Antihyperthermic Treatment in the Management of Malignant Infarction of the Middle Cerebral Artery
Abstract
1. Introduction
2. Materials and Methods
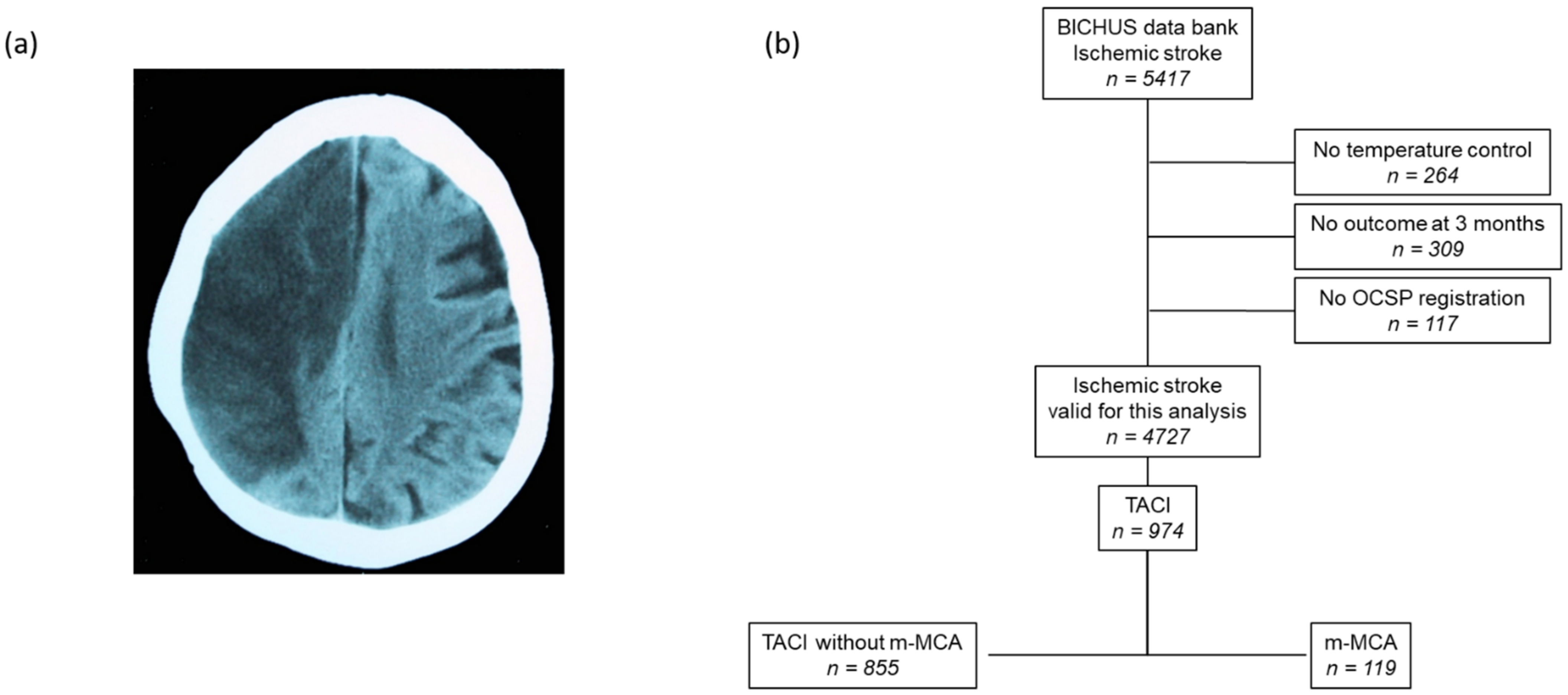
2.1. Patient Screening
2.2. Standard Protocol Approvals, Registrations, and Patients Consents
2.3. Clinical Variables
2.4. Neuroimaging Studies
2.5. Temperature Control
2.6. Treatment of m-MAC
2.7. Outcome Endpoints
2.8. Statistical Analyses
3. Results
3.1. Sample Description
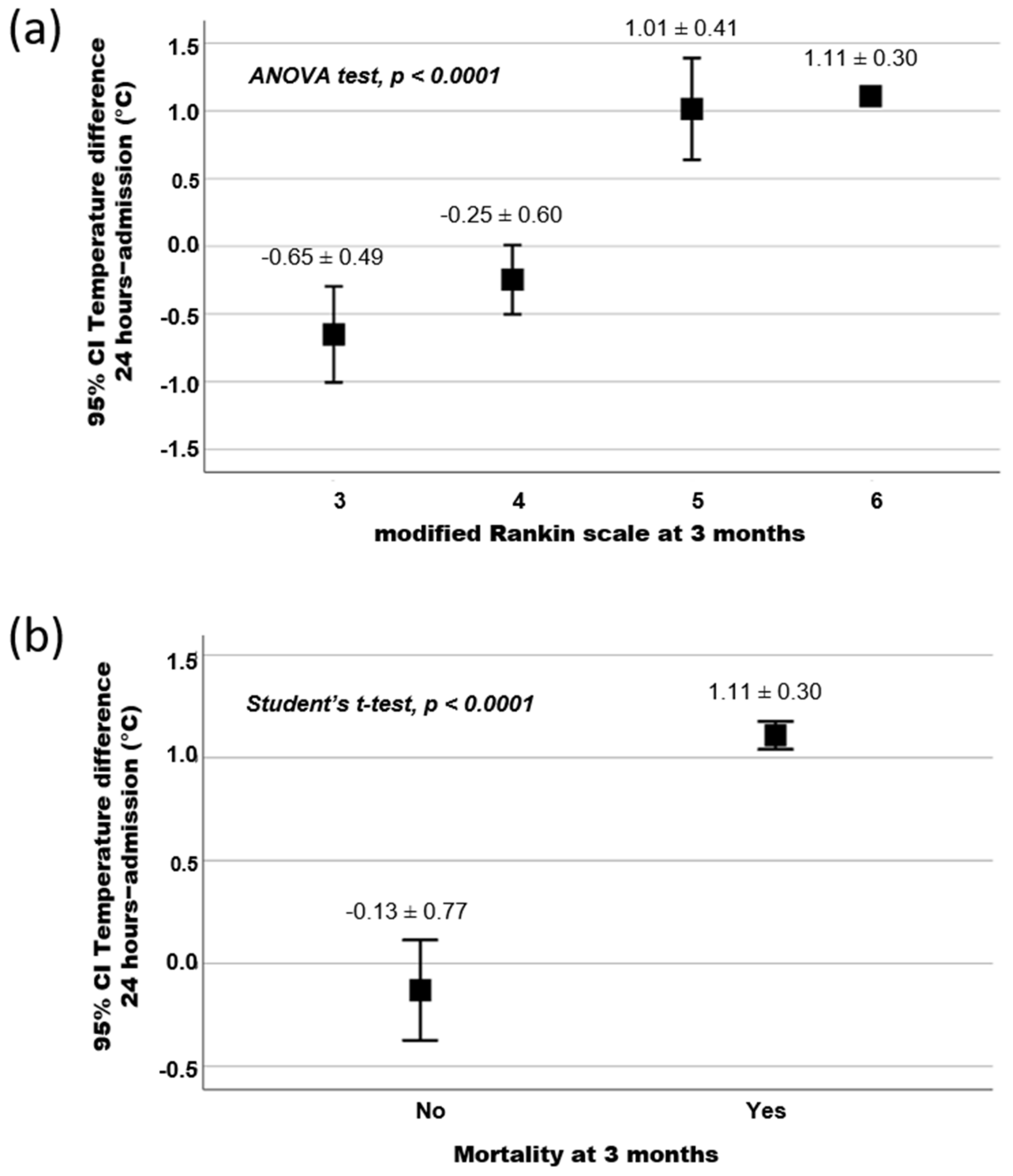
3.2. Association between Temperature and Functional Outcome at 3 Months
3.3. Influence of the Antihyperthermic Treatment and Its Repercussion on the Functional Outcome at 3 Months
3.4. Biomarkers Associated with Development of m-MCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hacke, W.; Schwab, S.; Horn, M.; Spranger, M.; de Georgia, M.; von Kummer, R. “Malignant” Middle Cerebral Artery Territory Infarction: Clinical Course and Prognostic Signs. Arch. Neurol. 1996, 53, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.; Piñero, G.; Cruz-Flores, S.; Alcalá Cerra, G.; Rabinstein, A. Malignant Hemispheric Infarction of the Middle Cerebral Artery. Diagnostic Considerations and Treatment Options. Neurologia 2016, 31, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Heiss, W.-D. Malignant MCA Infarction: Pathophysiology and Imaging for Early Diagnosis and Management Decisions. Cerebrovasc. Dis. 2016, 41, 1–7. [Google Scholar] [CrossRef]
- Wei, H.; Jia, F.-M.; Yin, H.-X.; Guo, Z.-L. Decompressive Hemicraniectomy versus Medical Treatment of Malignant Middle Cerebral Artery Infarction: A Systematic Review and Meta-Analysis. Biosci. Rep. 2020, 40, BSR20191448. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mitchell, P.; Ross, N.; Whitfield, P.C. Decompressive Hemicraniectomy in the Treatment of Malignant Middle Cerebral Artery Infarction: A Meta-Analysis. World Neurosurg. 2019, 123, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Els, T.; Oehm, E.; Voigt, S.; Klisch, J.; Hetzel, A.; Kassubek, J. Safety and Therapeutical Benefit of Hemicraniectomy Combined with Mild Hypothermia in Comparison with Hemicraniectomy Alone in Patients with Malignant Ischemic Stroke. Cerebrovasc. Dis. 2006, 21, 79–85. [Google Scholar] [CrossRef]
- Neugebauer, H.; Schneider, H.; Bösel, J.; Hobohm, C.; Poli, S.; Kollmar, R.; Sobesky, J.; Wolf, S.; Bauer, M.; Tittel, S.; et al. Outcomes of Hypothermia in Addition to Decompressive Hemicraniectomy in Treatment of Malignant Middle Cerebral Artery Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 571–579. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Liu, C. Outcomes of Therapeutic Hypothermia in Patients Treated with Decompressive Craniectomy for Malignant Middle Cerebral Artery Infarction: A Systematic Review and Meta-Analysis. Clin. Neurol. Neurosurg. 2020, 188, 105569. [Google Scholar] [CrossRef]
- Hwang, D.; Matouk, C.; Sheth, K. Management of the Malignant Middle Cerebral Artery Syndrome. Semin. Neurol. 2014, 33, 448–455. [Google Scholar] [CrossRef]
- Campos, F.; Blanco, M.; Barral, D.; Agulla, J.; Ramos-Cabrer, P.; Castillo, J. Influence of Temperature on Ischemic Brain: Basic and Clinical Principles. Neurochem. Int. 2012, 60, 495–505. [Google Scholar] [CrossRef]
- Vahedi, K.; Hofmeijer, J.; Juettler, E.; Vicaut, E.; George, B.; Algra, A.; Amelink, G.J.; Schmiedeck, P.; Schwab, S.; Rothwell, P.M.; et al. Early Decompressive Surgery in Malignant Infarction of the Middle Cerebral Artery: A Pooled Analysis of Three Randomised Controlled Trials. Lancet Neurol. 2007, 6, 215–222. [Google Scholar] [CrossRef]
- Bongiorni, G.T.; Hockmuller, M.C.J.; Klein, C.; Antunes, Á.C.M. Decompressive Craniotomy for the Treatment of Malignant Infarction of the Middle Cerebral Artery: Mortality and Outcome. Arq. Neuro-Psiquiatr. 2017, 75, 424–428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohan Rajwani, K.; Crocker, M.; Moynihan, B. Decompressive Craniectomy for the Treatment of Malignant Middle Cerebral Artery Infarction. Br. J. Neurosurg. 2017, 31, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.; Steiner, T.; Aschoff, A.; Schwarz, S.; Steiner, H.H.; Jansen, O.; Hacke, W. Early Hemicraniectomy in Patients with Complete Middle Cerebral Artery Infarction. Stroke 1998, 29, 1888–1893. [Google Scholar] [CrossRef]
- Wartenberg, K.E. Malignant Middle Cerebral Artery Infarction. Curr. Opin. Crit. Care 2012, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Bamford, J.; Sandercock, P.; Dennis, M.; Burn, J.; Warlow, C. Classification and Natural History of Clinically Identifiable Subtypes of Cerebral Infarction. Lancet 1991, 337, 1521–1526. [Google Scholar] [CrossRef]
- Montaner, J.; Alvarez-Sabín, J. NIH Stroke Scale and Its Adaptation to Spanish. Neurologia 2006, 21, 192–202. [Google Scholar]
- Van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver Agreement for the Assessment of Handicap in Stroke Patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Sims, J.R.; Gharai, L.R.; Schaefer, P.W.; Vangel, M.; Rosenthal, E.S.; Lev, M.H.; Schwamm, L.H. ABC/2 for Rapid Clinical Estimate of Infarct, Perfusion, and Mismatch Volumes. Neurology 2009, 72, 2104–2110. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR Signal Abnormalities at 1.5 T in Alzheimer’s Dementia and Normal Aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.J.; Cvoro, V.; MacLullich, A.M.J.; Shenkin, S.D.; Sandercock, P.A.G.; Sakka, E.; Wardlaw, J.M. Visual Rating Scales of White Matter Hyperintensities and Atrophy: Comparison of Computed Tomography and Magnetic Resonance Imaging. J. Stroke Cerebrovasc. Dis. 2018, 27, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Alonso de Leciñana, M.; Egido, J.A.; Casado, I.; Ribó, M.; Dávalos, A.; Masjuan, J.; Caniego, J.L.; Martínez Vila, E.; Díez Tejedor, E.; ad hoc Committee of the SEN Study Group for Cerebrovascular Diseases; et al. Guidelines for the Treatment of Acute Ischaemic Stroke. Neurologia 2014, 29, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Ávila-Gómez, P.; Hervella, P.; Da Silva-Candal, A.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Castillo, J.; Sobrino, T.; Iglesias-Rey, R.; et al. Temperature-Induced Changes in Reperfused Stroke: Inflammatory and Thrombolytic Biomarkers. J. Clin. Med. 2020, 9, 2108. [Google Scholar] [CrossRef]
- Miao, J.; Song, X.; Sun, W.; Qiu, X.; Lan, Y.; Zhu, Z. Predictors of Malignant Cerebral Edema in Cerebral Artery Infarction: A Meta-Analysis. J. Neurol. Sci. 2020, 409, 116607. [Google Scholar] [CrossRef]
- Hervella, P.; Rodríguez-Yáñez, M.; Pumar, J.M.; Ávila-Gómez, P.; da Silva-Candal, A.; López-Loureiro, I.; Rodríguez-Maqueda, E.; Correa-Paz, C.; Castillo, J.; Sobrino, T.; et al. Antihyperthermic Treatment Decreases Perihematomal Hypodensity. Neurology 2020, 94, e1738–e1748. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood-Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 594672. [Google Scholar] [CrossRef]
- Kassner, A.; Merali, Z. Assessment of Blood–Brain Barrier Disruption in Stroke. Stroke 2015, 46, 3310–3315. [Google Scholar] [CrossRef]
- Serena, J.; Blanco, M.; Castellanos, M.; Silva, Y.; Vivancos, J.; Moro, M.A.; Leira, R.; Lizasoain, I.; Castillo, J.; Dávalos, A. The Prediction of Malignant Cerebral Infarction by Molecular Brain Barrier Disruption Markers. Stroke 2005, 36, 1921–1926. [Google Scholar] [CrossRef]
- Rodríguez-Yáñez, M.; Castellanos, M.; Blanco, M.; Millán, M.; Nombela, F.; Sobrino, T.; Lizasoain, I.; Leira, R.; Serena, J.; Dávalos, A.; et al. Micro- and Macroalbuminuria Predict Hemorrhagic Transformation in Acute Ischemic Stroke. Neurology 2006, 67, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Hervella, P.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Sobrino, T.; Campos, F.; Castillo, J.; da Silva-Candal, A.; Iglesias-Rey, R. STWEAK as Predictor of Stroke Recurrence in Ischemic Stroke Patients Treated With Reperfusion Therapies. Front. Neurol. 2021, 12, 652867. [Google Scholar] [CrossRef] [PubMed]
- Silva-Candal, A.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Ávila-Gómez, P.; Sobrino, T.; Campos, F.; Castillo, J.; Hervella, P.; et al. The Presence of Leukoaraiosis Enhances the Association between STWEAK and Hemorrhagic Transformation. Ann. Clin. Transl. Neurol. 2020, 7, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Candal, A.; López-Dequidt, I.; Rodriguez-Yañez, M.; Ávila-Gómez, P.; Pumar, J.M.; Castillo, J.; Sobrino, T.; Campos, F.; Iglesias-Rey, R.; Hervella, P. STWEAK Is a Marker of Early Haematoma Growth and Leukoaraiosis in Intracerebral Haemorrhage. Stroke Vasc. Neurol. 2021, 6, 528–535. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Candal, A.; Custodia, A.; López-Dequidt, I.; Rodríguez-Yáñez, M.; Alonso-Alonso, M.L.; Ávila-Gómez, P.; Pumar, J.M.; Castillo, J.; Sobrino, T.; Campos, F.; et al. sTWEAK Is a Leukoaraiosis Biomarker Associated with Neurovascular Angiopathy. Ann. Clin. Transl. Neurol. 2022, 9, 171–180. [Google Scholar] [CrossRef]
- Beamer, N.B.; Coull, B.M.; Clark, W.M.; Wynn, M. Microalbuminuria in Ischemic Stroke. Arch. Neurol. 1999, 56, 699–702. [Google Scholar] [CrossRef]
- Thampy, A.; Pais, C.C. Early Clinical Implications of Microalbuminuria in Patients with Acute Ischaemic Stroke. J. Clin. Diagn. Res. 2016, 10, OC29–OC31. [Google Scholar] [CrossRef]
- Lee, M.; Saver, J.L.; Chang, K.-H.; Ovbiagele, B. Level of Albuminuria and Risk of Stroke: Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2010, 30, 464–469. [Google Scholar] [CrossRef]
- Turaj, W.; Slowik, A.; Szczudlik, A. Microalbuminuria in Cerebrovascular Diseases. Expert Rev. Neurother. 2003, 3, 215–223. [Google Scholar] [CrossRef]
- Li, F.; Chen, Q.-X.; Peng, B.; Chen, Y.; Yao, T.; Wang, G. Microalbuminuria in Patients with Acute Ischemic Stroke. Neurol. Res. 2019, 41, 498–503. [Google Scholar] [CrossRef]
- Gumbinger, C.; Sykora, M.; Diedler, J.; Ringleb, P.; Rocco, A. Microalbuminuria. Nervenarzt 2012, 83, 1357–1360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, M.; Saver, J.L.; Chang, K.-H.; Liao, H.-W.; Chang, S.-C.; Ovbiagele, B. Impact of Microalbuminuria on Incident Stroke. Stroke 2010, 41, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Ovbiagele, B. Microalbuminuria: Risk Factor and Potential Therapeutic Target for Stroke? J. Neurol. Sci. 2008, 271, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-N.; Fang, P.-T.; Hung, C.-H.; Hsu, C.-Y.; Chou, P.-S.; Yang, Y.-H. The Association between White Matter Changes and Development of Malignant Middle Cerebral Artery Infarction. Medicine 2021, 100, e25751. [Google Scholar] [CrossRef]
| Mortality | NO n = 41 | YES n = 78 | p |
|---|---|---|---|
| Age, years | 60.7 ± 12.2 | 65.6 ± 12.6 | 0.080 |
| Female gender, % | 36.6 | 69.2 | 0.001 |
| Latency time, min | 252.7 ± 165.1 | 309.6 ± 199.8 | 0.157 |
| Wake-up stroke, % | 12.2 | 14.1 | 0.772 |
| Previous TIA, % | 7.3 | 6.4 | 0.851 |
| Arterial hypertension, % | 63.4 | 79.5 | 0.058 |
| Diabetes, % | 24.4 | 28.2 | 0.656 |
| Smoking, % | 34.1 | 9.0 | 0.002 |
| Alcohol consumption, % | 31.7 | 25.6 | 0.071 |
| Dyslipidemia, % | 36.6 | 42.3 | 0.545 |
| Atrial fibrillation, % | 14.6 | 29.5 | 0.073 |
| NIHSS at admission | 18 (16, 21) | 21 (19, 23) | 0.006 |
| Temperature at admission, °C | 37.6 ± 0.6 | 37.3 ± 0.8 | 0.134 |
| Blood glucose, mg/dL | 169.3 ± 87.9 | 146.8 ± 81.7 | 0.206 |
| Leukocytes, ×103/mL | 10.7 ± 3.7 | 8.8 ± 0.9 | 0.695 |
| Fibrinogen, mg/dL | 459.6 ± 55.7 | 428.8 ± 69.1 | 0.244 |
| C-reactive protein, mg/L | 3.9 ± 3.7 | 3.9 ± 2.2 | 0.101 |
| Erythrocyte sedimentation rate, mm | 30.6 ± 15.4 | 31.6 ± 21.8 | 0.587 |
| Microalbuminuria, mg/24 h | 3.9 ± 10.4 | 11.6 ± 21.9 | 0.292 |
| 25-Hydroxy-vitamin D levels, ng/mL | 14.4 ± 5.5 | 15.2 ± 2.3 | 0.659 |
| TOAST | <0.0001 | ||
| Atherothrombotic, % | 36.6 | 30.8 | |
| Cardioembolic, % | 17.1 | 59.0 | |
| Indeterminate, % | 46.3 | 10.3 | |
| Antihyperthermic treatment, % | 78.0 | 50.0 | 0.003 |
| Systemic thrombolysis, % | 24.4 | 26.9 | 0.765 |
| Thrombectomy, % | 17.1 | 2.6 | 0.004 |
| Hemicraniectomy, % | 14.6 | 32.1 | 0.066 |
| Leukoaraiosis, % | 61.0 | 57.7 | 0.729 |
| Degree of leukoaraiosis | 0.114 | ||
| Grade I, % | 19.5 | 5.3 | |
| Grade II, % | 9.8 | 14.7 | |
| Grade III, % | 31.7 | 36.0 | |
| Infarct volume, mL | 179.8 ± 101.1 | 222.0 ± 97.3 | 0.036 |
| Temperature 24 h | 37.4 ± 0.9 | 38.5 ± 0.7 | <0.0001 |
| Temperature 24 h-admission | −0.14 ± 0.77 | 1.12 ± 0.29 | <0.0001 |
| Early neurological deterioration, % | 22.0 | 41.5 | 0.045 |
| Not Adjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p | OR | CI 95% | p | |
| Female gender | 3.90 | 1.76–8.65 | 0.001 | 6.23 | 0.93–41.81 | 0.060 |
| Smoking | 0.19 | 0.07–0.52 | 0.001 | 0.69 | 0.07–6.52 | 0.746 |
| NIHSS at admission | 1.12 | 1.01–1.24 | 0.026 | 1.08 | 0.87–1.35 | 0.493 |
| Cardioembolic | 4.11 | 1.47–11.43 | 0.007 | 1.39 | 0.21–9.27 | 0.730 |
| Thrombectomy | 0.13 | 0.02–0.65 | 0.013 | 0.74 | 0.01–54.79 | 0.891 |
| Temperature 24 h admission | 187.62 | 20.49–1718.39 | <0.0001 | 158.97 | 7.29–3465.61 | 0.001 |
| Infarct volume | 1.01 | 1.00–1.02 | 0.039 | 1.00 | 0.99–1.01 | 0.678 |
| Early neurological deterioration | 2.52 | 1.01–6.33 | 0.048 | 4.33 | 0.67–27.79 | 0.123 |
| Not Adjusted | Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Female gender | 3.90 | 1.76–8.65 | 0.001 | 5.04 | 1.23–20.52 | 0.024 |
| Smoking | 0.19 | 0.07–0.52 | 0.001 | 0.29 | 0.06–1.57 | 0.150 |
| NIHSS at admission | 1.12 | 1.01–1.24 | 0.026 | 1.09 | 0.93–1.28 | 0.273 |
| Cardioembolic | 4.11 | 1.47–11.43 | 0.007 | 2.70 | 0.58–12.65 | 0.207 |
| Thrombectomy | 0.13 | 0.02–0.65 | 0.013 | 0.11 | 0.01–1.46 | 0.095 |
| Antihypertensive treatment | 0.28 | 0.12–0.66 | 0.004 | 0.08 | 0.02–0.38 | 0.002 |
| Infarct volume | 1.01 | 1.00–1.02 | 0.039 | 1.00 | 0.99–1.01 | 0.103 |
| Early neurological deterioration | 2.52 | 1.01–6.33 | 0.048 | 11.47 | 2.21–59.46 | 0.004 |
| m-MCA n = 119 | TACI n = 855 | p | |
|---|---|---|---|
| Age, years | 64.5 ± 12.7 | 70.1 ± 13.5 | <0.0001 |
| Female gender, % | 58.0 | 38.8 | <0.0001 |
| Latency time, min | 253.3 ± 190.1 | 232.9 ± 154.2 | 0.003 |
| Wake-up stroke, % | 13.4 | 8.9 | 0.081 |
| Previous TIA, % | 6.7 | 7.8 | 0.419 |
| Arterial hypertension, % | 73.9 | 63.6 | 0.016 |
| Diabetes, % | 26.9 | 25.5 | 0.410 |
| Smoking, % | 17.6 | 20.8 | 0.251 |
| Alcohol consumption, % | 16.8 | 15.6 | 0.404 |
| Dyslipidemia, % | 40.3 | 35.7 | 0.186 |
| Atrial fibrillation, % | 24.4 | 13.1 | 0.002 |
| NIHSS at admission | 21 (18, 23) | 13 (9, 18) | <0.0001 |
| Temperature at admission, °C | 37.4 ± 0.7 | 36.5 ± 0.6 | <0.0001 |
| Blood glucose, mg/dL | 155.9 ± 82.4 | 142.4 ± 61.6 | 0.015 |
| Leukocytes, ×103/mL | 9.9 ± 3.0 | 9.7 ± 3.3 | 0.052 |
| Fibrinogen, mg/dL | 446.7 ± 60.6 | 451.3 ± 94.4 | 0.058 |
| C-reactive protein, mg/L | 3.9 ± 3.1 | 4.1 ± 3.8 | 0.102 |
| Erythrocyte sedimentation rate, mm | 31.0 ± 17.4 | 27.9 ± 21.2 | 0.012 |
| Microalbuminuria, mg/24 h | 16.3 ± 12.3 | 4.9 ± 23.7 | <0.0001 |
| 25-Hydroxy-vitamin D levels, ng/mL | 14.7 ± 4.3 | 15.2 ± 7.9 | 0.005 |
| TOAST | 0.001 | ||
| Atherothrombotic, % | 32.8 | 43.6 | |
| Cardioembolic, % | 44.5 | 28.4 | |
| Indeterminate, % | 22.7 | 27.6 | |
| Other, % | - | 0.4 | |
| Antihyperthermic treatment, % | 59.7 | 7.1 | <0.0001 |
| Systemic thrombolysis, % | 26.1 | 30.8 | 0.171 |
| Thrombectomy, % | 7.6 | 4.6 | 0.120 |
| Hemicraniectomy, % | 26.1 | - | |
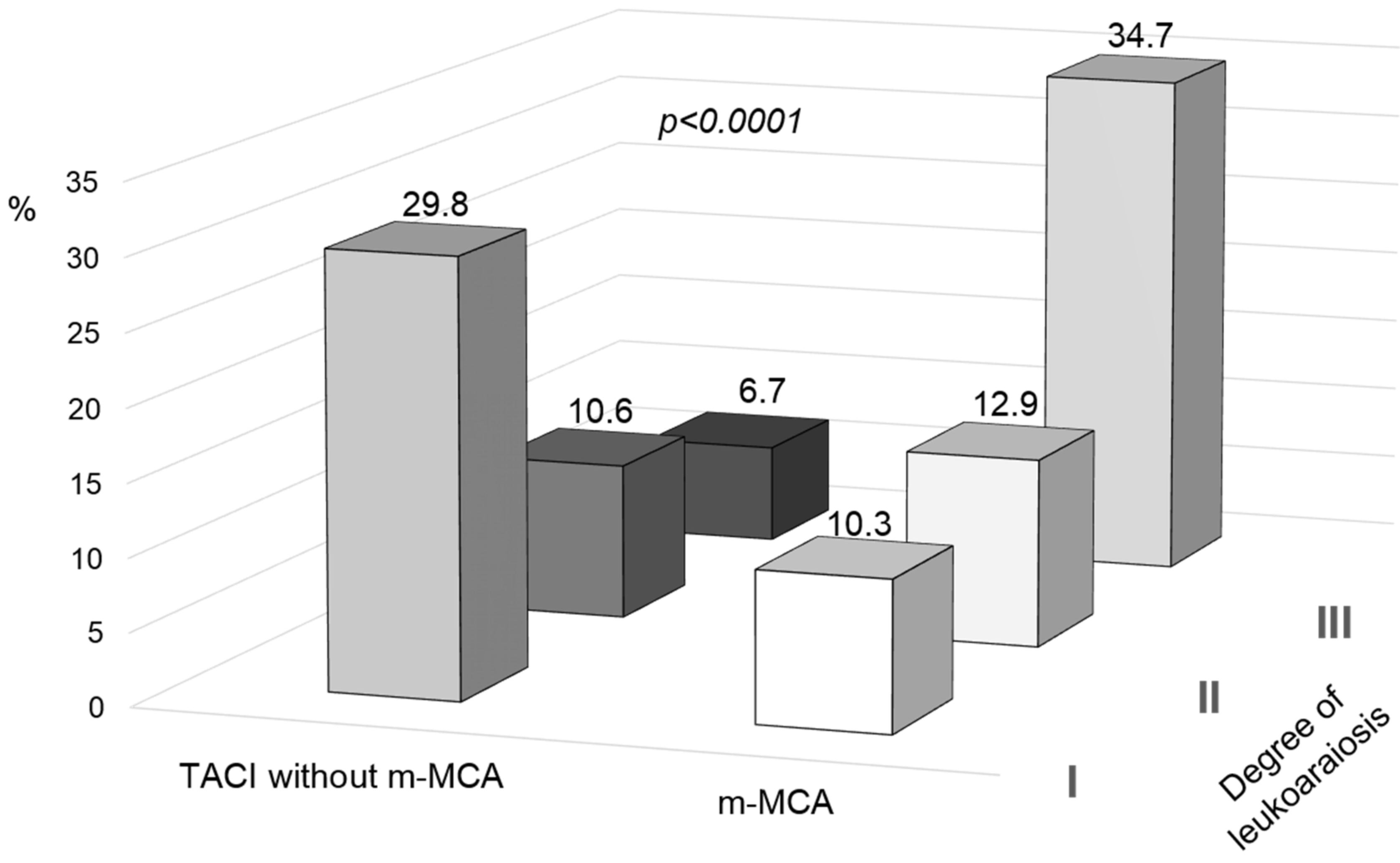
| Leukoaraiosis, % | 58.8 | 17.8 | <0.0001 |
| Degree of leukoaraiosis | |||
| Grade I, % | 10.3 | 29.8 | |
| Grade II, % | 12.9 | 10.6 | |
| Grade III, % | 34.7 | 6.7 | |
| Infarct volume, mL | 205.7 ± 100.4 | 47.4 ± 72.8 | <0.0001 |
| Temperature 24 h | 38.1 ± 0.9 | 36.5 ± 1.4 | <0.0001 |
| Temperature 24 h-admission | 0.7 ± 0.8 | 0.3 ± 1.3 | <0.0001 |
| Early neurological deterioration, % | 43.3 | 6.8 | <0.0001 |
| Rankin scale at 3 months | 6 (4–6) | 3 (1–4) | <0.0001 |
| Not Adjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p | OR | CI 95% | p | |
| Age | 0.97 | 0.96–0.98 | <0.0001 | 0.94 | 0.91–0.97 | <0.0001 |
| Female gender | 2.17 | 1.47–3.21 | <0.0001 | 2.06 | 0.97–4.36 | 0.061 |
| Latency time | 1.00 | 1.00–1.01 | 0.001 | 1.00 | 1.00–1.01 | 0.007 |
| Arterial hypertension | 1.62 | 1.05–2.50 | 0.028 | 2.62 | 1.07–6.43 | 0.035 |
| Atrial fibrillation | 2.13 | 1.34–3.39 | 0.001 | 2.06 | 0.76–5.60 | 0.158 |
| NIHSS at admission | 1.21 | 1.16–1.25 | <0.0001 | 1.28 | 1.18–1.38 | <0.0001 |
| Microalbuminuria | 1.01 | 1.01–1.03 | 0.019 | 1.01 | 1.00–1.03 | 0.005 |
| Cardioembolic | 2.08 | 1.34–3.25 | 0.001 | 0.45 | 0.17–1.17 | 0.104 |
| Not Adjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p | OR | CI 95% | p | |
| Age | 0.97 | 0.96–0.98 | <0.0001 | 0.93 | 0.91–0.95 | <0.0001 |
| Female gender | 2.17 | 1.47–3.21 | <0.0001 | 2.33 | 1.39–3.89 | 0.001 |
| Latency time | 1.00 | 1.00–1.01 | 0.001 | 1.00 | 1.00–1.01 | <0.0001 |
| Arterial hypertension | 1.62 | 1.05–2.50 | 0.028 | 3.94 | 2.07–7.51 | <0.0001 |
| Atrial fibrillation | 2.13 | 1.34–3.39 | 0.001 | 3.22 | 1.70–6.08 | <0.0001 |
| NIHSS at admission | 1.21 | 1.16–1.25 | <0.0001 | 1.21 | 1.15–1.27 | <0.0001 |
| Leukoaraiosis | 6.61 | 4.41–9.91 | <0.0001 | 3.07 | 1.84–5.13 | <0.0001 |
| Not Adjusted | * Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Age | 0.97 | 0.96–0.98 | <0.0001 | 0.94 | 0.92–0.96 | <0.0001 |
| Female gender | 2.17 | 1.47–3.21 | <0.0001 | 1.90 | 1.09–3.32 | 0.024 |
| Latency time | 1.00 | 1.00–1.01 | 0.001 | 1.00 | 1.00–1.01 | <0.0001 |
| Arterial hypertension | 1.62 | 1.05–2.50 | 0.028 | 3.42 | 1.73–6.75 | <0.0001 |
| Atrial fibrillation | 2.13 | 1.34–3.39 | 0.001 | 2.59 | 1.30–5.18 | 0.007 |
| NIHSS at admission | 1.21 | 1.16–1.25 | <0.0001 | 1.20 | 1.14–1.26 | <0.0001 |
| Degree of leukoaraiosis | 6.61 | 4.41–9.91 | <0.0001 | 3.07 | 1.84–5.13 | <0.0001 |
| Grade I | ref | ref | ||||
| Grade II | 6.61 | 4.41–9.91 | <0.0001 | 6.78 | 2.54–18.12 | <0.0001 |
| Grade III | 6.61 | 4.41–9.91 | <0.0001 | 13.49 | 6.01–30.29 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Alonso, M.L.; Sampedro-Viana, A.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Mosqueira, A.J.; Ouro, A.; Ávila-Gómez, P.; Sobrino, T.; Campos, F.; et al. Antihyperthermic Treatment in the Management of Malignant Infarction of the Middle Cerebral Artery. J. Clin. Med. 2022, 11, 2874. https://doi.org/10.3390/jcm11102874
Alonso-Alonso ML, Sampedro-Viana A, Rodríguez-Yáñez M, López-Dequidt I, Pumar JM, Mosqueira AJ, Ouro A, Ávila-Gómez P, Sobrino T, Campos F, et al. Antihyperthermic Treatment in the Management of Malignant Infarction of the Middle Cerebral Artery. Journal of Clinical Medicine. 2022; 11(10):2874. https://doi.org/10.3390/jcm11102874
Chicago/Turabian StyleAlonso-Alonso, Maria Luz, Ana Sampedro-Viana, Manuel Rodríguez-Yáñez, Iria López-Dequidt, José M. Pumar, Antonio J. Mosqueira, Alberto Ouro, Paulo Ávila-Gómez, Tomás Sobrino, Francisco Campos, and et al. 2022. "Antihyperthermic Treatment in the Management of Malignant Infarction of the Middle Cerebral Artery" Journal of Clinical Medicine 11, no. 10: 2874. https://doi.org/10.3390/jcm11102874
APA StyleAlonso-Alonso, M. L., Sampedro-Viana, A., Rodríguez-Yáñez, M., López-Dequidt, I., Pumar, J. M., Mosqueira, A. J., Ouro, A., Ávila-Gómez, P., Sobrino, T., Campos, F., Castillo, J., Hervella, P., & Iglesias-Rey, R. (2022). Antihyperthermic Treatment in the Management of Malignant Infarction of the Middle Cerebral Artery. Journal of Clinical Medicine, 11(10), 2874. https://doi.org/10.3390/jcm11102874










