The Role of Heparin in COVID-19: An Update after Two Years of Pandemics
Abstract
:1. Introduction
2. Endothelial Damage in COVID-19
3. Sustained Prothrombotic Changes in COVID-19
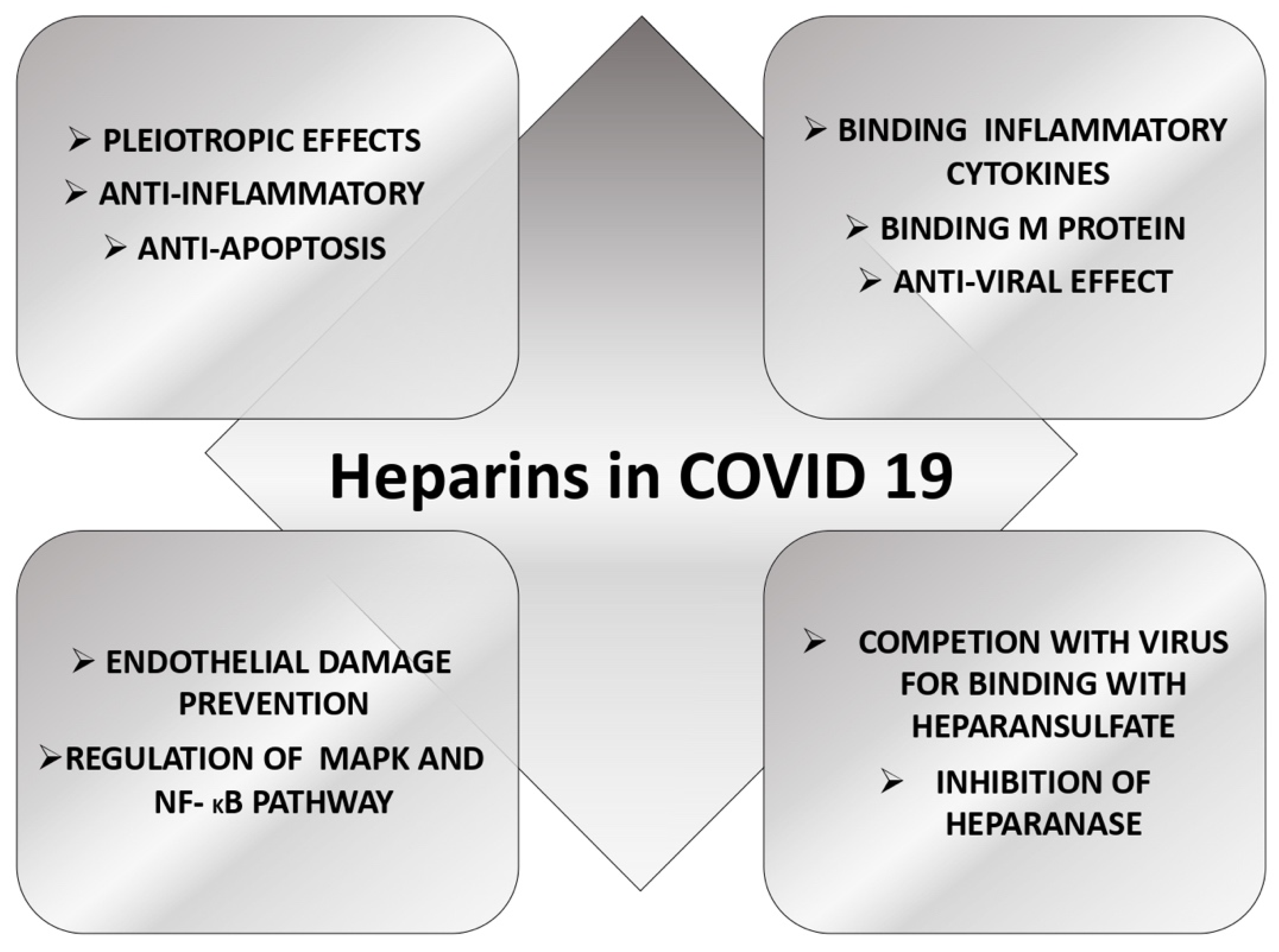
4. The Role of Heparin in COVID-19
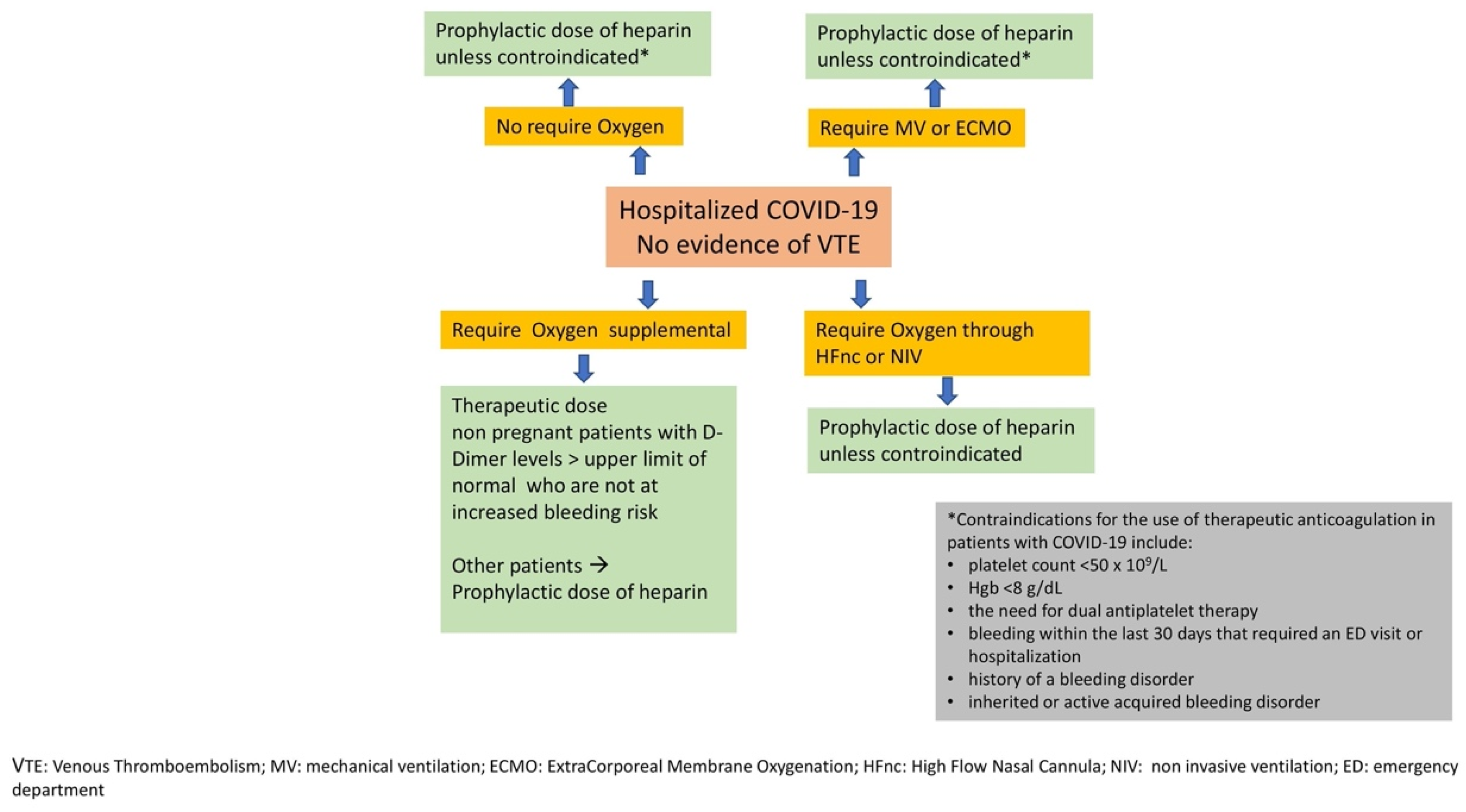
5. Evidence of Heparin Use in COVID-19 Acute Illness
6. Postdischarge Prophylaxis with Heparin
7. Discussion
Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, S.Q.; Peng, H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus-2019-nCoV-sixth public health emergency of international concern. Eurosurveillance 2020, 25, 200131e. [Google Scholar] [CrossRef]
- WHO Site. Available online: https://covid19.who.int/ (accessed on 18 March 2022).
- Ccc Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154, Erratum in Nat. Rev. Microbiol. 2022, 20, 315. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Gungor, B.; Atici, A.; Baycan, O.F.; Alici, G.; Ozturk, F.; Tugrul, S.; Asoglu, R.; Cevik, E.; Sahin, I.; Barman, H.A. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 39, 173–179. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.; Van der Meer, N.J.; Arbous, M.S.; Gommers, D.A.; Kant, K.M.; Kaptein, F.H.; van Paassen, J.; Stals, M.A.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Roberts, L.N.; Navaratnam, A.V.; Arya, R.; Briggs, T.W.R.; Gray, W.K. Venous thromboembolism in patients hospitalised with COVID-19 in England. Thromb. Res. 2022, 213, 138–144. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Cai, X.; Zhang, W.; Li, Y.; Fu, C. Prevalence of Venous Thromboembolism in Critically Ill Patients with Coronavirus Disease 2019: A Meta-Analysis. Front. Med. 2021, 8, 603558. [Google Scholar] [CrossRef]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.D.; Sacco, C.; Bertuzzi, A.; et al. Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef]
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Le Mao, R.; Rodríguez, C.; Hunt, B.J.; Monreal, M. Incidence of VTE and Bleeding Among Hospitalized Patients with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest 2021, 159, 1182–1196. [Google Scholar] [CrossRef]
- Chowdhury, J.F.; Moores, L.K.; Connors, J.M. Anticoagulation in hospitalized patients with COVID-19. N. Engl. J. Med. 2020, 383, 1675–1678. [Google Scholar] [CrossRef]
- Heinrich, F.; Roedl, K.; Jarczak, D.; Goebels, H.-L.; Heinemann, A.; Schäfer, U.; Ludwig, F.; Bachmann, M.; Bein, B.; Weber, C.F.; et al. New Insights in the Occurrence of Venous Thromboembolism in Critically Ill Patients with COVID-19—A Large Postmortem and Clinical Analysis. Viruses 2022, 14, 811. [Google Scholar] [CrossRef]
- Hull, R.D.; Merali, T.; Mills, A.; Stevenson, A.L.; Liang, J. Venous thromboembolism in elderly high-risk medical patients: Time course of events and influence of risk factors. Clin. Appl. Thromb. Hemost. 2013, 19, 357–362. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Baldwin, A.L.; Thurston, G. Mechanics of endothelial cell architecture and vascular permeability. Crit. Rev. Biomed. Eng. 2001, 29, 247–278. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [Green Version]
- Mangana, C.; Lorigo, M.; Cairrao, E. Implications of Endothelial Cell-Mediated Dysfunctions in Vasomotor Tone Regulation. Biologics 2021, 1, 231–251. [Google Scholar] [CrossRef]
- Del Turco, S.; Vianello, A.; Ragusa, R.; Caselli, C.; Basta, G. COVID-19 and cardiovascular consequences: Is the endothelial dysfunction the hardest challenge? Thromb. Res. 2020, 196, 143–151. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Mackman, N.; Antoniak, S.; Wolberg, A.S.; Kasthuri, R.; Key, N.S. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2033–2044. [Google Scholar] [CrossRef]
- Pavoni, V.; Gianesello, L.; Pazzi, M.; Stera, C.; Meconi, T.; Frigieri, F.C. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J. Thromb. Thrombolysis 2020, 50, 281–286. [Google Scholar] [CrossRef]
- Nougier, C.; Benoit, R.; Simon, M.; Desmurs-Clavel, H.; Marcotte, G.; Argaud, L.; David, J.S.; Bonnet, A.; Negrier, C.; Dargaud, Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020, 18, 2215–2219. [Google Scholar] [CrossRef]
- Bachler, M.; Bösch, J.; Stürzel, D.P.; Hell, T.; Giebl, A.; Ströhle, M.; Klein, S.J.; Schäfer, V.; Lehner, G.F.; Joannidis, M.; et al. Impaired fibrinolysis in critically ill COVID-19 patients. Br. J. Anaesth. 2021, 126, 590–598. [Google Scholar] [CrossRef]
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an acute to chronic disease? Potential long-term health consequences. Crit. Rev. Clin. Lab. Sci. 2021, 58, 297–310. [Google Scholar] [CrossRef]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. 2020, 26, e928996. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Li, P.; Zhao, W.; Kaatz, S.; Latack, K.; Schultz, L.; Poisson, L. Factors Associated with Risk of Postdischarge Thrombosis in Patients with COVID-19. JAMA Netw. Open. 2021, 4, e2135397. [Google Scholar] [CrossRef]
- von Meijenfeldt, F.A.; Havervall, S.; Adelmeijer, J.; Lundström, A.; Magnusson, M.; Mackman, N.; Thalin, C.; Lisman, T. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. 2021, 5, 756–759. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Irish COVID-19 Vasculopathy Study (iCVS) investigators. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef]
- Engelen, M.M.; Vandenbriele, C.; Balthazar, T.; Claeys, E.; Gunst, J.; Guler, I.; Jacquemin, M.; Janssens, S.; Lorent, N.; Liesenborghs, L.; et al. Venous Thromboembolism in Patients Discharged after COVID-19 Hospitalization. Semin. Thromb. Hemost. 2021, 47, 362–371. [Google Scholar] [CrossRef]
- Rungjirajittranon, T.; Owattanapanich, W.; Leelakanok, N.; Sasijareonrat, N.; Suwanawiboon, B.; Chinthammitr, Y.; Ruchutrakool, T. Thrombotic and Hemorrhagic Incidences in Patients After Discharge from COVID-19 Infection: A Systematic Review and Meta-Analysis. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211069082. [Google Scholar] [CrossRef]
- Paschoa, A.F. Heparin: 100 years of pleiotropic effects. J. Thromb. Thrombolysis 2016, 41, 636–643. [Google Scholar] [CrossRef]
- Bal Dit Sollier, C.; Dillinger, J.G.; Drouet, L. Anticoagulant activity and pleiotropic effects of heparin. J. Med. Vasc. 2020, 45, 147–157. [Google Scholar] [CrossRef]
- Mousavi, S.; Moradi, M.; Khorshidahmad, T.; Motamedi, M. Anti-inflammatory effects of heparin and its derivatives: A systematic review. Adv. Pharmacol. Sci. 2015, 2015, 507151. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, L.; Palumbo, F.P.; Ardita, G.; Antignani, P.L.; Arosio, E.; Failla, G. Italian Society for Vascular Investigation and the Italian Society of Vascular Medicine. Coagulopathy, thromboembolic complications, and the use of heparin in COVID-19 pneumonia. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 711–716. [Google Scholar] [CrossRef]
- Thachil, J. The versatile heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Bai, J. Protective effects of heparin on endothelial cells in sepsis. Int. J. Clin. Exp. Med. 2015, 8, 5547–5552. [Google Scholar]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Baba, M.; Sato, A.; Pauwels, R.; De Clercq, E.; Shigeta, S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antivir. Res. 1987, 7, 361–367. [Google Scholar] [CrossRef]
- Modhiran, N.; Gandhi, N.S.; Wimmer, N.; Cheung, S.; Stacey, K.; Young, P.R.; Ferro, V.; Watterson, D. Dual targeting of dengue virus virions and NS1 protein with the heparan sulfate mimic PG545. Antivir. Res. 2019, 168, 121–127. [Google Scholar] [CrossRef]
- Sasaki, M.; Anindita, P.D.; Ito, N.; Sugiyama, M.; Carr, M.; Fukuhara, H.; Ose, T.; Maenaka, K.; Takada, A.; Hall, W.W.; et al. The role of heparan sulfate proteoglycans as an attachment factor for rabies virus entry and infection. J. Infect. Dis. 2018, 217, 1740–1749. [Google Scholar] [CrossRef] [Green Version]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ash-pole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. J. Virol. 2021, 95, e01987. [Google Scholar] [CrossRef]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 2020, 183, 1043–1057. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Pang, H.; Li, S.J. Heparin interacts with the main protease of SARS-CoV-2 and inhibits its activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267 Pt 2, 120595. [Google Scholar] [CrossRef]
- Potje, S.R.; Costa, T.J.; Fraga-Silva, T.F.C.; Martins, R.B.; Benatti, M.N.; Almado, C.E.L.; de Sá, K.S.G.; Bonato, V.L.D.; Arruda, E.; Louzada-Junior, P.; et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021, 276, 119376. [Google Scholar] [CrossRef]
- Buijsers, B.; Yanginlar, C.; de Nooijer, A.; Grondman, I.; Maciej-Hulme, M.L.; Jonkman, I.; Janssen, N.A.F.; Rother, N.; de Graaf, M.; Pickkers, P.; et al. Increased Plasma Heparanase Activity in COVID-19 Patients. Front. Immunol. 2020, 11, 575047. [Google Scholar] [CrossRef]
- Lemos, A.C.B.; do Espírito Santo, D.A.; Salvetti, M.C.; Gilio, R.N.; Agra, L.B.; Pazin-Filho, A.; Miranda, C.H. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb. Res. 2020, 196, 359–366. [Google Scholar] [CrossRef]
- Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; Moghadam, K.G.; et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients with COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar] [CrossRef]
- Bikdeli, B.; Talasaz, A.H.; Rashidi, F.; Bakhshandeh, H.; Rafiee, F.; Rezaeifar, P.; Baghizadeh, E.; Matin, S.; Jamalkhani, S.; Tahamtan, O.; et al. Intermediate-Dose versus Standard-Dose Prophylactic Anticoagulation in Patients with COVID-19 Admitted to the Intensive Care Unit: 90-Day Results from the INSPIRATION Randomized Trial. Thromb. Haemost. 2022, 122, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; de Barros ESilva, P.G.M.; Furtado, R.H.M.; Macedo, A.V.S.; Bronhara, B.; Damiani, L.P.; Barbosa, L.M.; de Aveiro Morata, J.; Ramacciotti, E.; de Aquino Martins, P.; et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet 2021, 397, 2253–2263. [Google Scholar] [CrossRef]
- Goligher, E.C.; Bradbury, C.A.; McVerry, B.J.; Lawler, P.R.; Berger, J.S.; Gong, M.N.; Carrier, M.; Reynolds, H.R.; Kumar, A.; Turgeon, A.F.; et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with COVID-19. N Engl. J. Med. 2021, 385, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Goligher, E.C.; Berger, J.S.; Neal, M.D.; McVerry, B.J.; Nicolau, J.C.; Gong, M.N.; Carrier, M.; Rosenson, R.S.; Reynolds, H.R.; et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar] [CrossRef]
- Perepu, U.S.; Chambers, I.; Wahab, A.; Ten Eyck, P.; Wu, C.; Dayal, S.; Sutamtewagul, G.; Bailey, S.R.; Rosenstein, L.J.; Lentz, S.R. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: A multi-center, open-label, randomized controlled trial. J. Thromb. Haemost. 2021, 19, 2225–2234. [Google Scholar] [CrossRef]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Áinle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. RAPID trial investigators. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. HEP-COVID Investigators. Efficacy and Safety of Therapeutic-Dose Heparin vs. Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients with COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612–1620, Erratum in JAMA Intern. Med. 2022, 182, 239. [Google Scholar] [CrossRef]
- Marcos-Jubilar, M.; Carmona-Torre, F.; Vidal, R.; Ruiz-Artacho, P.; Filella, D.; Carbonell, C.; Jiménez-Yuste, V.; Schwartz, J.; Llamas, P.; Alegre, F.; et al. Therapeutic versus Prophylactic Bemiparin in Hospitalized Patients with Nonsevere COVID-19 Pneumonia (BEMICOP Study): An Open-Label, Multicenter, Randomized, Controlled Trial. Thromb. Haemost. 2022, 122, 295–299. [Google Scholar] [CrossRef]
- Morici, N.; Podda, G.; Birocchi, S.; Bonacchini, L.; Merli, M.; Trezzi, M.; Massaini, G.; Agostinis, M.; Carioti, G.; Saverio Serino, F.; et al. Enoxaparin for thromboprophylaxis in hospitalized COVID-19 patients: The X-COVID-19 Randomized Trial. Eur. J. Clin. Investig. 2021, 52, e13735. [Google Scholar] [CrossRef]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. The effect of higher-intensity dosing of anticoagulation on the clinical outcomes in hospitalized patients with COVID-19: A meta-analysis of randomized controlled trials. J. Infect. Chemother. 2022, 28, 257–265. [Google Scholar] [CrossRef]
- Hasan, S.S.; Radford, S.; Kow, C.S.; Zaidi, S.T.R. Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2020, 50, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.K.; Vegunta, R.; Rokkam, V.R.P.; Meyyur Aravamudan, V.; Vegunta, R.; Khan, S.R.; Ponnada, S.; Boregowda, U.; Prudhvi, K.; Chamarthi, G.; et al. Venous Thromboembolism in Hospitalized COVID-19 Patients. Am. J. Ther. 2020, 27, e599–e610. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maboud, M.; Menshawy, A.; Elgebaly, A.; Bahbah, E.I.; Ashal, G.; Negida, A. Should we consider heparin prophylaxis in COVID-19 patients? A systematic review and meta-analysis. J. Thromb. Thrombolysis 2021, 51, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Costanzo, S.; Di Castelnuovo, A.; de Gaetano, G.; Donati, M.B.; Iacoviello, L. Different Anticoagulant Regimens, Mortality, and Bleeding in Hospitalized Patients with COVID-19: A Systematic Review and an Updated Meta-Analysis. Semin. Thromb. Hemost. 2021, 47, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Giannis, D.; Allen, S.L.; Tsang, J.; Flint, S.; Pinhasov, T.; Williams, S.; Tan, G.; Thakur, R.; Leung, C.; Snyder, M.; et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: The CORE-19 registry. Blood 2021, 137, 2838–2847. [Google Scholar] [CrossRef]
- Courtney, L.A.; Trujillo, T.C.; Saseen, J.J.; Wright, G.; Palkimas, S. Evaluation of the Clinical Impact of Thromboprophylaxis in Patients with COVID-19 Following Hospital Discharge. Ann. Pharmacother. 2022, 10600280211064306. [Google Scholar] [CrossRef]
- Zhang, R.; Ni, L.; Di, X.; Wang, X.; Ma, B.; Niu, S.; Liu, C. Systematic review and meta-analysis of the prevalence of venous thromboembolic events in novel coronavirus disease-2019 patients. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 289.e5–298.e5. [Google Scholar] [CrossRef]
- Wu, T.; Zuo, Z.; Yang, D.; Luo, X.; Jiang, L.; Xia, Z.; Xiao, X.; Liu, J.; Ye, M.; Deng, M. Venous thromboembolic events in patients with COVID-19: A systematic review and meta-analysis. Age Ageing 2021, 50, 284–293. [Google Scholar] [CrossRef]
- Kollias, A.; Kyriakoulis, K.G.; Lagou, S.; Kontopantelis, E.; Stergiou, G.S.; Syrigos, K. Venous thromboembolism in COVID-19: A systematic review and meta-analysis. Vasc. Med. 2021, 26, 415–425. [Google Scholar] [CrossRef]
- Giossi, R.; Menichelli, D.; Pani, A.; Tratta, E.; Romandini, A.; Roncato, R.; Nani, A.; Schenardi, P.; Diani, E.; Fittipaldo, V.A.; et al. A Systematic Review and a Meta-Analysis Comparing Prophylactic and Therapeutic Low Molecular Weight Heparins for Mortality Reduction in 32,688 COVID-19 Patients. Front. Pharmacol. 2021, 12, 698008. [Google Scholar] [CrossRef]
- Spini, A.; Giudice, V.; Brancaleone, V.; Morgese, M.G.; De Francia, S.; Filippelli, A.; Ruggieri, A.; Ziche, M.; Ortona, E.; Cignarella, A.; et al. Sex-tailored pharmacology and COVID-19: Next steps towards appropriateness and health equity. Pharmacol. Res. 2021, 173, 105848. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Banerjee, M. Immune Thrombocytopenia Secondary to COVID-19: A Systematic Review. SN Compr. Clin. Med. 2020, 2, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: May 2021 update on the use of intermediate-intensity anticoagulation in critically ill patients. Blood Adv. 2021, 5, 3951–3959. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 19 May 2022).
- Russo, V.; Cardillo, G.; Viggiano, G.V.; Mangiacapra, S.; Cavalli, A.; Fontanella, A.; Agrusta, F.; Bellizzi, A.; Amitrano, M.; Iannuzzo, M.; et al. Thromboprofilaxys with Fondaparinux vs. Enoxaparin in Hospitalized COVID-19 Patients: A Multicenter Italian Observational Study. Front. Med. 2020, 7, 569567. [Google Scholar] [CrossRef]
- Russo, V.; Cardillo, G.; Viggiano, G.V.; Mangiacapra, S.; Cavalli, A.; Fontanella, A.; Agrusta, F.; Bellizzi, A.; Amitrano, M.; Iannuzzo, M.; et al. Fondaparinux Use in Patients with COVID-19: A Preliminary Multicenter Real-World Experience. J. Cardiovasc. Pharmacol. 2020, 76, 369–371. [Google Scholar] [CrossRef]
- Prandoni, P.; Cattelan, A.M.; Carrozzi, L.; Leone, L.; Filippi, L.; De Gaudenzi, E.; Villalta, S.; Pesavento, R.; Fondacovit Investigators [All in Italy]. The hazard of fondaparinux in non-critically ill patients with COVID-19: Retrospective controlled study versus enoxaparin. Thromb Res. 2020, 196, 395–397. [Google Scholar] [CrossRef]
- Dai, M.F.; Guo, S.T.; Ke, Y.J.; Wang, B.Y.; Yu, F.; Xu, H.; Gu, Z.C.; Ge, W.H. The Use of Oral Anticoagulation Is Not Associated With a Reduced Risk of Mortality in Patients With COVID-19: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Pharmacol. 2022, 13, 781192. [Google Scholar] [CrossRef]
- Ananworanich, J.; Mogg, R.; Dunne, M.W.; Bassyouni, M.; David, C.V.; Gonzalez, E.; Rogalski-Salter, T.; Shih, H.; Silverman, J.; Medema, J.; et al. Randomized study of rivaroxaban vs. placebo on disease progression and symptoms resolution in high-risk adults with mild COVID-19. Clin. Infect. Dis. 2021, ciab813. [Google Scholar] [CrossRef]
- Gremese, E.; Ferraccioli, G. The pathogenesis of microthrombi in COVID-19 cannot be controlled by DOAC: NETosis should be the target. J. Intern. Med. 2021, 289, 420–421. [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: July 2021 update on postdischarge thromboprophylaxis. Blood Adv. 2022, 6, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Patell, R.; Bogue, T.; Koshy, A.; Bindal, P.; Merrill, M.; Aird, W.C.; Bauer, K.A.; Zwicker, J.I. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood 2020, 136, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.N.; Whyte, M.B.; Georgiou, L.; Giron, G.; Czuprynska, J.; Rea, C.; Vadher, B.; Patel, R.K.; Gee, E.; Arya, R. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood 2020, 136, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Giannis, D.; Douketis, J.D.; Spyropoulos, A.C. Anticoagulant therapy for COVID-19: What we have learned and what are the unanswered questions? Eur. J. Intern. Med. 2022, 96, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Ramacciotti, E.; Agati, L.B.; Calderaro, D.; Volpiani, G.G.; de Oliveira, C.C.C.; Aguiar, V.C.R.; Rodrigues, E.; Sobreira, M.L.; Joviliano, E.E.; Dusilek, C.; et al. Medically Ill hospitalized Patients for COVID-19 THrombosis Extended ProphyLaxis with rivaroxaban ThErapy: Rationale and Design of the MICHELLE Trial. Am. Heart J. 2021, 242, 115–122. [Google Scholar] [CrossRef]
| TRIAL | Methods | Mean Age/Male | Interventions | Results |
|---|---|---|---|---|
| HESACOVID [51] REBEC RBR-949z6v PHASE 2 | 20 PTS, RCT, OL, LMWH | Td: 55 years/90% Pd: 58 years/70% | Td vs. Pd | Td reduces the need for mechanical ventilation and improves blood gas parameters |
| INSPIRATION [52,53] NCT04486508 PHASE 3 | 562 PTS, RCT, OL, LMWH | Id: 62 years/58.7% Pd: 61 years/57% | Id vs. Pd | No difference in the 30-day outcomes (ICU) No difference in the 90-days outcomes (ICU) |
| ACTION [54] NCT04394377 PHASE 4 | 615 PTS, RCT, OL, LMWH, UH, DOAC | Td: 56.7 years/62% Pd: 56.5 years/58% | Td vs. Pd | No difference in primary outcome between Td and Pd Bleeding increased statistically with Td (DOAC) |
| The REMAP-CAP/ACTIV-4a/ATTACC trial (severe) [55] NCT02735707 PHASE 3, NCT04505774 PHASE 4, NCT04359277 PHASE 4, NCT04372589 PHASE 2/3 | 1098 PTS, RCT, OL, LMWH, UH | Td: 60.4 years/72.2% Pd: 61.7 years/67.9% | Td vs. Pd | No difference in mortality |
| The REMAP-CAP/ACTIV-4a/ATTACC trial (moderate) [56] NCT02735707 PHASE 3, NCT04505774 PHASE 4, NCT04359277 PHASE 4, NCT04372589 PHASE 2/3 | 2131 PTS, RCT, OL, LMWH, UH | Td: 59 years/60.4% Pd: 58.8 years/56.9% | Td vs. Pd | Reduction in mortality and disease intensity with Td |
| Perepu et al. [57] NCT04360824 PHASE 4 | 173PTS, RCT, OL, LMWH | Id: 65 years/54% Pd: 63.5 years/58% | Id vs. Pd | No difference in ICU patients |
| RAPID [58] NCT04362085 PHASE 3 | 465 PTS, RCT, OL, LMWH, UH | Td: 60.4 years/53.9% Pd: 59.6 years/59.5% | Td vs. Pd | 28 days mortality reduction with Td in moderately ill patients |
| HEP-COVID [59] NCT04401293 PHASE 3 | 253 PTS, RCT, DB, LMWH, UH | Td: 65.8 years/52.7% Pd: 67.7 years/54.8% | Td vs. Pd | 30 days reduction in thromboembolic events and death with Td in moderately ill patients |
| BEMICOP STUDY [60] NCT04604327 PHASE 3 | 65 PTS, RCT, OL, LMWH | Td: 63 years/53.1% Pd: 62.3 years/72.7% | Td vs. Pd | Td does improve clinical outcomes |
| X-COVID 19 [61] NCT04366960 PHASE 3 | 183 PTS, RCT, OL, LMWH | Id: 60 years/61.5% Pd: 59 years/64.1% | Id vs. Pd | No DVT in both groups; 6 vs. 0 PE in Pd group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangiafico, M.; Caff, A.; Costanzo, L. The Role of Heparin in COVID-19: An Update after Two Years of Pandemics. J. Clin. Med. 2022, 11, 3099. https://doi.org/10.3390/jcm11113099
Mangiafico M, Caff A, Costanzo L. The Role of Heparin in COVID-19: An Update after Two Years of Pandemics. Journal of Clinical Medicine. 2022; 11(11):3099. https://doi.org/10.3390/jcm11113099
Chicago/Turabian StyleMangiafico, Marco, Andrea Caff, and Luca Costanzo. 2022. "The Role of Heparin in COVID-19: An Update after Two Years of Pandemics" Journal of Clinical Medicine 11, no. 11: 3099. https://doi.org/10.3390/jcm11113099
APA StyleMangiafico, M., Caff, A., & Costanzo, L. (2022). The Role of Heparin in COVID-19: An Update after Two Years of Pandemics. Journal of Clinical Medicine, 11(11), 3099. https://doi.org/10.3390/jcm11113099






