Measurement of Lipid Peroxidation Products and Creatine Kinase in Blood Plasma and Saliva of Athletes at Rest and following Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Measurement of Exercise Performance
2.4. Sample Collection
2.5. Measurement of Lipid Peroxidation Products
2.6. Measurement of Creatine Kinase Activity
2.7. Statistical Analysis
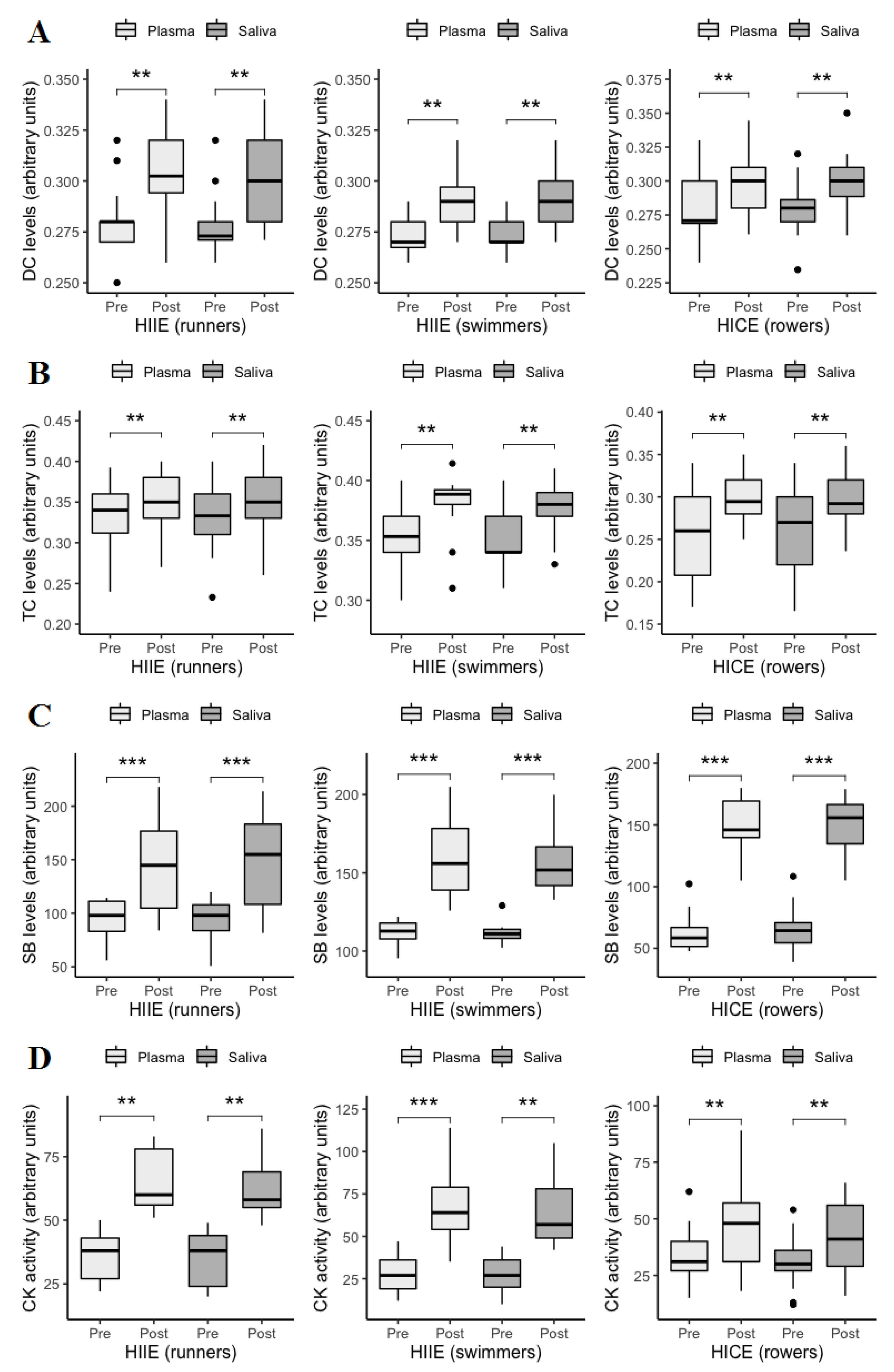
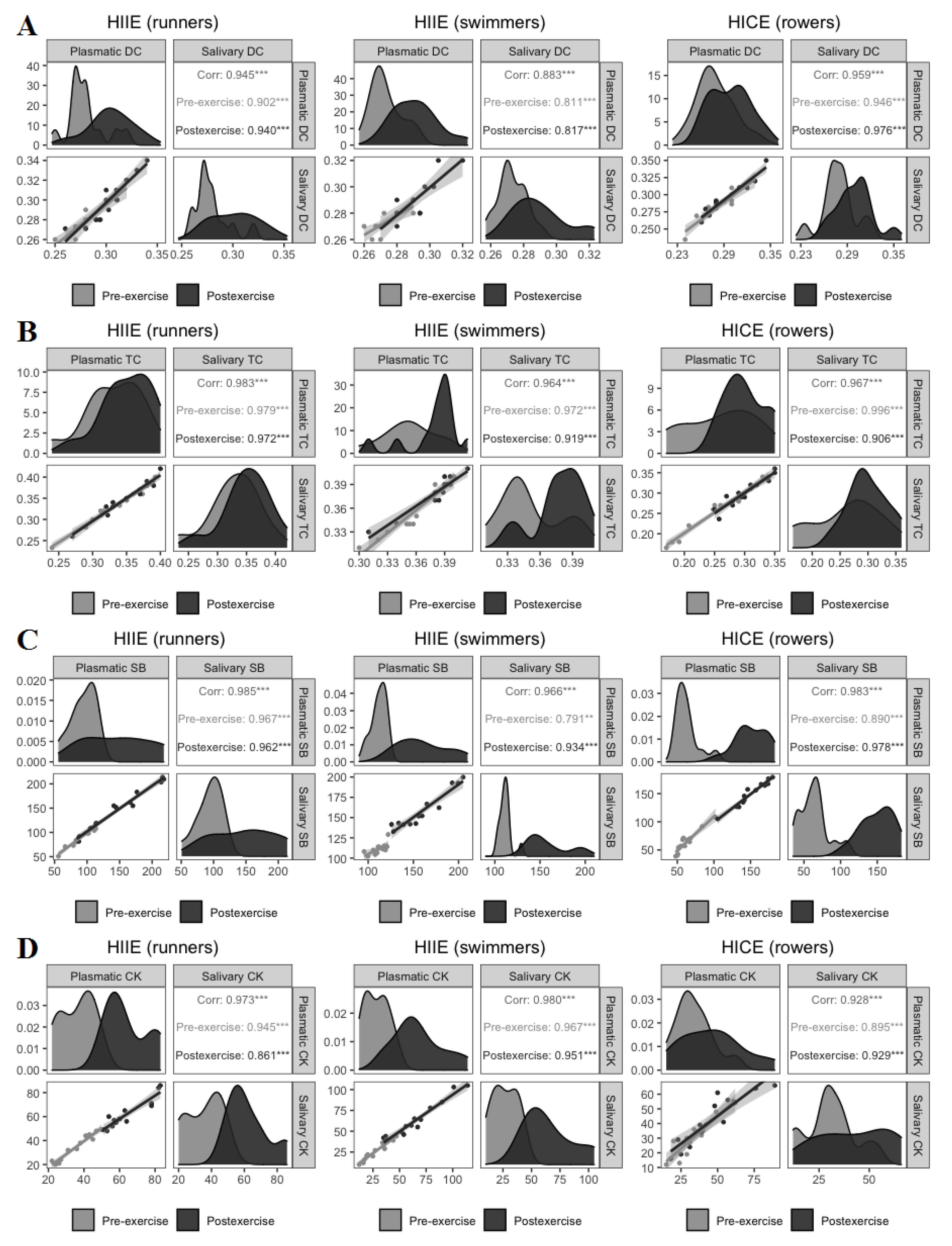
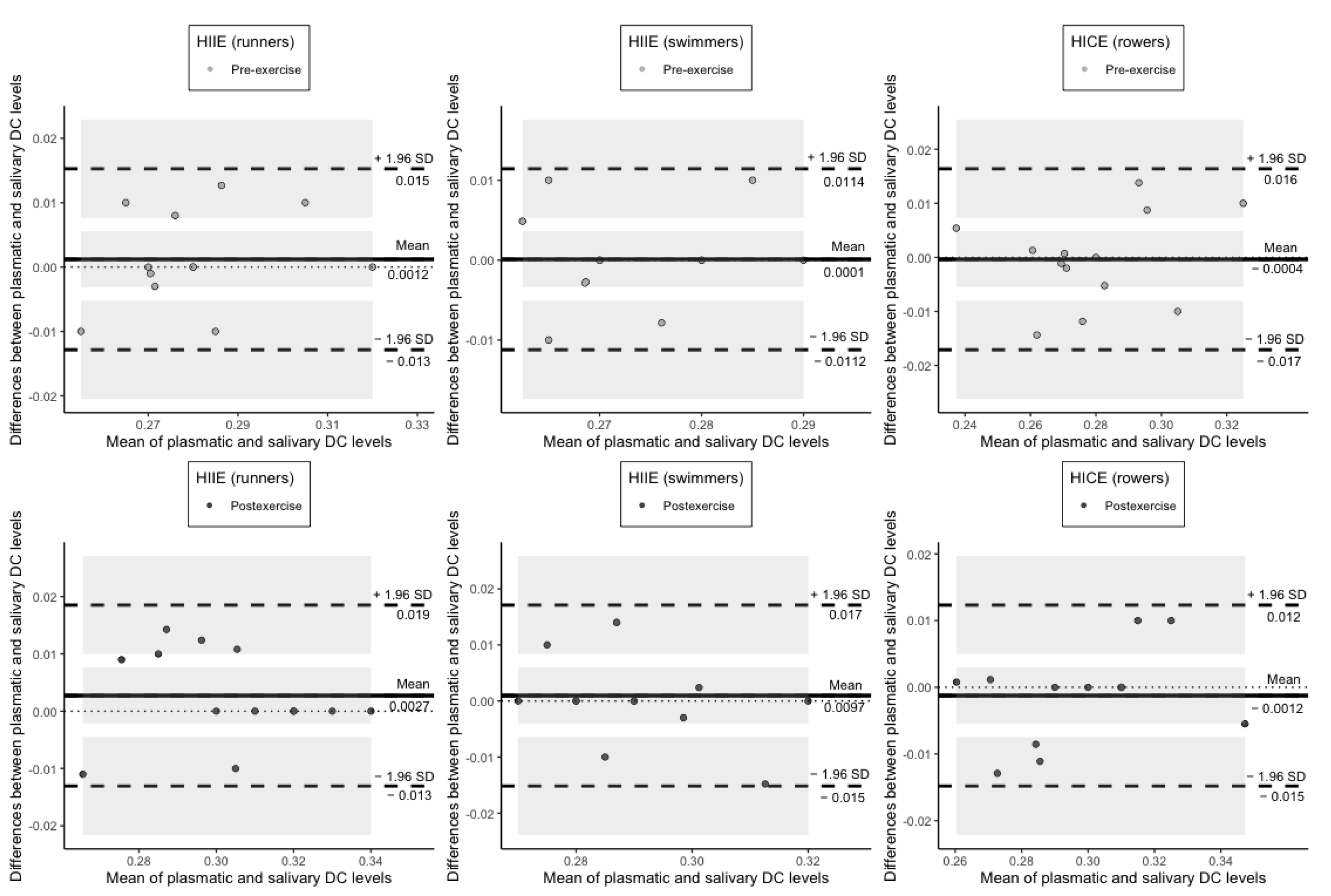
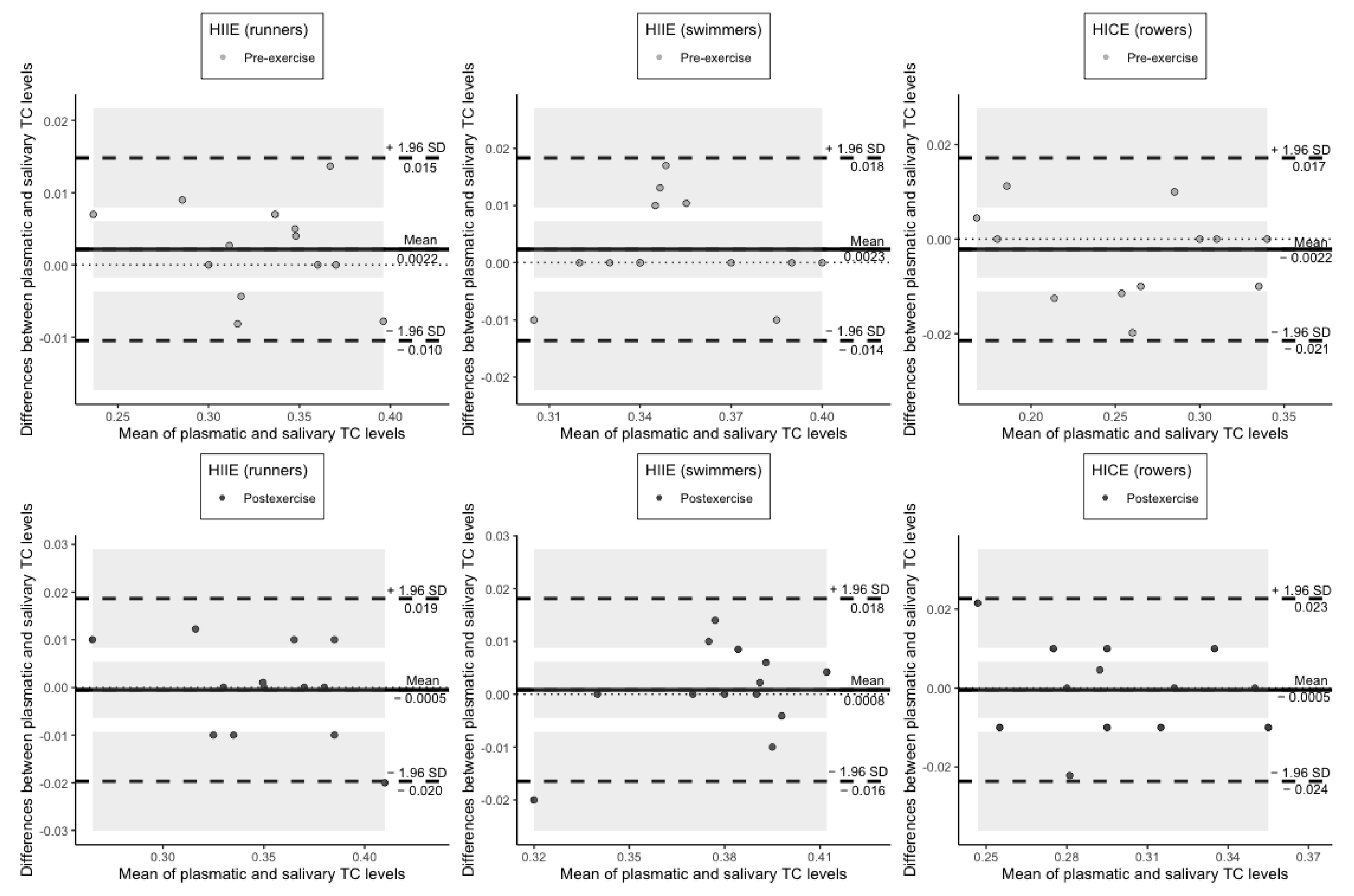
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsay, A.; Costello, J.T. Realising the Potential of Urine and Saliva as Diagnostic Tools in Sport and Exercise Medicine. Sports Med. 2017, 47, 11–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. NPJ Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovchinnikov, A.N.; Deryugina, A.V. Saliva as highly informative substrate for non-invasive analysis of lipoperoxide processes and muscle damage in highly skilled athletes. Klin. Lab. Diagn. 2019, 64, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.; Lewis, J.; Scarrott, C.; Gill, N.; Gieseg, S.P.; Draper, N. Assessing the effectiveness of selected biomarkers in the acute and cumulative physiological stress response in professional rugby union through non-invasive assessment. Int. J. Sports Med. 2015, 36, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Mortatti, A.L.; Moreira, A.; Aoki, M.S.; Crewther, B.T.; Castagna, C.; de Arruda, A.F.S.; Filho, J.M. Effect of competition on salivary cortisol, immunoglobulin A, and upper respiratory tract infections in elite young soccer players. J. Strength Cond. Res. 2012, 26, 1396–1401. [Google Scholar] [CrossRef]
- Elloumi, M.; Maso, F.; Michaux, O.; Robert, A.; Lac, G. Behaviour of saliva cortisol [C], testosterone [T] and the T/C ratio during a rugby match and during the post-competition recovery days. Eur. J. Appl. Physiol. 2003, 90, 23–28. [Google Scholar] [CrossRef]
- Beaven, C.M.; Gill, N.D.; Cook, C.J. Salivary testosterone and cortisol responses in professional rugby players after four resistance exercise protocols. J. Strength Cond. Res. 2008, 22, 426–432. [Google Scholar] [CrossRef]
- Ghigiarelli, J.J.; Sell, K.M.; Raddock, J.M.; Taveras, K. Effects of strongman training on salivary testosterone levels in a sample of trained men. J. Strength Cond. Res. 2013, 27, 738–747. [Google Scholar] [CrossRef]
- Neville, V.; Gleeson, M.; Folland, J.P. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med. Sci. Sports Exerc. 2008, 40, 1228–1236. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, D.; Marquina, R.; Rondon, N.; Rodriguez-Malaver, A.J.; Reyes, R. Effects of aerobic exercise on uric acid, total antioxidant activity, oxidative stress, and nitric oxide in human saliva. Res. Sports Med. 2008, 16, 128–137. [Google Scholar] [CrossRef]
- Deminice, R.; Sicchieri, T.; Payao, P.O.; Jordao, A.A. Blood and salivary oxidative stress biomarkers following an acute session of resistance exercise in humans. Int. J. Sports Med. 2010, 31, 599–603. [Google Scholar] [CrossRef]
- Sant’Anna, M.; Casimiro-Lopes, G.; Boaventura, G.; Marques, S.T.; Sorenson, M.M.; Simão, R.; Pinto, V.S. Anaerobic exercise affects the saliva antioxidant/oxidant balance in high performance pentathlon athletes. Hum. Mov. 2016, 17, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.V.; Giolo, J.S.; Teixeira, R.R.; Vilela, D.D.; Peixoto, L.G.; Justino, A.B.; Caixeta, D.C.; Puga, G.M.; Espindola, F.S. Salivary and Plasmatic Antioxidant Profile following Continuous, Resistance, and High-Intensity Interval Exercise: Preliminary Study. Oxid. Med. Cell. Longev. 2019, 2019, 5425021. [Google Scholar] [CrossRef] [PubMed]
- Hofman, L.F. Human saliva as a diagnostic specimen. J. Nutr. 2001, 131, 1621S–1625S. [Google Scholar] [CrossRef]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- McLellan, C.P.; Lovell, D.I.; Gass, G.C. Creatine kinase and endocrine responses of elite players pre, during, and post rugby league match play. J. Strength Cond. Res. 2010, 24, 2908–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadore, E.; Lhullier, F.; Brentano, M.; Silva, E.; Ambrosini, M.; Spinelli, R.; Silva, R.; Kruel, L. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J. Sports Sci. 2008, 26, 1067–1072. [Google Scholar] [CrossRef]
- Hough, J.; Robertson, C.; Gleeson, M. Blunting of exercise-induced salivary testosterone in elite-level triathletes with a 10-day training camp. Int. J. Sports Physiol. Perform. 2015, 10, 935–938. [Google Scholar] [CrossRef]
- De Oliveira, V.; Bessa, A.; Lamounier, R.; de Santana, M.G.; de Mello, M.T.; Espindola, F.S. Changes in the salivary biomarkers induced by an effort test. Int. J. Sports Med. 2010, 31, 377–381. [Google Scholar] [CrossRef]
- Kivlighan, K.T.; Granger, D.A. Salivary a-amylase response to competition: Relation to gender, previous experience, and attitudes. Psychoneuroendocrinology 2006, 31, 703–714. [Google Scholar] [CrossRef]
- MacKinnon, L.T.; Jenkins, D.G. Decreased salivary immunoglobulins after intense interval exercise before and after training. Med. Sci. Sports Exerc. 1993, 25, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, L.T.; Chick, T.W.; Van As, A.; Tomasi, T.B. Decreased secretory immunoglobulins following intense endurance exercise. Res. Sports Med. 1989, 1, 209–218. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Corongiu, F.P.; Banni, S. Detection of conjugated dienes by second derivative ultraviolet spectrophotometry. Methods Enzymol. 1994, 233, 303–310. [Google Scholar] [PubMed]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G. Endogenous Intoxication and Saliva Lipid Peroxidation in Patients with Lung Cancer. Diagnostics 2016, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [Green Version]
- Ovchinnikov, A.N. The Effect of the Combination of Royal Jelly and Exogenous Coenzyme Q10 on the Indicators of Functional Status among Athletes under Exercise. PhD Thesis, Lobachevsky University, Nizhny Novgorod, Russia, 2019. [Google Scholar]
- Aoi, W.; Naito, Y.; Yoshikawa, T. Role of oxidative stress in impaired insulin signaling associated with exercise-induced muscle damage. Free Radic. Biol. Med. 2013, 65, 1265–1272. [Google Scholar] [CrossRef]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [Green Version]
- Kontorshchikova, K.N.; Tikhomirova, Y.R.; Ovchinnikov, A.N.; Kolegova, T.I.; Churkina, N.N.; Kuznetsova, S.Y.; Krylov, V.N. Indices of Free Radical Oxidation in the Oral Fluid as Markers of Athletes’ Functional State. Sovrem. Tehnol. V Med. 2017, 9, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Barranco, T.; Tvarijonaviciute, A.; Tecles, F.; Carrillo, J.M.; Sánchez-Resalt, C.; Jimenez-Reyes, P.; Rubio, M.; García-Balletbó, M.; Cerón, J.J.; Cugat, R. Changes in creatine kinase, lactate dehydrogenase and aspartate aminotransferase in saliva samples after an intense exercise: A pilot study. J. Sports Med. Phys. Fit. 2017, 58, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.N.; Paoli, A.; Deryugina, A.V.; Yarygina, D.A. Salivary and Plasmatic Creatine Kinase and Lactate Dehydrogenase Responses Following High-intensity Continuous Exercise. Med. Sci. Sports Exerc. 2021, 53, 377. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997, 277, 925–926. [Google Scholar] [CrossRef]
- Kontorschikova, K.; Tikhomirova, J.; Ovchinnikov, A.; Okrut, I.; Krylov, V.; Kolegova, T. The evaluation of highly qualified sportsmen’s biochemical homeostasis. Clin. Chem. Lab. Med. 2017, 55, S811. [Google Scholar]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatta, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Vasilaki, A.; Jackson, M.J. Role of reactive oxygen species in the defective regeneration seen in aging muscle. Free Radic. Biol. Med. 2013, 65, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef]
- Beckendorf, L.; Linke, W.A. Emerging importance of oxidative stress in regulating striated muscle elasticity. J. Muscle Res. Cell Motil. 2015, 36, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Schipper, H.M.; Velly, A.M.; Mohit, S.; Gornitsky, M. Salivary biomarkers of oxidative stress: A critical review. Free Radic. Biol. Med. 2015, 85, 95–104. [Google Scholar] [CrossRef]
- Fernández, A.G.; de la Rubia Ortí, J.E.; Franco-Martinez, L.; Ceron, J.J.; Mariscal, G.; Barrios, C. Changes in Salivary Levels of Creatine Kinase, Lactate Dehydrogenase, and Aspartate Aminotransferase after Playing Rugby Sevens: The Influence of Gender. Int. J. Environ. Res. Public Health 2020, 17, 8165. [Google Scholar] [CrossRef]
- Gissel, H.; Clausen, T. Excitation-induced Ca2+ influx and skeletal muscle cell damage. Acta Physiol. Scand. 2001, 171, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gissel, H. The role of Ca2+ in muscle cell damage. Ann. N. Y. Acad. Sci. 2005, 1066, 166–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inserte, J.; Hernando, V.; Garcia-Dorado, D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2012, 96, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanum, R.; Thevanayagam, H. Lipid peroxidation: Its effects on the formulation and use of pharmaceutical emulsions. Asian J. Pharm. Sci. 2017, 12, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaur, R.J. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Asp. Med. 2003, 24, 149–159. [Google Scholar] [CrossRef]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993, 57, 715S–724S. [Google Scholar] [CrossRef] [Green Version]
- Roche, L.D. Oxidative stress: The dark side of soybean-oil-based emulsions used in parenteral nutrition. Oxid. Antioxid. Med. Sci. 2012, 1, 11–14. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Mougios, V. Reference intervals for serum creatine kinase in athletes. Br. J. Sports Med. 2007, 41, 674–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, C.H.; Wang, K.T.; Lee, P.Y.; Liu, C.C.; Hsieh, Y.C.; Kuo, J.Y.; Bulwer, B.E.; Hung, C.L.; Chang, S.C.; Shih, S.C.; et al. Gender-differences in the associations between circulating creatine kinase, blood pressure, body mass and non-alcoholic fatty liver disease in asymptomatic asians. PLoS ONE 2017, 12, e0179898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosthuyse, T.; Bosch, A.N. The Effect of Gender and Menstrual Phase on Serum Creatine Kinase Activity and Muscle Soreness Following Downhill Running. Antioxidants 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
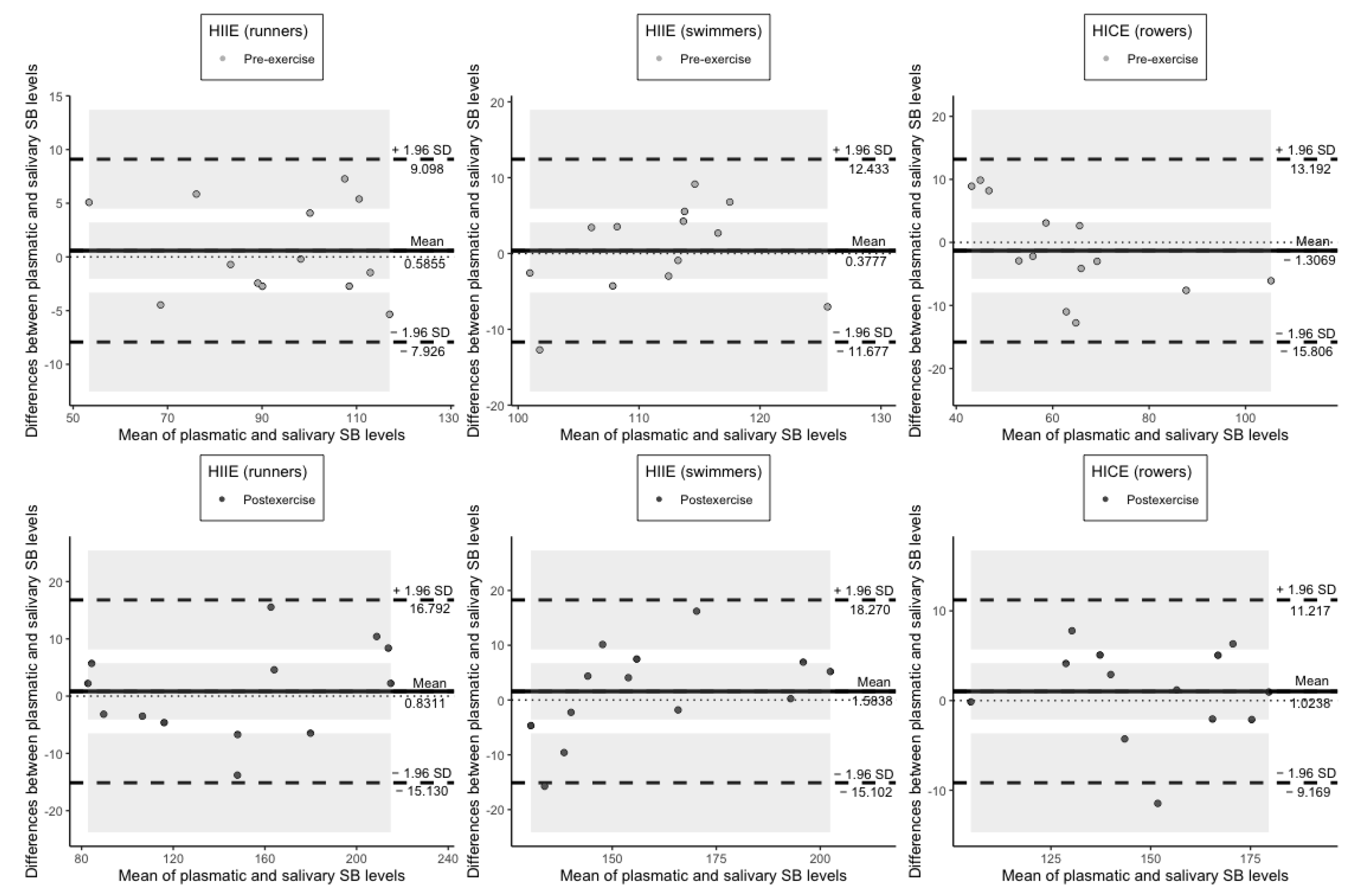
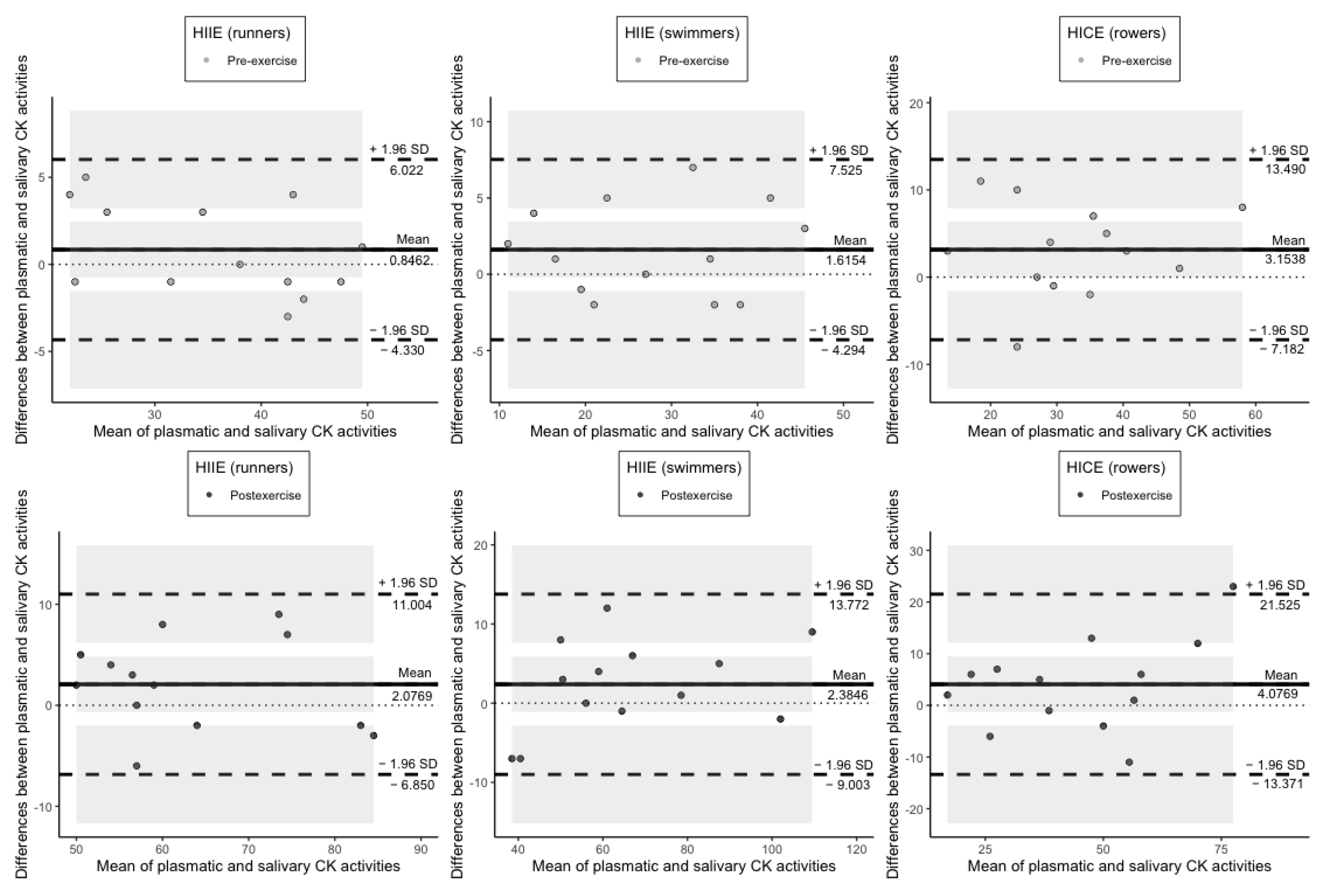
| Runners | Swimmers | Rowers | |
|---|---|---|---|
| Age (years) | 19.7 ± 1.2 | 19.4 ± 1.0 | 19.6 ± 1.0 |
| Height (cm) | 173.2 ± 3.1 | 173.7 ± 1.3 | 173.0 ± 3.0 |
| Body mass (kg) | 63.4 ± 1.7 | 64.6 ± 2.4 | 63.0 ± 2.3 |
| BMI (kg/m2) | 21.2 ± 0.5 | 21.4 ± 0.6 | 21.0 ± 0.6 |
| HIIE (Runners) | HIIE (Swimmers) | HICE (Rowers) | |
|---|---|---|---|
| Time to completion (s) | 11.15 ± 0.14 | - | - |
| FINA points (a.u.) | - | 621.00 ± 34.23 | - |
| Time to completion (min) | - | - | 6.36 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovchinnikov, A.N.; Paoli, A.; Seleznev, V.V.; Deryugina, A.V. Measurement of Lipid Peroxidation Products and Creatine Kinase in Blood Plasma and Saliva of Athletes at Rest and following Exercise. J. Clin. Med. 2022, 11, 3098. https://doi.org/10.3390/jcm11113098
Ovchinnikov AN, Paoli A, Seleznev VV, Deryugina AV. Measurement of Lipid Peroxidation Products and Creatine Kinase in Blood Plasma and Saliva of Athletes at Rest and following Exercise. Journal of Clinical Medicine. 2022; 11(11):3098. https://doi.org/10.3390/jcm11113098
Chicago/Turabian StyleOvchinnikov, Aleksandr N., Antonio Paoli, Vladislav V. Seleznev, and Anna V. Deryugina. 2022. "Measurement of Lipid Peroxidation Products and Creatine Kinase in Blood Plasma and Saliva of Athletes at Rest and following Exercise" Journal of Clinical Medicine 11, no. 11: 3098. https://doi.org/10.3390/jcm11113098
APA StyleOvchinnikov, A. N., Paoli, A., Seleznev, V. V., & Deryugina, A. V. (2022). Measurement of Lipid Peroxidation Products and Creatine Kinase in Blood Plasma and Saliva of Athletes at Rest and following Exercise. Journal of Clinical Medicine, 11(11), 3098. https://doi.org/10.3390/jcm11113098








