greco M, Rossi C, Garbero E, Nattino G, Minniti G, Del Sarto P, Bertolini G, Finazzi S, et al. Benchmark of Intraoperative Activity in Cardiac Surgery: A Comparison between Pre- and Post-Operative Prognostic Models. Journal of Clinical Medicine. 2022; 11(11):3231. https://doi.org/10.3390/jcm11113231
Chicago/Turabian StyleZamperoni, Anna, Greta Carrara, Massimiliano Greco, Carlotta Rossi, Elena Garbero, Giovanni Nattino, Giuseppe Minniti, Paolo Del Sarto, Guido Bertolini, Stefano Finazzi, and et al. 2022. "Benchmark of Intraoperative Activity in Cardiac Surgery: A Comparison between Pre- and Post-Operative Prognostic Models" Journal of Clinical Medicine 11, no. 11: 3231. https://doi.org/10.3390/jcm11113231
APA StyleZamperoni, A., Carrara, G., Greco, M., Rossi, C., Garbero, E., Nattino, G., Minniti, G., Del Sarto, P., Bertolini, G., Finazzi, S., & Cardiac Surgical Intensive Care Writing Committee (GiViTI). (2022). Benchmark of Intraoperative Activity in Cardiac Surgery: A Comparison between Pre- and Post-Operative Prognostic Models. Journal of Clinical Medicine, 11(11), 3231. https://doi.org/10.3390/jcm11113231






