Triglyceride and Glucose Index as a Screening Tool for Nonalcoholic Liver Disease in Patients with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alba, L.M.; Lindor, K. Review article: Nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2003, 17, 977–986. [Google Scholar] [CrossRef] [PubMed]
- De Alwis, N.M.W.; Day, C.P. Non-alcoholic fatty liver disease: The mist gradually clears. J. Hepatol. 2008, 48, S104–S112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugianesi, E.; McCullough, A.; Marchesini, G. Insulin Resistance: A Metabolic Pathway to Chronic Liver Disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Neagoe, C.D.; Avramescu, E.T.; Amzolini, A.M.; Singer, C.E.; Ianosi, S.L.; Genunche, A.V.; Ianosi, G.N.; Farmazon, A.S.; Cazacu, S.M.; Popescu, M. The accuracy of NAFLD diagnosis using mathematical scores in active young obese patients. J. Sport Kinet. Mov. 2019, 34, 24–33. [Google Scholar]
- Zhang, S.; Du, T.; Zhang, J.; Lu, H.; Lin, X.; Xie, J.; Yang, Y.; Yu, X. The Triglyceride and Glucose Index (TyG) Is an Effective Biomarker to Identify Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2017, 16, 15. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.; Du, Z.; Wang, M.; Mao, Y.; Mao, W. A longitudinal epidemiological study on the triglyceride and glucose index and the incident nonalcoholic fatty liver disease. Lipids Health Dis. 2018, 17, 262. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, M.K.; Kang, S.; Park, K.; Kim, J.H.; Baik, S.J.; Nam, J.S.; Ahn, C.W.; Park, J.S. Triglyceride Glucose Index Is Superior to the Homeostasis Model Assessment of Insulin Resistance for Predicting Nonalcoholic Fatty Liver Disease in Korean Adults. Endocrinol. Metab. 2019, 34, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Inavolu, P.; Singla, N.; Nunsavata, K.; Bhashyakarla, R.K. 14. Triglyceride and Glucose Index (TYG) Index as an Screening Biomarker to Identify Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2018, 8, S41. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, J.H.; Park, K.; Lee, S.B.; Nam, J.S.; Kang, S.; Park, J.S.; Ahn, C.W.; Kim, Y.S. Relationship between the Triglyceride Glucose Index and the Presence and Fibrosis of Nonalcoholic Fatty Liver Disease in Korean Adults. Diabetes 2018, 67, 612. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation, Task Force on Epidemiology and Prevention National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The Product of Fasting Glucose and Triglycerides as Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, S.K. Nonalcoholic fatty liver disease. J. Lipid Res. 2009, 50, S412–S416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarantino, G.; Saldalamacchia, G.; Conca, P.; Arena, A. Non-alcoholic fatty liver disease: Further expression of the metabolic syndrome. J. Gastroenterol. Hepatol. 2007, 22, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Oka, R.; Miura, K.; Sakurai, M.; Nakamura, K.; Yagi, K.; Miyamoto, S.; Moriuchi, T.; Mabuchi, H.; Yamagishi, M.; Takeda, Y.; et al. Comparison of waist circumference with body mass index for predicting abdominal adipose tissue. Diabetes Res. Clin. Pract. 2009, 83, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Dowman, J.K.; Tomlinson, J.W.; Newsome, P.N. Systematic review: The diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2011, 33, 525–540. [Google Scholar] [CrossRef] [Green Version]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [Green Version]
- Simental-Mendía, L.E.; Simental-Mendía, E.; Rodríguez-Hernández, H.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of triglycerides and glucose as biomarker for screening simple steatosis and NASH in asymptomatic women. Ann. Hepatol. 2016, 15, 715–720. [Google Scholar] [CrossRef]
- Hossain, S.; Sultana, S.; Zaman, K.; Shafiq, S.; Rahman, A.; Hossain, S.; Islam, S.; Uddin, M.; Faroque, M.; Ferdoushi, S.; et al. Triglyceride and Glucose Index (TyG) is a Reliable Biomarker to Predict Non-Alcoholic Fatty Liver Disease. J. Biosci. Med. 2020, 8, 124–136. [Google Scholar] [CrossRef]
- Guo, W.; Lu, J.; Qin, P.; Li, X.; Zhu, W.; Wu, J.; Xu, N.; Zhang, Q. The triglyceride-glucose index is associated with the severity of hepatic steatosis and the presence of liver fibrosis in non-alcoholic fatty liver disease: A cross-sectional study in Chinese adults. Lipids Health Dis. 2020, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, I.O.; Biciuşcă, V.; Taisescu, C.I.; Alexandru, D.O.; Taisescu, O.; Comănescu, M.V.; Petrescu, F.; Popescu, I.A.; Traşcă, D.M.; Forţofoiu, M.C.; et al. Histological factors that predict the liver fibrosis in patients with chronic hepatitis C. Rom. J. Morphol. Embryol. 2016, 57, 759–765. [Google Scholar] [PubMed]
- Fortofoiu, M.; Fortofoiu, M.C. Clinical, histological, immunohistochemical and morphometric aspects in patients with chronic hepatitis of alcoholic and viral etiology. In Proceedings of the 16th International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 28 June–7 July 2016; Volume 1, pp. 471–478. [Google Scholar]
- Masarone, M.; Rosato, V.; Aglitti, A.; Bucci, T.; Caruso, R.; Salvatore, T.; Sasso, F.C.; Tripodi, M.F.; Persico, M. Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS ONE 2017, 12, e0178473. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Nevola, R.; Coppola, C.; Narciso, V.; Rinaldi, L.; Calvaruso, V.; Pafundi, P.C.; Lombardi, R.; et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 2020, 22, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Narciso, V.; Nevola, R.; Rinaldi, L.; Calvaruso, V.; Staiano, L.; Di Marco, V.; et al. Impact of hepatitis C virus clearance by direct-acting antiviral treatment on the incidence of major cardiovascular events: A prospective multicentre study. Atherosclerosis 2020, 296, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Sasso, F.C.; Pafundi, P.C.; Caturano, A.; Galiero, R.; Vetrano, E.; Nevola, R.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Di Marco, V.; et al. Impact of direct acting antivirals (DAAs) on cardiovascular events in HCV cohort with pre-diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2345–2353. [Google Scholar] [CrossRef]

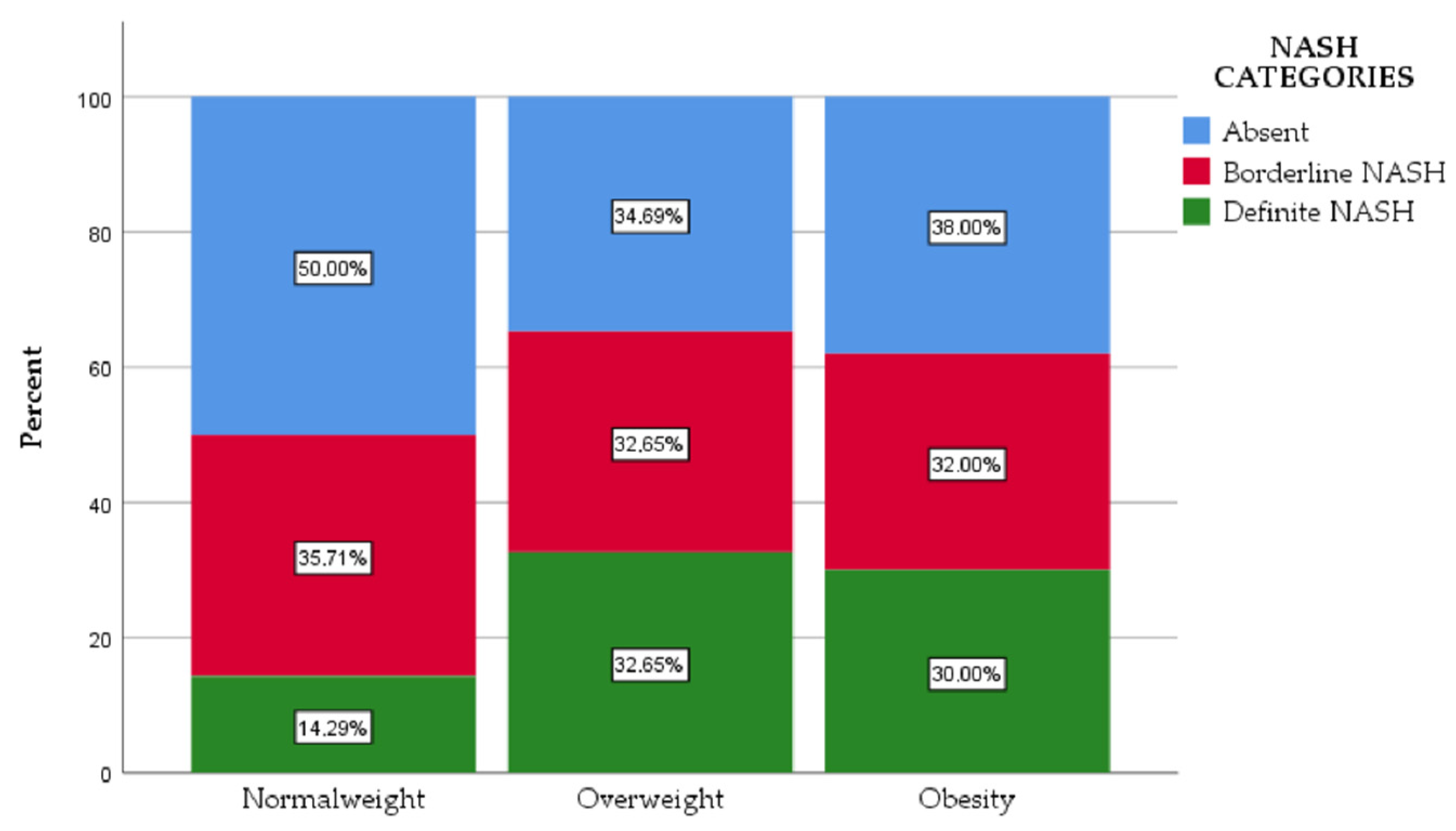

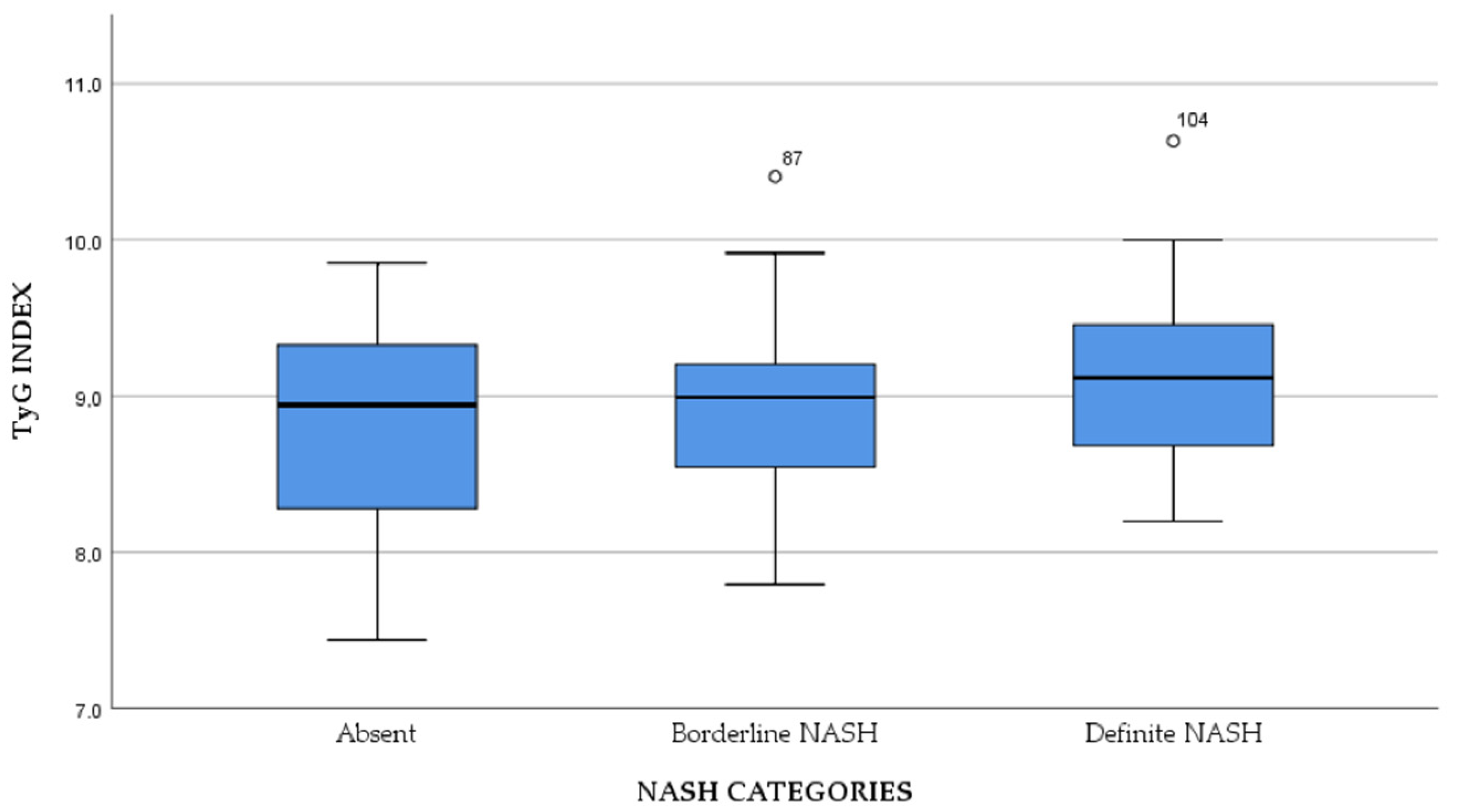
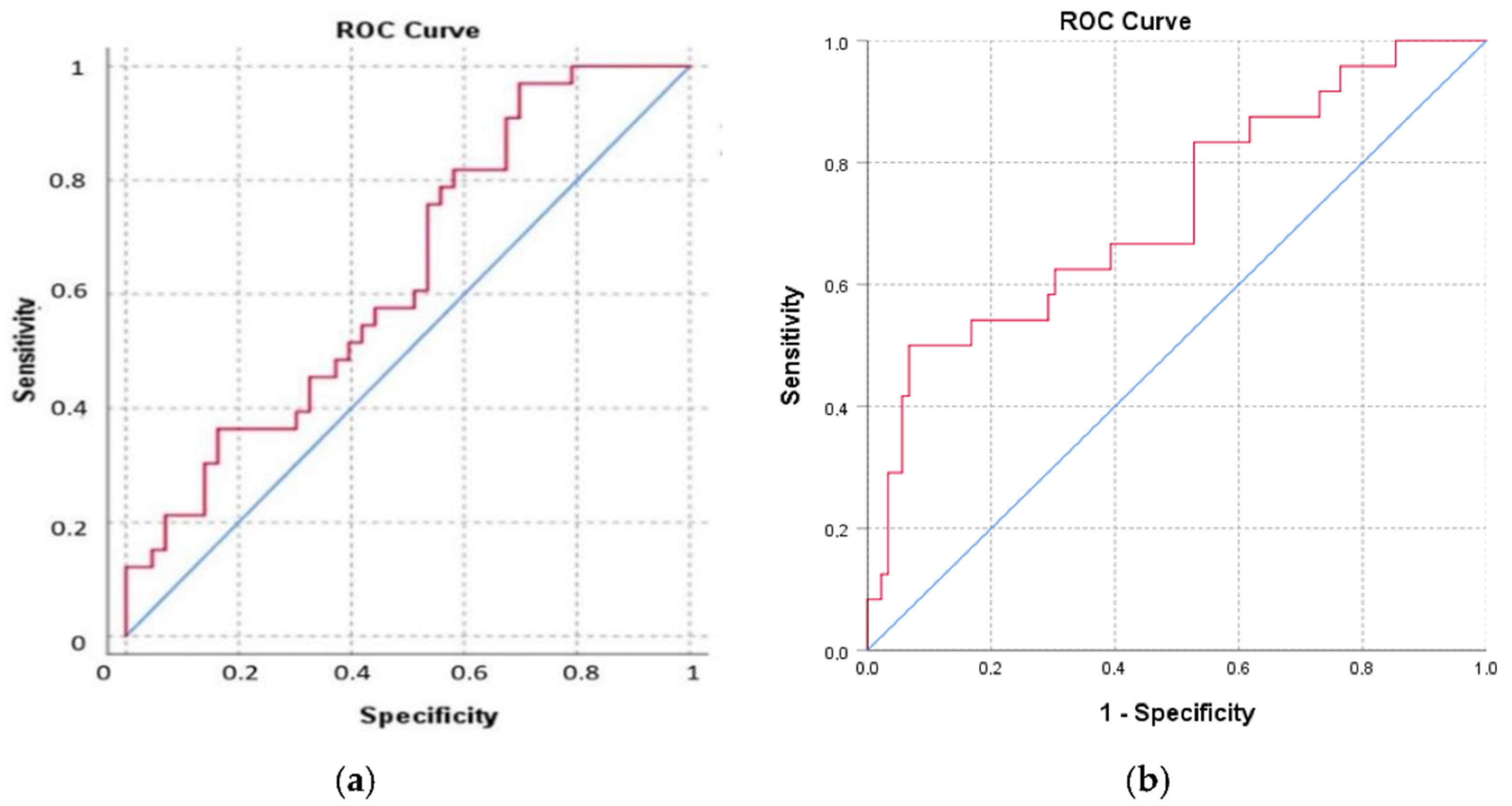
| Characteristics | Normal Weight | Overweight | Obesity | p |
|---|---|---|---|---|
| Gender (%) | ||||
| Male (n = 41) | 14.6 | 53.7 | 31.7 | - |
| Female (n = 72) | 11.1 | 37.5 | 51.4 | |
| Age (years) * | 55 [20] | 51 [20] | 56 [21] | 0.016 |
| Waist circumference (cm) † | ||||
| Male | 101 ± 7.34 | 102.23 ± 6.1 | 115.85 ± 6.95 | 0.001 |
| Female | 82.38 ± 1.68 | 97 ± 9.84 | 110.57 ± 9.61 | |
| FPG (mg/dL) * | 91 [20] | 97 [43] | 108 [44] | 0.032 |
| Triglycerides (mg/dL) * | 111 [98] | 171 [109] | 163 [123] | 0.102 |
| Hypertension (%) | 20 | 42.9 | 54.8 | 0.317 |
| HDL (mg/dL) * | 47 [20] | 47 [18] | 45 [16] | 0.759 |
| ALT (U/L) * | 30 [29] | 29 [24] | 42 [28] | 0.024 |
| GROUP | TyG Index |
|---|---|
| Normal weight | 8.46 ± 0.61 |
| Overweight | 8.93 ± 0.55 |
| Obesity | 9.05 ± 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amzolini, A.M.; Forțofoiu, M.-C.; Alhija, A.B.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Forțofoiu, M.; Matei, D.; Diaconu, M.; Tudor, M.S.; et al. Triglyceride and Glucose Index as a Screening Tool for Nonalcoholic Liver Disease in Patients with Metabolic Syndrome. J. Clin. Med. 2022, 11, 3043. https://doi.org/10.3390/jcm11113043
Amzolini AM, Forțofoiu M-C, Alhija AB, Vladu IM, Clenciu D, Mitrea A, Forțofoiu M, Matei D, Diaconu M, Tudor MS, et al. Triglyceride and Glucose Index as a Screening Tool for Nonalcoholic Liver Disease in Patients with Metabolic Syndrome. Journal of Clinical Medicine. 2022; 11(11):3043. https://doi.org/10.3390/jcm11113043
Chicago/Turabian StyleAmzolini, Anca Maria, Mircea-Cătălin Forțofoiu, Anca Barău Alhija, Ionela Mihaela Vladu, Diana Clenciu, Adina Mitrea, Maria Forțofoiu, Daniela Matei, Magdalena Diaconu, Marinela Sinziana Tudor, and et al. 2022. "Triglyceride and Glucose Index as a Screening Tool for Nonalcoholic Liver Disease in Patients with Metabolic Syndrome" Journal of Clinical Medicine 11, no. 11: 3043. https://doi.org/10.3390/jcm11113043
APA StyleAmzolini, A. M., Forțofoiu, M.-C., Alhija, A. B., Vladu, I. M., Clenciu, D., Mitrea, A., Forțofoiu, M., Matei, D., Diaconu, M., Tudor, M. S., & Micu, E. S. (2022). Triglyceride and Glucose Index as a Screening Tool for Nonalcoholic Liver Disease in Patients with Metabolic Syndrome. Journal of Clinical Medicine, 11(11), 3043. https://doi.org/10.3390/jcm11113043









