Reverse Shoulder Arthroplasty for Proximal Humerus Head-Split Fractures—A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
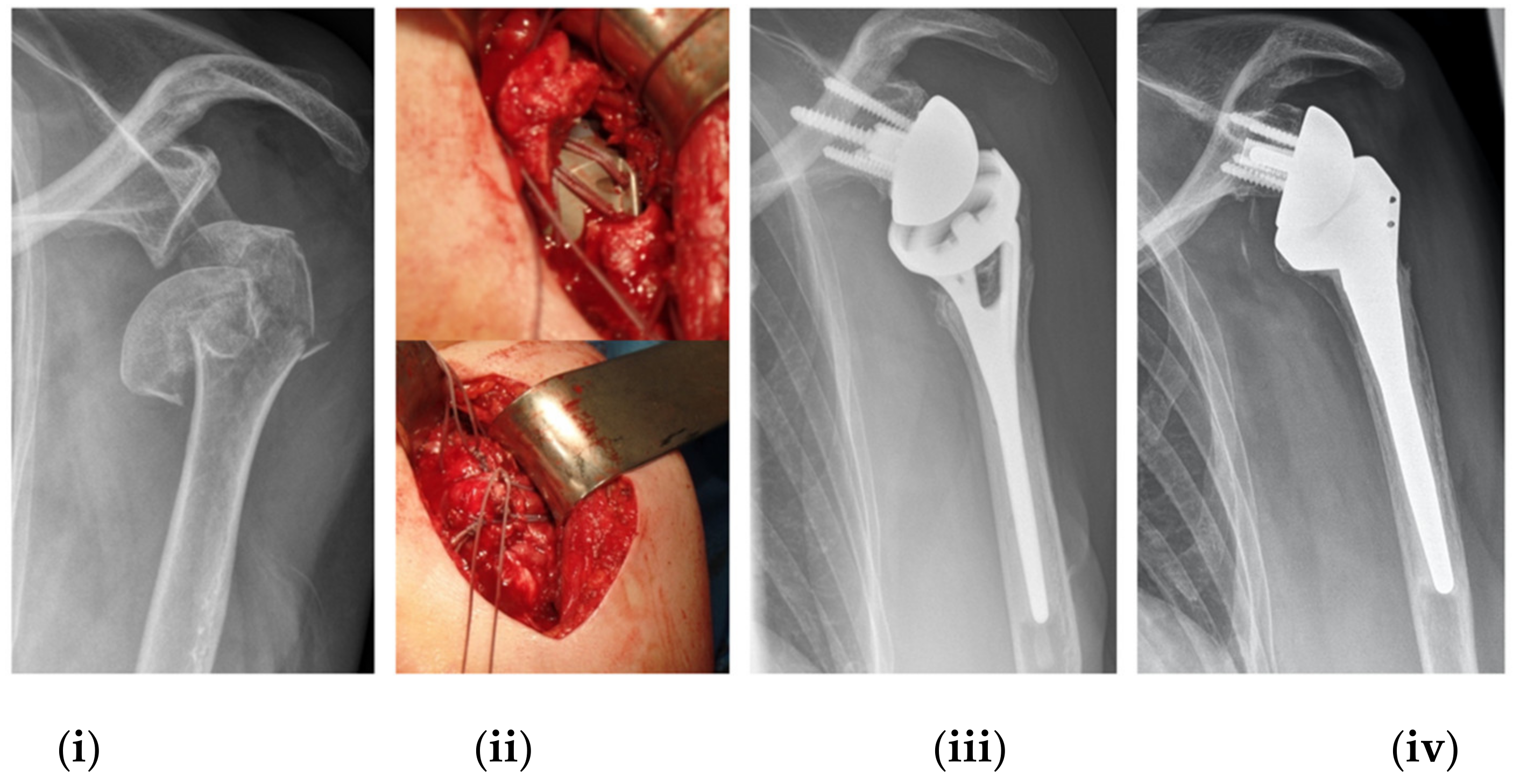
2.2. Implant Description, Surgical Procedure and Postoperative Rehabilitation Protocol
2.3. Clinical Assessment
2.4. Radiographic Evaluation
2.5. Complication
2.6. Statistical Analysis
3. Results
3.1. Clinical Results
3.2. Radiographic Results
3.3. Comparison of Head-Split Types
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aaron, D.; Shatsky, J.; Paredes, J.C.; Jiang, C.; Parsons, B.O.; Flatow, E.L. Proximal Humeral Fractures: Internal Fixation. Instr. Course Lect. 2013, 62, 143–154. [Google Scholar]
- Greiwe, R.M.; Vargas-Ariza, R.; Bigliani, L.U.; Levine, W.N.; Ahmad, C.S. Hemiarthroplasty for head-split fractures of the proximal humerus. Orthopedics 2013, 36, e905–e911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, C.R.; Zarins, B. Chronic unreduced dislocations of the shoulder. J. Bone Jt. Surg. Am. 1982, 64, 494–505. (In English) [Google Scholar] [CrossRef]
- Randelli, M. La Fracture-Luxation Postérieure de L’épaule: Noveux Éléments de Classification et Thérapeutiques. In Proceedings of the 2nd Congrés de la Société Europénne de Chirurgie de l’éPaule et du Coude, Berné, France, 1–2 October 1988. [Google Scholar]
- Walch, G.; Boileau, P.; Martin, B.; Dejour, H. Unreduced Posterior Luxations and Fractures-Luxations of the Shoulder. Apropos of 30 cases. Revue Chir. Orthop. Reparatrice L’appareil Mot. 1990, 76, 546–558. [Google Scholar]
- Chesser, T.J.; Langdon, I.J.; Ogilvie, C.; Sarangi, P.P.; Clarke, A.M. Fractures Involving Splitting of the Humeral Head. J. Bone Jt. Surg. Br. 2001, 83, 423–426. [Google Scholar] [CrossRef]
- Scheibel, M.; Peters, P.; Moro, F.; Moroder, P. Head-split fractures of the proximal humerus. Obere Extremität 2019, 14, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Gokkus, K.; Agar, E.; Sagtas, E.; Aydin, A.T. Proximal humerus head-splitting fracture associated with single-part anterior dislocation. BMJ Case Rep. 2014, 2014, bcr2013202188. [Google Scholar] [CrossRef] [Green Version]
- Bufquin, T.; Hersan, A.; Hubert, L.; Massin, P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: A prospective review of 43 cases with a short-term follow-up. J. Bone Jt. Surg. Br. 2007, 89, 516–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazeneuve, J.F.; Cristofari, D.J. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop. Traumatol. Surg. Res. 2014, 100, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, P.N.; Slikker, W., III; Mall, N.A.; Gupta, A.K.; Rahman, Z.; Enriquez, D.; Nicholson, G.P. Reverse total shoulder arthroplasty for acute proximal humeral fracture: Comparison to open reduction-internal fixation and hemiarthroplasty. J. Shoulder Elb. Surg. 2014, 23, 197–204. [Google Scholar] [CrossRef]
- Cuff, D.J.; Pupello, D.R. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J. Bone Jt. Surg. Am. 2013, 95, 2050–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata-Fink, A.; Meinke, M.; Jones, C.; Kim, B.; Bell, J.E. Reverse shoulder arthroplasty for treatment of proximal humeral fractures in older adults: A systematic review. J. Shoulder Elb. Surg. 2013, 22, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Imiolczyk, J.P.; Moroder, P.; Scheibel, M. Fracture-Specific and Conventional Stem Designs in Reverse Shoulder Arthroplasty for Acute Proximal Humerus Fractures-A Retrospective, Observational Study. J. Clin. Med. 2021, 10, 175. [Google Scholar] [CrossRef]
- Peters, P.-M.; Plachel, F.; Danzinger, V.; Nove, M.; Märdian, S.; Scheibel, M.; Moroder, P. Clinical and Radiographic Outcomes After Surgical Treatment of Proximal Humeral Fractures with Head-Split Component. J. Bone Jt. Surg. Am. 2020, 102, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.R.; Murley, A.H. A Clinical Method of Functional Assessment of the Shoulder. Clin. Orthop. Relat. Res. 1987, 214, 160–164. [Google Scholar] [CrossRef]
- Michener, L.A.; McClure, P.W.; Sennett, B.J. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: Reliability, validity, and responsiveness. J. Shoulder Elb. Surg. 2002, 11, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Gilbart, M.K.; Gerber, C. Comparison of the subjective shoulder value and the Constant score. J. Shoulder Elb. Surg. 2007, 16, 717–721. [Google Scholar] [CrossRef]
- Godfrey, J.; Hamman, R.; Lowenstein, S.; Briggs, K.; Kocher, M. Reliability, validity, and responsiveness of the simple shoulder test: Psychometric properties by age and injury type. J. Shoulder Elb. Surg. 2007, 16, 260–267. [Google Scholar] [CrossRef]
- Boileau, P.; Chuinard, C.; Roussanne, Y.; Bicknell, R.T.; Rochet, N.; Trojani, C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin. Orthop. Relat. Res. 2008, 466, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Tavakkolizadeh, A.; Ghassemi, A.; Colegate-Stone, T.; Latif, A.; Sinha, J. Gender-specific Constant score correction for age. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 529–533. [Google Scholar] [CrossRef]
- Kennedy, J.; Klifto, C.S.; Ledbetter, L.; Bullock, G.S. Reverse total shoulder arthroplasty clinical and patient-reported outcomes and complications stratified by preoperative diagnosis: A systematic review. J. Shoulder Elb. Surg. 2021, 30, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.P.; Mannan, S.S.; Dharmarajan, R.; Rangan, A. Tuberosity healing after reverse shoulder arthroplasty for complex proximal humeral fractures in elderly patients-does it improve outcomes? A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2019, 28, e78–e91. (In English) [Google Scholar] [CrossRef]
- Gokkus, K.; Sagtas, E.; Kara, H.; Aydin, A.T. Posterior Shoulder Dislocation Associated with the Head (Splitting) and Humeral Neck Fracture: Impact of Understanding Radiologic Signs and Experience With an Extended Deltopectoral Approach. Tech. Hand Up. Extrem. Surg. 2018, 22, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J. Das Gesetz der Transformation des Knochen. DMW Dtsch. Med. Wochenschr. 1893, 19, 1222–1224. [Google Scholar] [CrossRef] [Green Version]
- Antuña, S.A.; Sperling, J.W.; Cofield, R.H. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: A minimum five-year follow-up. J. Shoulder Elb. Surg. 2008, 17, 202–209. (In English) [Google Scholar] [CrossRef]
- Shukla, D.R.; McAnany, S.; Kim, J.; Overley, S.; Parsons, B.O. Hemiarthroplasty versus reverse shoulder arthroplasty for treatment of proximal humeral fractures: A meta-analysis. J. Shoulder Elb. Surg. 2016, 25, 330–340. [Google Scholar] [CrossRef]
- Bonnevialle, N.; Tournier, C.; Clavert, P.; Ohl, X.; Sirveaux, F.; Saragaglia, D. Hemiarthroplasty versus reverse shoulder arthroplasty in 4-part displaced fractures of the proximal humerus: Multicenter retrospective study. Orthop. Traumatol. Surg. Res. 2016, 102, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Gavaskar, A.S.; Tummala, N.C. Locked plate osteosynthesis of humeral head-splitting fractures in young adults. J. Shoulder Elb. Surg. 2015, 24, 908–914. [Google Scholar] [CrossRef]


| N (women in %) | 26 (77%) | |
| Age at surgery (years) (mean ± SD) | 73.4 ± 7.8 | |
| (range) | 56–91 | |
| Follow-up period (months) (mean ± SD) | 50 ± 22 | |
| (range) | 12–142 | |
| Trauma mechanism | Low energy | High energy |
| N | 13 (50%) | 13 (50%) |
| (Women in %) | 85% | 69% |
| Age at surgery (years) (mean ± SD) | 77.8 ± 7.8 | 68.5 ± 7.3 |
| (range) | (64–91) | (56–78) |
| Follow-up period (months) (mean ± SD) | 52.6 ± 36.7 | 46.8 ± 22.0 |
| (range) | (14–142) | (12–93) |
| Head-split classification * (n) | ||
| I | 3 | 7 |
| II | 3 | 0 |
| III | 2 | 1 |
| IV | 5 | 5 |
| Additional glenoid rim fracture (n) | 2 | 0 |
| Mean (SD) | Range | |
|---|---|---|
| Absolute CS (points) | 73.7 (11.2) | 43–92 |
| Absolute CS of opposite shoulder (points) | 80.3 (10.4) | 58–97 |
| Relative CS compared to opposite shoulder (%) | 92.4 (14.1) | 67–141 |
| Age- and gender-modified CS (%) | 79.1 (10.0) | 53–95 |
| ASES score (points) | 89.1 (13.8) | 53–100 |
| SSV (%) | 82.0 (13.0) | 50–100 |
| SST (%) | 77.3 (19.4) | 33–100 |
| ADLER score (0–30 points) | 27.7 (4.0) | 12–30 |
| Pain scale (0–15 points) | 14.3 (2.0) | 8–15 |
| Abduction strength (kg) | 4.0 (1.9) | 0–8.7 |
| Range of motion | ||
| Anterior forward elevation (°) | 148 (25) | 100–175 |
| Abduction (°) | 144 (27) | 80–180 |
| External rotation in 0° abduction (°) | 15 (16) | −10–60 |
| Internal rotation (CS points) | 6.1 (2.7) | 0–10 |
| Satisfaction (1–4) | 3.8 (0.4) | 3–4 |
| c | Type 1 | Type 2 | Type 3 | Type 4 | p-Value * | p-Value ** Type 1 vs. 4 |
|---|---|---|---|---|---|---|
| Mean (SD) Range | ||||||
| n | 10 | 3 | 3 | 10 | ||
| Age at surgery | 77 (6.1) | 76 (7.8) | 71 (7.9) | 69 (10.7) | ||
| 68–87 | 70–85 | 62–77 | 56–91 | |||
| Follow-up period | 43 (25.4) | 80 (55.0) | 47 (12.8) | 49 (27.3) | ||
| 14–95 | 37–142 | 33–58 | 12–93 | |||
| High-energy (n)/low-energy trauma setting (n) | 7/3 | 0/3 | 1/2 | 5/5 | ||
| Age and gender modified CS (%) | 87 (4.8) | 73 (11.8) | 78 (10.1) | 74 (9.3) | 0.010 | >0.001 |
| 80–95 | 60–81 | 67–86 | 53–86 | |||
| Absolute CS (points) | 81 (6.3) | 68 (10.0) | 75 (7.8) | 68 (12.7) | 0.033 | 0.006 |
| 72–92 | 57–75 | 66–81 | 43–84 | |||
| Relative CS to opposite shoulder (%) | 100 (15.0) | 82 (8.1) | 90 (12.8) | 89 (13.0) | 0.2 | 0.06 |
| 91–141 | 73–88 | 75–99 | 67–112 | |||
| ASES score (points) | 98 (1.7) | 86 (16.4) | 77 (20.4) | 85 (14.2) | 0.047 | 0.002 |
| 88–100 | 68–100 | 62–100 | 53–98 | |||
| SSV (%) | 88 (9.5) | 78 (25.7) | 73 (19) | 80 (9.1) | 0.3 | 0.03 |
| 70–100 | 50–100 | 60–95 | 70–90 | |||
| SST (%) | 90 (12.3) | 72 (21.0) | 72 (17.5) | 68 (20.6) | 0.2 | 0.1 |
| 67–100 | 50–92 | 58–92 | 33–92 | |||
| ADLER score (0–30 points) | 29 (1.7) | 24 (10.4) | 25 (3.8) | 28 (2.2) | 0.053 | 0.050 |
| 26–30 | 12–30 | 21–28 | 24–30 | |||
| Anterior forward elevation (°) | 154 (23) | 142 (28) | 162 (3) | 140 (28) | 0.5 | 0.1 |
| 110–170 | 110–160 | 160–165 | 100–175 | |||
| Abduction (°) | 155 (22) | 150 (17) | 157 (23) | 128 (29) | 0.1 | 0.04 |
| 120–180 | 130–160 | 130–170 | 80–165 | |||
| External rotation in 0° abduction (°) | 16 (21) | 13 (12) | 10 (15) | 16 (14) | 0.9 | 0.4 |
| 0–60 | 0–20 | −5–25 | 100–175 | |||
| Internal rotation (CS points) | 8.2 (1.4) | 3.3 (2.3) | 6.7 (2.3) | 4.6 (2.7) | 0.002 | <0.001 |
| 6–10 | 2–6 | 4–8 | 0–8 | |||
| Abduction strength (kg) | 4.9 (1.9) | 2.7 (1.0) | 3.9 (0.8) | 3.5 (2.2) | 0.3 | 0.08 |
| 2.5–8.7 | 1.5–3.4 | 3.3–4.8 | 0–7.2 | |||
| GT healing | 4 out of 8 (50%) | 1 out of 3 (33%) | 2 out of 3 (67%) | 7 out of 9 (78%) | 0.5 | 0.3 |
| LT healing | 7 out of 8 (88%) | 2 out of 3 (67%) | 2 out of 3 (67%) | 7 out of 9 (78%) | 0.9 | 0.3 |
| Scapular notching | 0% | 1 × Grade 1 (33%) | 0% | 1 × Grade 1 (11%) | 0.02 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imiolczyk, J.-P.; Brunner, U.; Imiolczyk, T.; Freislederer, F.; Endell, D.; Scheibel, M. Reverse Shoulder Arthroplasty for Proximal Humerus Head-Split Fractures—A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 2835. https://doi.org/10.3390/jcm11102835
Imiolczyk J-P, Brunner U, Imiolczyk T, Freislederer F, Endell D, Scheibel M. Reverse Shoulder Arthroplasty for Proximal Humerus Head-Split Fractures—A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(10):2835. https://doi.org/10.3390/jcm11102835
Chicago/Turabian StyleImiolczyk, Jan-Philipp, Ulrich Brunner, Tankred Imiolczyk, Florian Freislederer, David Endell, and Markus Scheibel. 2022. "Reverse Shoulder Arthroplasty for Proximal Humerus Head-Split Fractures—A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 10: 2835. https://doi.org/10.3390/jcm11102835
APA StyleImiolczyk, J.-P., Brunner, U., Imiolczyk, T., Freislederer, F., Endell, D., & Scheibel, M. (2022). Reverse Shoulder Arthroplasty for Proximal Humerus Head-Split Fractures—A Retrospective Cohort Study. Journal of Clinical Medicine, 11(10), 2835. https://doi.org/10.3390/jcm11102835





