Efficacy of Nondiuretic Pharmacotherapy for Improving the Treatment of Congestion in Patients with Acute Heart Failure: A Systematic Review of Randomised Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Screening and Selection
2.3. Data Extraction
2.4. Eligibility Criteria
2.5. Quality Assessment
3. Results
3.1. Overview of the Included Studies
3.2. Effect of Adjuvant Therapy on Dyspnea
3.3. Effect of Adjuvant Therapies on Natriuretic Peptides
3.4. Effect of Adjuvant Therapies on Body Weight Change and Urine Output
3.5. Methodological Quality of Studies
4. Discussion
4.1. Optimising Vascular Resistance: Novel Vasodilatory Therapies
4.1.1. Vasopressin Antagonists
4.1.2. Serelaxin
4.1.3. Rolofylline
4.1.4. Nesiritide
4.1.5. Tezosentan
4.2. Optimising Inotropy
4.2.1. Optimising Inotropy: Novel Calcitrope Therapies
Levosimendan
Istaroxime
Cimlanod
4.2.2. Optimising Inotropy: Novel Myotrope Therapies
Omecamtiv Mecarbil
4.3. Novel Drug Targets in Clinical Development AHF—Sodium-Glucose Transporters (SGLT2 (Sodium-Glucose Cotransporter 2)) Inhibitors
4.4. The Gap in Evidence and Implications of Future Research
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurmani, S.; Squire, I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr. Heart Fail. Rep. 2017, 14, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute heart failure. Nat. Rev. Dis. Primers 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Di Nora, C.; Livi, U. Heart transplantation in cardiac storage diseases: Data on Fabry disease and cardiac amyloidosis. Curr. Opin. Organ Transpl. 2020, 25, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transpl. 2016, 35, 1–23. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Rubio-Gracia, J.; Demissei, B.G.; ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef]
- Felker, G.M.; Ellison, D.H.; Mullens, W.; Cox, Z.L.; Testani, J.M. Diuretic Therapy for Patients With Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1178–1195. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Ellison, D.H. Diuretic Resistance. Am. J. Kidney Dis. 2017, 69, 136–142. [Google Scholar] [CrossRef]
- Patoulias, D.; Papadopoulos, C.; Zografou, I.; Doumas, M. Acute heart failure, type 2 diabetes and loop diuretic use: Any adjunct role for sodium–glucose cotransporter-2 inhibitors? J. Cardiovasc. Med. 2020, 21, 343. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Valente, M.A.; Damman, K.; Hillege, H.L.; Navis, G.; Voors, A.A. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat. Rev. Cardiol. 2015, 12, 184–192. [Google Scholar] [CrossRef]
- Reed, B.N.; Devabhakthuni, S. Diuretic Resistance in Acute Decompensated Heart Failure: A Challenging Clinical Conundrum. Crit. Care Nurs. Q 2017, 40, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.W. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef]
- Valente, M.A.E.; Voors, A.A.; Damman, K.; Van Veldhuisen, D.J.; Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur. Heart J. 2014, 35, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Elnaem, M.H.; Mansour, N.O.; Nahas, A.F.; Baraka, M.A.; Elkalmi, R.; Cheema, E. Renal Outcomes Associated with the Use of Non-Insulin Antidiabetic Pharmacotherapy: A Review of Current Evidence and Recommendations. Int. J. Gen. Med. 2020, 13, 1395–1409. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.S.; Lam, C.S.P.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; et al. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients With Heart Failure by Baseline Diabetes Status: Results From the EMPEROR-Reduced Trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef]
- Cox, Z.L.; Collins, S.P.; Aaron, M.; Hernandez, G.A., III; Davidson, B.T.; Fowler, M.; Lindsell, C.J.; Frank, E.H., Jr.; Jenkins, C.A.; Kampe, A.; et al. Efficacy and safety of dapagliflozin in acute heart failure: Rationale and design of the DICTATE-AHF trial. Am. Heart J. 2021, 232, 116–124. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Zhang, H.; Liu, W.; Zhang, J.; Chen, Z.; You, L.; Wu, Y.; Zhou, K.; Zhang, L.; et al. Dyspnea Measurement in Acute Heart Failure: A Systematic Review and Evidence Map of Randomized Controlled Trials. Front. Med. 2021, 8, 728772. [Google Scholar] [CrossRef]
- Pollesello, P.; Gal, T.B.; Bettex, D.; Cerny, V.; Comin-Colet, J.; Eremenko, A.A.; Farmakis, D.; Fedele, F.; Fonseca, C.; Harjola, V.-P.; et al. Short-Term Therapies for Treatment of Acute and Advanced Heart Failure-Why so Few Drugs Available in Clinical Use, Why Even Fewer in the Pipeline? J. Clin. Med. 2019, 8, 1834. [Google Scholar] [CrossRef]
- Ali, S.; Jung, S.; Nandkeolyar, S.; Stoletniy, L.; Sakr, A.; Verbrugge, F.H.; Hilliard, A.; Abramov, D. Inpatient Diuretic Management of Acute Heart Failure: A Practical Review. Am. J. Cardiovasc. Drugs 2021, 21, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G. Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons: Hoboken, NJ, USA, 2016; pp. 187–241. [Google Scholar]
- Matsue, Y.; Suzuki, M.; Torii, S.; Yamaguchi, S.; Fukamizu, S.; Ono, Y.; Fujii, H.; Kitai, T.; Nishioka, T.; Sugi, K.; et al. Clinical Effectiveness of Tolvaptan in Patients With Acute Heart Failure and Renal Dysfunction. J. Card. Fail. 2016, 22, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Mentz, R.J.; Cole, R.T.; Adams, K.F.; Egnaczyk, G.F.; Fiuzat, M.; Patel, C.B.; Echols, M.; Khouri, M.G.; Tauras, J.M.; et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized With Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1399–1406. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Konstam, M.A.; Burnett, J.C.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST Clinical Status Trials. JAMA 2007, 297, 1332–1343. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kiernan, M.; Chandler, A.; Dhingra, R.; Mody, F.V.; Eisen, H.; Haught, W.H.; Wagoner, L.; Gupta, D.; Patten, R.; et al. Short-Term Effects of Tolvaptan in Patients With Acute Heart Failure and Volume Overload. J. Am. Coll. Cardiol. 2017, 69, 1409–1419. [Google Scholar] [CrossRef]
- Inomata, T.; Ikeda, Y.; Kida, K.; Shibagaki, Y.; Sato, N.; Kumagai, Y.; Shinagawa, H.; Ako, J.; Izumi, T. Effects of Additive Tolvaptan vs. Increased Furosemide on Heart Failure With Diuretic Resistance and Renal Impairment—Results From the K-STAR Study. Circ. J. 2017, 82, 159–167. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Gattis, W.; O’Connor, C. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004, 291, 1963–1967. [Google Scholar] [CrossRef]
- Goldsmith, S.R.; Elkayam, U.; Haught, W.H.; Barve, A.; He, W. Efficacy and Safety of the Vasopressin V1A/V2-Receptor Antagonist Conivaptan in Acute Decompensated Heart Failure: A Dose-Ranging Pilot Study. J. Card. Fail. 2008, 14, 641–647. [Google Scholar] [CrossRef]
- McMurray, J.J.; Teerlink, J.R.; Cotter, G.; Bourge, R.C.; Cleland, J.G.; Jondeau, G.; Krum, H.; Metra, M.; O’Connor, C.M.; Parker, J.D.; et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: The VERITAS randomized controlled trials. JAMA 2007, 298, 2009–2019. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Metra, M.; Felker, G.; Ponikowski, P.; Voors, A.A.; Weatherley, B.D.; Marmor, A.; Katz, A.; Grzybowski, J.; Unemori, E.; et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): A multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 2009, 373, 1429–1439. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Ponikowski, P.; Unemori, E.; Voors, A.A.; Adams, K.F.; et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 2013, 381, 29–39. [Google Scholar] [CrossRef]
- Publication Committee for the VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. JAMA 2002, 287, 1531–1540. [Google Scholar]
- O’Connor, C.; Starling, R.; Hernandez, A.F.; Armstrong, P.; Dickstein, K.; Hasselblad, V.; Heizer, G.; Komajda, M.; Massie, B.; Mcmurray, J.; et al. Effect of Nesiritide in Patients with Acute Decompensated Heart Failure. N. Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yi, S.; Zhu, B.; Wang, L.; Wang, H.; Bai, Y.; Ye, P.; Luo, L. Efficacy and safety of a modified dosage regimen of nesiritide in patients older than 75 years with acute heart failure. Aging Clin. Exp. Res. 2012, 24, 524–529. [Google Scholar] [PubMed]
- Cotter, G.; Dittrich, H.C.; Weatherley, B.D.; Bloomfield, D.M.; O’Connor, C.M.; Metra, M.; Massie, B.M. The PROTECT Pilot Study: A Randomized, Placebo-Controlled, Dose-Finding Study of the Adenosine A1 Receptor Antagonist Rolofylline in Patients With Acute Heart Failure and Renal Impairment. J. Card. Fail. 2008, 14, 631–640. [Google Scholar] [CrossRef]
- Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Weatherley, B.D.; Cleland, J.G.F.; Givertz, M.M.; Voors, A.; et al. Rolofylline, an Adenosine A1−Receptor Antagonist, in Acute Heart Failure. N. Engl. J. Med. 2010, 363, 1419–1428. [Google Scholar] [CrossRef]
- Packer, M.; Colucci, W.; Fisher, L.; Massie, B.M.; Teerlink, J.R.; Young, J.; Padley, R.J.; Thakkar, R.; Delgado-Herrera, L.; Salon, J.; et al. Effect of Levosimendan on the Short-Term Clinical Course of Patients With Acutely Decompensated Heart Failure. JACC Heart Fail. 2013, 1, 103–111. [Google Scholar] [CrossRef]
- Carubelli, V.; Zhang, Y.; Metra, M.; Lombardi, C.; Felker, G.M.; Filippatos, G.; Segal, R.; Malfatto, G.; la Rovere, M.T.; Li, D.; et al. Treatment with 24 hour istaroxime infusion in patients hospitalised for acute heart failure: A randomised, placebo-controlled trial. Eur. J. Heart Fail. 2020, 22, 1684–1693. [Google Scholar] [CrossRef]
- Felker, G.M.; McMurray, J.J.; Cleland, J.G.; O’Connor, C.M.; Teerlink, J.R.; Voors, A.A.; Belohlavek, J.; Bohm, M.; Borentain, M.; Bueno, H.; et al. Effects of a Novel Nitroxyl Donor in Acute Heart Failure: The STAND-UP AHF Study. J. Am. Coll. Cardiol. Heart Fail. 2021, 9, 146–157. [Google Scholar]
- Teerlink, J.R.; Felker, G.M.; McMurray, J.J.; Ponikowski, P.; Metra, M.; Filippatos, G.S.; Ezekowitz, J.A.; Dickstein, K.; Cleland, J.G.F.; Kim, J.B.; et al. Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: The ATOMIC-AHF Study. J. Am. Coll. Cardiol. 2016, 67, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.; Elvan, A.; Van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Smithline, H.A.; Donnino, M.; Blank, F.S.J.; Barus, R.; Coute, R.A.; Knee, A.B.; Visintainer, P. Supplemental thiamine for the treatment of acute heart failure syndrome: A randomized controlled trial. BMC Complement. Altern. Med. 2019, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Peacock, W.F.; Chandra, A.; Char, D.; Collins, S.; Der Sahakian, G.; Ding, L.; Dunbar, L.; Fermann, G.; Fonarow, G.; Garrison, N.; et al. Clevidipine in acute heart failure: Results of the A Study of Blood Pressure Control in Acute Heart Failure—A Pilot Study (PRONTO). Am. Heart J. 2014, 167, 529–536. [Google Scholar] [CrossRef]
- Liu, C.; Liu, K. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J. Cardiovasc. Pharmacol. 2014, 63, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hamo, C.E.; O’Connor, C.; Metra, M.; Udelson, J.E.; Gheorghiade, M.; Butler, J. A Critical Appraisal of Short-Term End Points in Acute Heart Failure Clinical Trials. J. Card. Fail. 2018, 24, 783–792. [Google Scholar] [CrossRef]
- Verbrugge, F.H. Editor’s Choice-Diuretic resistance in acute heart failure. Eur. Heart Acute Cardiovasc. Care 2018, 7, 379–389. [Google Scholar] [CrossRef]
- Luo, X.; Jin, Q.; Wu, Y. Tolvaptan add-on therapy in patients with acute heart failure: A systematic review and meta-analysis. Pharmacol. Res. Perspect. 2020, 8, e00614. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Davison, B.A.; Cotter, G.; Maggioni, A.P.; Sato, N.; Chioncel, O.; Ertl, G.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; et al. Effects of serelaxin in patients admitted for acute heart failure: A meta-analysis. Eur. J. Heart Fail. 2020, 22, 315–329. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Voors, A.A.; et al. Effects of Serelaxin in Patients with Acute Heart Failure. N. Engl. J. Med. 2019, 381, 716–726. [Google Scholar] [CrossRef]
- Maggioni, A.P.; López-Sendón, J.; Nielsen, O.W.; Hallén, J.; Aalamian-Mattheis, M.; Wang, Y.; Ertl, G. Efficacy and safety of serelaxin when added to standard of care in patients with acute heart failure: Results from a PROBE study, RELAX-AHF-EU. Eur. J. Heart Fail. 2019, 21, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.P.; Butler, J. The other serelaxin in acute heart failure study: Lessons from a pragmatic clinical trial. Eur. J. Heart Fail. 2019, 21, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Ennezat, P.V.; Stewart, M.; Samson, R.; Bouabdallaoui, N.; Maréchaux, S.; Banfi, C.; Bouvaist, H.; Le Jemtel, T.H. Editor’s Choice-Recent therapeutic trials on fluid removal and vasodilation in acute heart failure. Eur. Heart Acute Cardiovasc. Care 2014, 5, 86–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teerlink, J.R.; Iragui, V.J.; Mohr, J.P.; Carson, P.E.; Hauptman, P.J.; Lovett, D.H.; Miller, A.B.; Pina, I.L.; Thomson, S.; Varosy, P.D.; et al. The safety of an adenosine A(1)-receptor antagonist, rolofylline, in patients with acute heart failure and renal impairment: Findings from PROTECT. Drug Saf. 2012, 35, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, U.; Ng, T.M.; Hatamizadeh, P.; Janmohamed, M.; Mehra, A. Renal Vasodilatory Action of Dopamine in Patients With Heart Failure: Magnitude of Effect and Site of Action. Circulation 2008, 117, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Maskin, C.S.; Ocken, S.; Chadwick, B.; LeJemtel, T.H. Comparative systemic and renal effects of dopamine and angiotensin-converting enzyme inhibition with enalaprilat in patients with heart failure. Circulation 1985, 72, 846–852. [Google Scholar] [CrossRef]
- Wan, S.-H.; Stevens, S.R.; Borlaug, B.A.; Anstrom, K.J.; Deswal, A.; Felker, G.M.; Givertz, M.M.; Bart, B.A.; Tang, W.W.; Redfield, M.M.; et al. Differential Response to Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure With Reduced or Preserved Ejection Fraction: Results From the ROSE AHF Trial (Renal Optimization Strategies Evaluation in Acute Heart Failure). Circ. Heart Fail. 2016, 9, e002593. [Google Scholar] [CrossRef]
- Pashkovetsky, E.; Gupta, C.A.; Aronow, W.S. Use of levosimendan in acute and advanced heart failure: Short review on available real-world data. Ther. Clin. Risk Manag. 2019, 15, 765–772. [Google Scholar] [CrossRef]
- Harjola, V.-P.; Giannakoulas, G.; von Lewinski, D.; Matskeplishvili, S.; Mebazaa, A.; Papp, Z.; Schwinger, R.H.G.; Pollesello, P.; Parissis, J.T. Use of levosimendan in acute heart failure. Eur. Heart J. Suppl. 2018, 20, I2–I10. [Google Scholar] [CrossRef]
- Gong, B.; Li, Z.; Wong, P.C.Y. Levosimendan Treatment for Heart Failure: A Systematic Review and Meta-Analysis. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1415–1425. [Google Scholar] [CrossRef]
- Greenberg, B.; Urey, M.A. Stand [Up] and Stand by for New Strategies for Treating Acute Heart Failure. JACC Heart Fail. 2021, 9, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, D.H.; Boraii, S.; Cheema, E.; Elnaem, M.H.; Omar, T.; Abdelraouf, A.; Mansour, N.O. The intraperitoneal ondansetron for postoperative pain management following laparoscopic cholecystectomy: A proof-of-concept, double-blind, placebo-controlled trial. Biomed. Pharmacother. 2021, 140, 111725. [Google Scholar] [CrossRef] [PubMed]

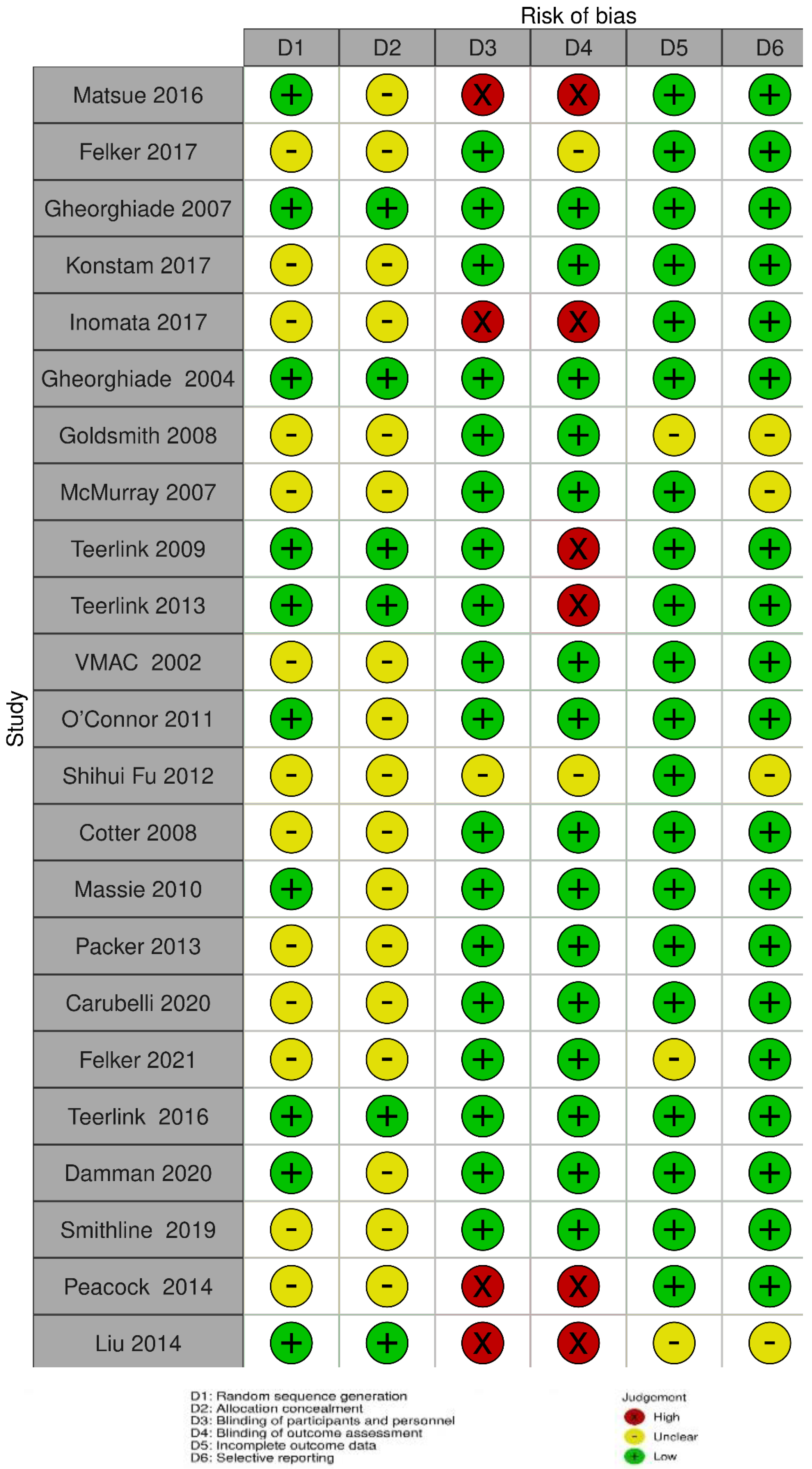
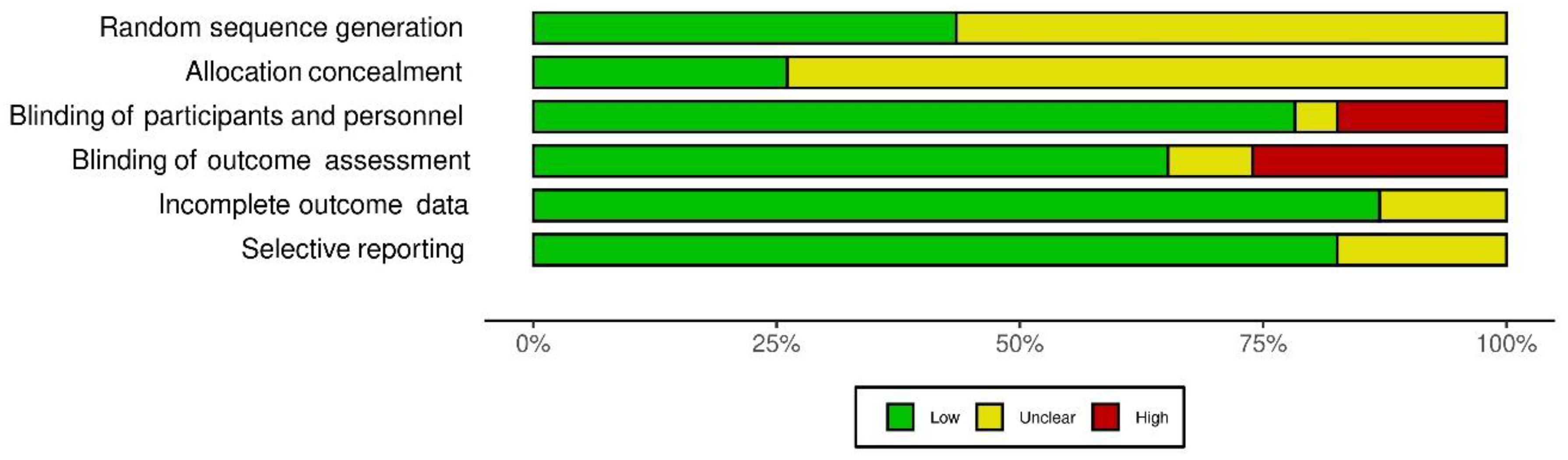
| Trial | Sample Size | Outcome(s) | Conclusion | |
|---|---|---|---|---|
| Tolvaptan | ||||
| 1 | AQUAMARINE | Matsue et al., 2016 [24] Japan (n = 217) | Primary endpoint: urine output (UOP) within 48 h of hospitalisation. Secondary endpoints: Improvement of dyspnea from baseline measured on patient-reported 7-point Likert scale up to 48 h after enrollment. Change in B-type natriuretic peptide (BNP) Change in body weight | In AHF patients with renal dysfunction, adding tolvaptan to conventional therapy increased diuresis and alleviated dyspnea symptoms. |
| 2 | TACTICS-HF | Felker et al., 2017 [25] USA (n = 257) | Primary endpoint: The proportion of patients who improved at least moderately in dyspnea on a 7-point Likert scale after 8 and 24 h. Secondary endpoints: Dyspnea relief, fluid loss, change in body weight, the proportion of patients free from clinical congestion at 48 and 72 h | Tolvaptan did not improve the proportion of AHF patients classified as responders. |
| 3 | EVEREST | Gheorghiade, et al., 2007 [26] Multi. (n = 4133) | Primary endpoints: Composite score of changes from baseline in patient-assessed global clinical status and body weight. | Tolvaptan improved symptoms in AHF patients. |
| 4 | SECRET | Konstam et al., 2017 [27] USA (n = 250) | Primary endpoint: Change in dyspnea score (Likert scale). Secondary endpoints: Change in body weight. Other endpoints: Change in BNP | Tolvaptan showed improvement in dyspnea and weight loss. |
| 5 | K STAR | Inomata et al., 2017 [28] Japan (n = 81) | Primary endpoint: The average change in UOP compared with its baseline values. Secondary endpoints: Changes in body weight, and congestive signs and symptoms | Tolvaptan increased diuresis without further renal impairment. |
| 6 | ACTIV | Gheorghiade et al., 2004 [29] USA (n = 250) | Primary endpoints: Change in body weight at 24 h Worsening heart failure (WHF) Secondary endpoints: Changes in dyspnea, oedema, UOP, diuretics use, patient- and physician-assessed symptom scales. | Tolvaptan decreased bodyweight more effectively than standard therapy. |
| Conivaptan | ||||
| 7 | Goldsmith et al., 2008 [30] USA (n = 170) | Did not specify a primary endpoint Change in patient-assessed severity of dyspnea Change in global status (VAS score) UOP | Conivaptan safely improves UOP but does not relieve dyspnea. | |
| Tezosentan | ||||
| 8 | VERITAS I, II | McMurray et al., 2007 [31] Multi. (n = 1435) | The primary endpoint of the individual studies: changes in dyspnea using a VAS over 24 h. | Tezosentan did not improve symptoms in AHF patients. |
| Serelaxin | ||||
| 9 | Pre-RELAX AHF | Teerlink et al., 2009 [32] Multi. (n = 234) | Primary endpoints (not prespecified): the overall effect of relaxin across several clinical domains: Relief of dyspnea (Likert scale and VAS). In-hospital WHF | Relaxin (30μg/kg) use relieved dyspnea. |
| 10 | RELAX-AHF | Teerlink et al., 2013 [33] Multi. (n = 1161) | Primary endpoints: Relief of dyspnea (Likert scale), and by VAS. | Treatment with serelaxin was associated with dyspnea relief. |
| Neseritide | ||||
| 11 | VMAC | Publication Committee for the VMAC Investigators, 2002 [34] (n = 489) | Primary endpoints: The absolute changes in PCWP The patient’s self-evaluation of dyspnea | Nesiritide improves hemodynamic function and dyspnea more effectively than placebo. |
| 12 | ASCEND-HF | O’Connor et al., 2011 [35] Multi. (n = 7141) | Primary endpoint: Co-primary endpoints of dyspnea change after six and 24 h (Likert scale). | Nesiritide has a nonsignificant effect on dyspnea. |
| 13 | Fu et al., 2012 [36] China (n = 140) | Primary endpoints not specified Dyspnea using the medical research council (MRC) scales. Assessment of oedema Assessment of water loss volume | Nesiritide was associated with better symptoms relief, such as dyspnea and oedema. | |
| Rolofylline | ||||
| 14 | PROTECT pilot PROTECT pilot study | Cotter et al., 2008 [37] Multi. (n = 301) | Composite primary trichotomous endpoint: Patient-reported dyspnea (7-point Likert scale), WHF and worsening renal insufficiency. Patients were classified as improved, worse, or unchanged). | Rolofylline improved dyspnea relief and decreased worsening heart failure or renal function. |
| 15 | PROTECT | Massie et al., 2010 [38] Multi. (n = 2033) | The primary endpoint (clinical composite) Treatment success, i.e., moderate/marked improvement in dyspnea. Treatment failure, death or readmission for heart failure (HF) or worsening heart failure WHF No change in the patient’s condition. | Rolofylline does not show promise in treating patients AHF with renal dysfunction. |
| Trial | Sample Size | Outcome(s) | Conclusion | |
|---|---|---|---|---|
| Calcitrope trials | ||||
| Levosimendan | ||||
| 16 | REVIVE I and II | Packer et al., 2013 [39] USA Revive (I) (n = 100), (II) (n = 600) | Composite endpoint of clinically Patient-reported measures: Improved: moderate/markedly improvement Worse: Persistent/unresponsive symptoms Unchanged | levosimendan can produce significant symptomatic benefits. |
| Istaroxime | ||||
| 17 | Carubelli et al., 2020 [40] Multi. (n = 120) | Secondary endpoints Changes in dyspnea by VAS, changes in NT-proBNP, WHF | Istaroxime use did not add benefit to the diuretic response. | |
| Cimlanod | ||||
| 18 | STAND-UP AHF | Felker et al., 2021 [41] Multi. (n = 322) | Secondary endpoints: Change in plasma concentration of NT-proBNP. Change in patient-reported resting dyspnea using the AUC of the numeric rating scale. | Cimlanod marginally improved some parameters related to congestion. |
| Myotrope trials: Omecamtiv mecarbil | ||||
| 19 | ATOMIC-AHF | Teerlink et al., 2016 [42] Multi. (n = 606) | Primary endpoint Dyspnea relief (Likert scale) Secondary endpoints Dyspnea numerical response AUC Patient global assessment response NT-proBNP change from baseline. | In patients with AHF, omecamtiv mecarbil had no significant effect on dyspnea. |
| Trial | Sample Size | Outcome(s) | Conclusion | |
|---|---|---|---|---|
| Empagliflozin | ||||
| 20 | EMPA-RESPONSE-AHF | Damman et al., 2020 [43] Netherlands (n = 80) | Primary endpoints Change in the AUC of dyspnea visual analogue scale Diuretic response Percentage change in NT-proBNP | Empagliflozin did not enhance diuretic response. |
| Thiamine | ||||
| 21 | Smithline et al., 2019 [44] Multi. (n = 118) | Primary endpoint Dyspnea severity using VAS in three positions: sitting upright on supplemental oxygen, sitting upright off oxygen, or lying supine off oxygen. | The results of this study do not support the adjuvant use of thiamine in AHF. | |
| Clevidipine | ||||
| 22 | PRONTO | Peacock et al., 2014 [45] Multi. (n = 104) | Secondary endpoint: Dyspnea reduction (VAS score) at different time points up to 720 min after administration | Clevidipine effectively lowers blood pressure and improves dyspnea in hypertensive AHF patients. |
| Glucocorticoid | ||||
| 23 | COPE-ADHF | Liu et al., 2014 [46] (n = 102) | Other Outcomes Patient-assessed dyspnea (7-point scale). Physician-assessed global clinical (7-point scale). | This preliminary trial shows the potential benefit of short-term glucocorticoid use in patients with ADHF. |
| Common Pitfalls in AHF Studies | Recommendations |
|---|---|
| Lack of standardised algorithm for diuretic therapy | Trials with primary efficacy indicators of diuresis require a protocolised algorithm that guides dose adjustment based on response (Na in urine and UOP) to reduce the impact of prescribing variations on the diuretic therapy results. The most recent European society of cardiology practice guidelines [5] and ongoing clinical trials have endorsed a similar approach [18]. |
Heterogeneity in efficacy indicators impeded the evaluation of potential therapies:
| Variability could be minimised [19] via:
|
| Time-to-treatment [5] | As with acute coronary syndrome, current guidelines advocate a ‘time-to-treatment’ concept and recommend early treatment in patients with AHF, ideally prior to hospital admission. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emara, A.N.; Mansour, N.O.; Elnaem, M.H.; Wadie, M.; Dehele, I.S.; Shams, M.E.E. Efficacy of Nondiuretic Pharmacotherapy for Improving the Treatment of Congestion in Patients with Acute Heart Failure: A Systematic Review of Randomised Controlled Trials. J. Clin. Med. 2022, 11, 3112. https://doi.org/10.3390/jcm11113112
Emara AN, Mansour NO, Elnaem MH, Wadie M, Dehele IS, Shams MEE. Efficacy of Nondiuretic Pharmacotherapy for Improving the Treatment of Congestion in Patients with Acute Heart Failure: A Systematic Review of Randomised Controlled Trials. Journal of Clinical Medicine. 2022; 11(11):3112. https://doi.org/10.3390/jcm11113112
Chicago/Turabian StyleEmara, Abdelrahman N., Noha O. Mansour, Mohamed Hassan Elnaem, Moheb Wadie, Inderpal Singh Dehele, and Mohamed E. E. Shams. 2022. "Efficacy of Nondiuretic Pharmacotherapy for Improving the Treatment of Congestion in Patients with Acute Heart Failure: A Systematic Review of Randomised Controlled Trials" Journal of Clinical Medicine 11, no. 11: 3112. https://doi.org/10.3390/jcm11113112
APA StyleEmara, A. N., Mansour, N. O., Elnaem, M. H., Wadie, M., Dehele, I. S., & Shams, M. E. E. (2022). Efficacy of Nondiuretic Pharmacotherapy for Improving the Treatment of Congestion in Patients with Acute Heart Failure: A Systematic Review of Randomised Controlled Trials. Journal of Clinical Medicine, 11(11), 3112. https://doi.org/10.3390/jcm11113112






