Impact of Immunosuppressive Drugs on Fibroblasts: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
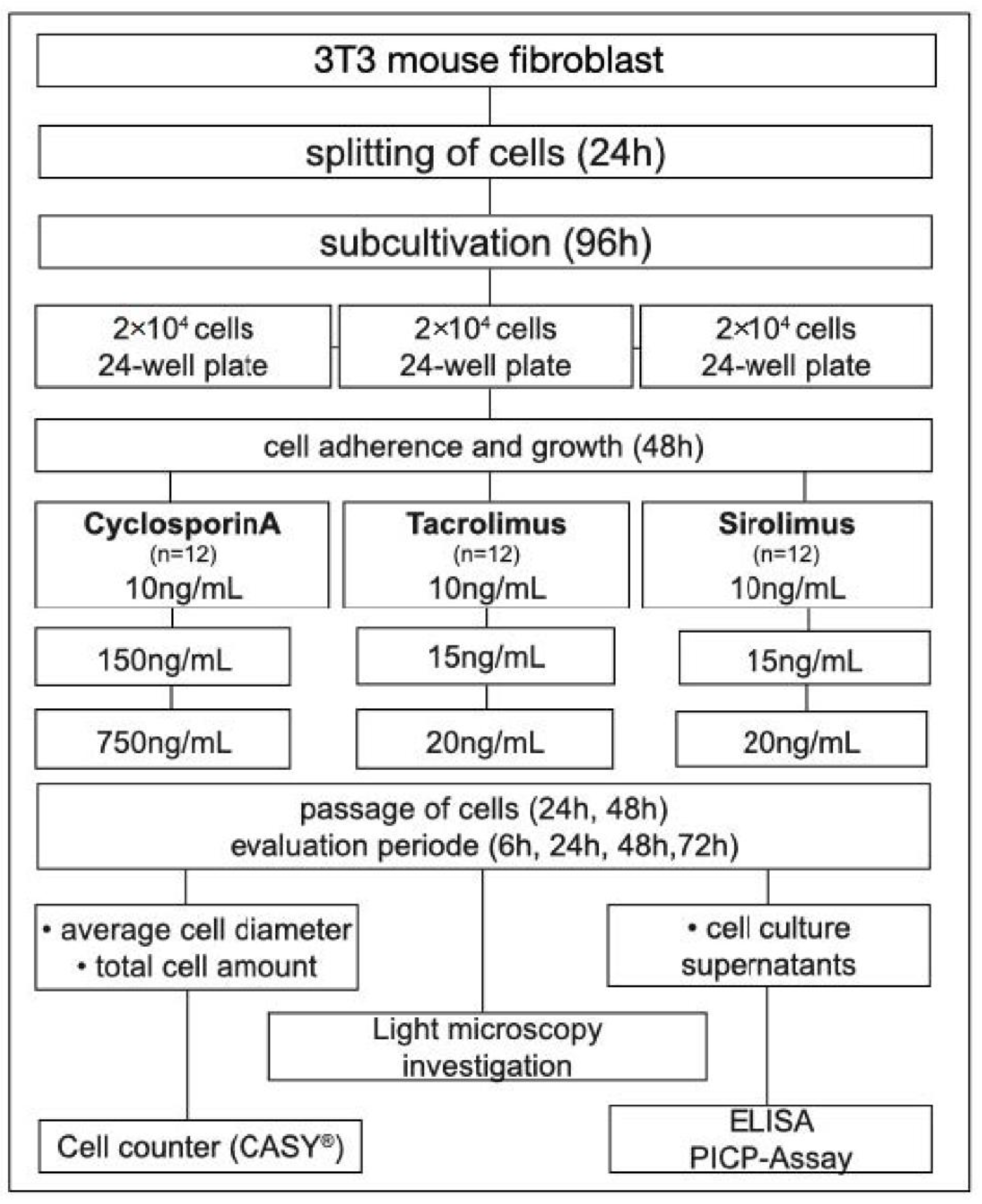
2.1. Study Design
2.2. Immunosuppressive Drugs
2.3. Cell Culture and Treatment
2.4. Cellular Analysis
2.5. Procollagen Type 1 Assay (PICP)
2.6. Statistical Analysis
3. Results
3.1. CsA
3.2. TaC
3.3. SiR
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayter, S.M.; Cook, M.C. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun. Rev. 2012, 11, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Cajanding, R. Immunosuppression following organ transplantation. Part 1: Mechanisms and immunosuppressive agents. Br. J. Nurs. 2018, 27, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Cajanding, R. Immunosuppression following organ transplantation. Part 2: Complications and their management. Br. J. Nurs. 2018, 27, 1059–1065. [Google Scholar] [CrossRef]
- Petti, S.; Polimeni, A.; Berloco, P.B.; Scully, C. Orofacial diseases in solid organ and hematopoietic stem cell transplant recipients. Oral Dis. 2013, 19, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Wendorff, H.; Berisha, L.; Meisel, A.; Widmer, F.; Marcinkowski, A.; Teschler, H.; Sommerwerck, U.; Haak, R.; Kollmar, O.; et al. Association between the time after transplantation and different immunosuppressive medications with dental and periodontal treatment need in patients after solid organ transplantation. Transpl. Infect. Dis. 2018, 20, e12832. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S74–S84. [Google Scholar] [CrossRef]
- Marshall, R.I.; Bartold, P.M. A clinical review of drug-induced gingival overgrowths. Aust. Dent. J. 1999, 44, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, S.; Okanobu, A.; Hatano, S.; Kajiya, M.; Sasaki, S.; Hamamoto, Y.; Iwata, T.; Ouhara, K.; Takeda, K.; Mizuno, N.; et al. Relationship between periodontal inflammation and calcium channel blockers induced gingival overgrowth-a cross-sectional study in a Japanese population. Clin. Oral Investig. 2019, 23, 4099–4105. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Cesarano, F.; Arazzi, M.; Liberato, L.D.; Scacco, S.; Grassi, R.; Grassi, F.R.; Gnoni, A.; et al. Periodontal Microbiological Status Influences the Occurrence of Cyclosporine-A and Tacrolimus-Induced Gingival Overgrowth. Antibiotics 2019, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Hatahira, H.; Abe, J.; Hane, Y.; Matsui, T.; Sasaoka, S.; Motooka, Y.; Hasegawa, S.; Fukuda, A.; Naganuma, M.; Ohmori, T.; et al. Drug-induced gingival hyperplasia: A retrospective study using spontaneous reporting system databases. J. Pharm. Health Care Sci. 2017, 3, 19. [Google Scholar] [CrossRef]
- Paixão, C.G.; Sekiguchi, R.T.; Saraiva, L.; Pannuti, C.M.; Silva, H.T.; Medina-Pestana, J.; Romito, G.A. Gingival overgrowth among patients medicated with cyclosporin A and tacrolimus undergoing renal transplantation: A prospective study. J. Periodontol. 2011, 82, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.S.; Seymour, R.A.; Taylor, J.J.; Thomason, J.M. Prevalence of gingival overgrowth in transplant patients immunosuppressed with tacrolimus. J. Clin. Periodontol. 2004, 31, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Cota, L.O.; Aquino, D.R.; Franco, G.C.; Cortelli, J.R.; Cortelli, S.C.; Costa, F.O. Gingival overgrowth in subjects under immunosuppressive regimens based on cyclosporine, tacrolimus, or sirolimus. J. Clin. Periodontol. 2010, 37, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Thomason, J.M.; Ellis, J.S. The pathogenesis of drug-induced gingival overgrowth. J. Clin. Periodontol. 1996, 23 Pt 1, 165–175. [Google Scholar] [CrossRef]
- Jung, J.Y.; Jeong, Y.J.; Jeong, T.S.; Chung, H.J.; Kim, W.J. Inhibition of apoptotic signals in overgrowth of human gingival fibroblasts by cyclosporin A treatment. Arch. Oral Biol. 2008, 53, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Moreo, G.; Limongelli, L.; Palmieri, A.; Carinci, F. Drug-Induced Gingival Overgrowth: The Effect of Cyclosporin A and Mycophenolate Mophetil on Human Gingival Fibroblasts. Biomedicines 2020, 8, 221. [Google Scholar] [CrossRef]
- Todaro, G.J.; Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 1963, 17, 299–313. [Google Scholar] [CrossRef]
- Beaumont, J.; Chesterman, J.; Kellett, M.; Durey, K. Gingival overgrowth: Part 1: Aetiology and clinical diagnosis. Br. Dent. J. 2017, 222, 85–91. [Google Scholar] [CrossRef]
- Hallmon, W.W.; Rossmann, J.A. The role of drugs in the pathogenesis of gingival overgrowth. A collective review of current concepts. Periodontology 2000 1999, 21, 176–196. [Google Scholar] [CrossRef]
- Cotrim, P.; Martelli-Junior, H.; Graner, E.; Sauk, J.J.; Coletta, R.D. Cyclosporin A induces proliferation in human gingival fibroblasts via induction of transforming growth factor-beta1. J. Periodontol. 2003, 74, 1625–1633. [Google Scholar] [CrossRef]
- Ponnaiyan, D.; Jegadeesan, V. Cyclosporine A: Novel concepts in its role in drug-induced gingival overgrowth. Dent. Res. J. 2015, 12, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Naruishi, K.; Yamada-Naruishi, H.; Omori, K.; Nishimura, F.; Takashiba, S. Long-term cyclosporin A exposure suppresses cathepsin-B and -L activity in gingival fibroblasts. J. Periodontal Res. 2004, 39, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A.; Hassell, T.; Jacobs, D.; Manning, C.J.; Hefti, A.F. Cyclosporin A and hydroxycyclosporine (M-17) affect the secretory phenotype of human gingival fibroblasts. J. Oral Pathol. Med. 1998, 27, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Palmieri, A.; Lucchese, A.; Di Stasio, D.; Moreo, G.; Carinci, F. Role of Cyclosporine in Gingival Hyperplasia: An In Vitro Study on Gingival Fibroblasts. Int. J. Mol. Sci. 2020, 21, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliano, N.; Moscheni, C.; Tartaglia, G.M.; Selleri, S.; Chiriva-Internati, M.; Cobos, E.; Torri, C.; Costa, F.; Pettinari, L.; Gioia, M. A therapeutic dose of FK506 does not affect collagen turnover pathways in healthy human gingival fibroblasts. Transplant. Proc. 2008, 40, 1419–1424. [Google Scholar] [CrossRef]
- Nassar, C.A.; Nassar, P.O.; Andia, D.C.; Guimarães, M.R.; Spolidorio, L.C. The effects of up to 240 days of tacrolimus therapy on the gingival tissues of rats—A morphological evaluation. Oral Dis. 2008, 14, 67–72. [Google Scholar] [CrossRef]
- Pamuk, F.; Cetinkaya, B.O.; Ayas, B.; Keles, G.C.; Gacar, A. Evaluation of gingival alterations in rats medicated with cyclosporine A, tacrolimus and sirolimus: A stereological study. J. Periodontal. Res. 2015, 50, 629–636. [Google Scholar] [CrossRef]
- Sam, W.J.; Chamberlain, C.E.; Lee, S.J.; Goldstein, J.A.; Hale, D.A.; Mannon, R.B.; Kirk, A.D.; Hon, Y.Y. Associations of ABCB1 3435C>T and IL-10-1082G>A polymorphisms with long-term sirolimus dose requirements in renal transplant patients. Transplantation 2011, 92, 1342–1347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Z.; Fan, J.; Liu, G.; Peng, Z. Impact of interleukin-10 gene polymorphisms on tacrolimus dosing requirements in Chinese liver transplant patients during the early posttransplantation period. Eur. J. Clin. Pharmacol. 2011, 67, 803–813. [Google Scholar] [CrossRef]
- Crews, K.R.; Hicks, J.K.; Pui, C.H.; Relling, M.V.; Evans, W.E. Pharmacogenomics and individualized medicine: Translating science into practice. Clin. Pharmacol. Ther. 2012, 92, 467–475. [Google Scholar] [CrossRef] [Green Version]
- García, M.; Macías, R.M.; Cubero, J.J.; Benítez, J.; Caravaca, F.; Gervasini, G. ABCB1 polymorphisms are associated with cyclosporine-induced nephrotoxicity and gingival hyperplasia in renal transplant recipients. Eur. J. Clin. Pharmacol. 2013, 69, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Klein, T.E. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011, 89, 464–467. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, G.; Sievers, L.; Tiburcy, M.; Zimmermann, W.H.; Kollmar, O.; Schmalz, G.; Ziebolz, D. Impact of Immunosuppressive Drugs on Fibroblasts: An In Vitro Study. J. Clin. Med. 2022, 11, 3107. https://doi.org/10.3390/jcm11113107
Wagner G, Sievers L, Tiburcy M, Zimmermann WH, Kollmar O, Schmalz G, Ziebolz D. Impact of Immunosuppressive Drugs on Fibroblasts: An In Vitro Study. Journal of Clinical Medicine. 2022; 11(11):3107. https://doi.org/10.3390/jcm11113107
Chicago/Turabian StyleWagner, Gunar, Lisa Sievers, Malte Tiburcy, Wolfram Hubertus Zimmermann, Otto Kollmar, Gerhard Schmalz, and Dirk Ziebolz. 2022. "Impact of Immunosuppressive Drugs on Fibroblasts: An In Vitro Study" Journal of Clinical Medicine 11, no. 11: 3107. https://doi.org/10.3390/jcm11113107
APA StyleWagner, G., Sievers, L., Tiburcy, M., Zimmermann, W. H., Kollmar, O., Schmalz, G., & Ziebolz, D. (2022). Impact of Immunosuppressive Drugs on Fibroblasts: An In Vitro Study. Journal of Clinical Medicine, 11(11), 3107. https://doi.org/10.3390/jcm11113107




