Flow Cytometry Analysis of Blood Large Extracellular Vesicles in Patients with Multiple Sclerosis Experiencing Relapse of the Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Antibodies and Solutions
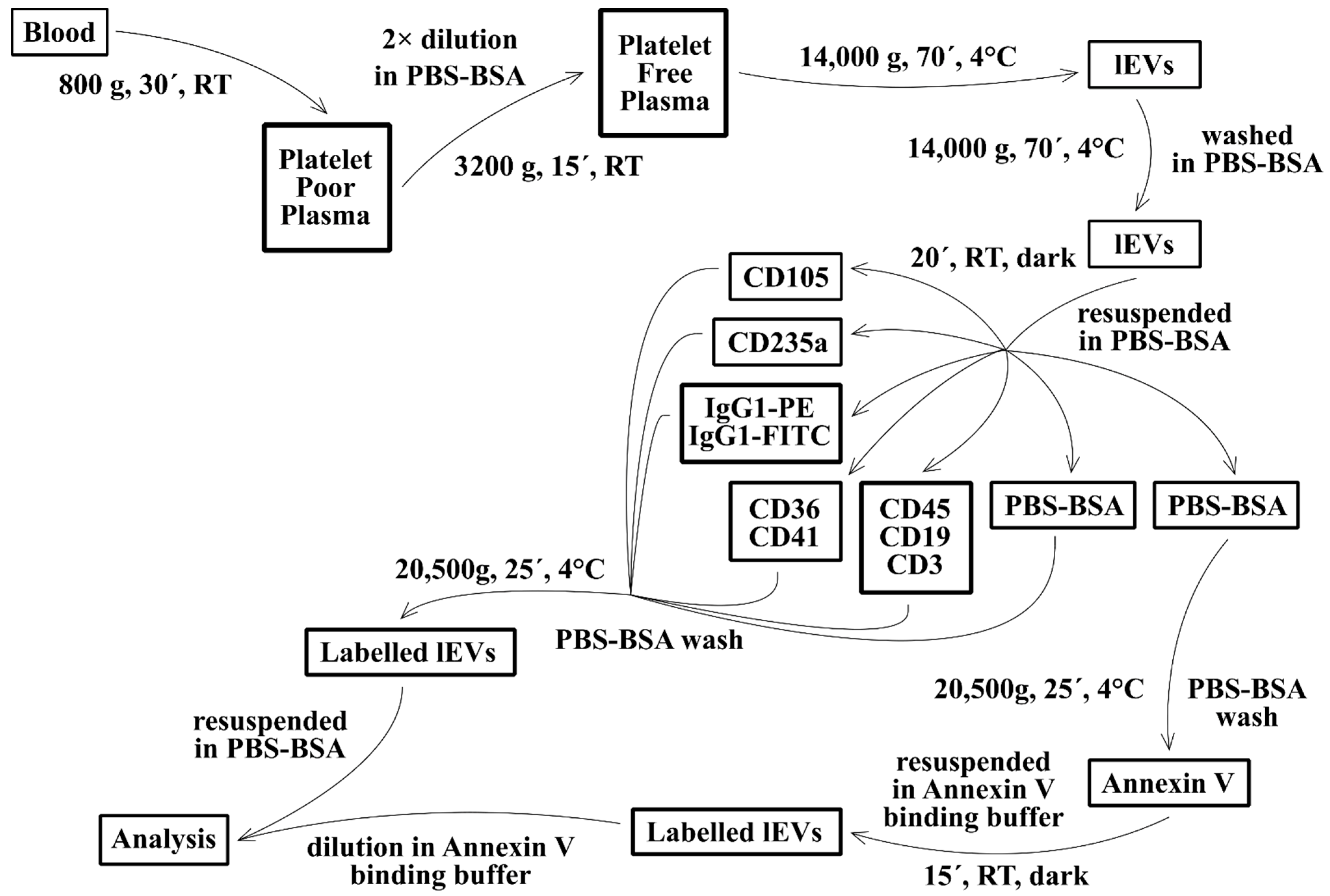
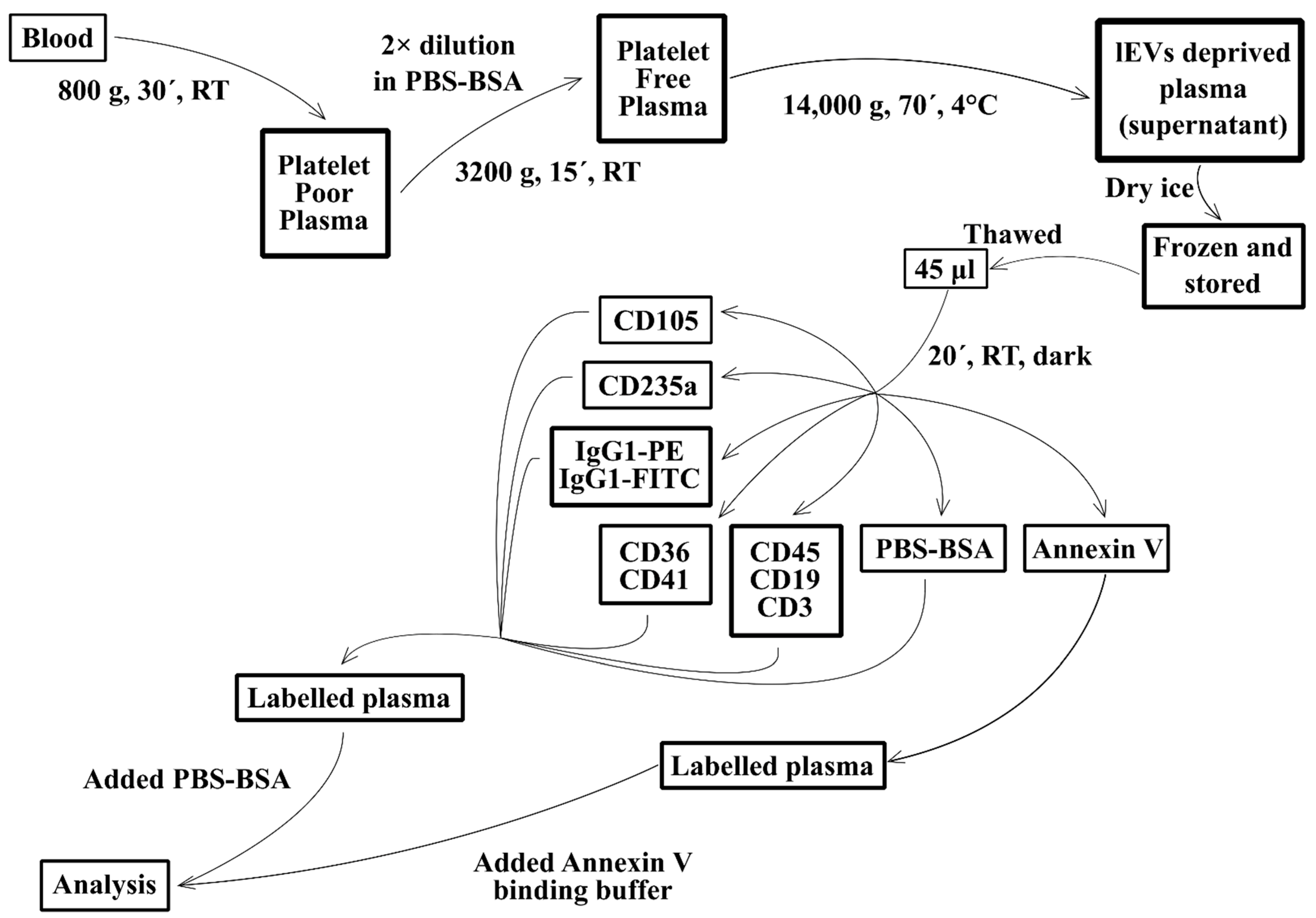
2.3. Isolation of Fresh lEVs
2.4. lEVs Labelling
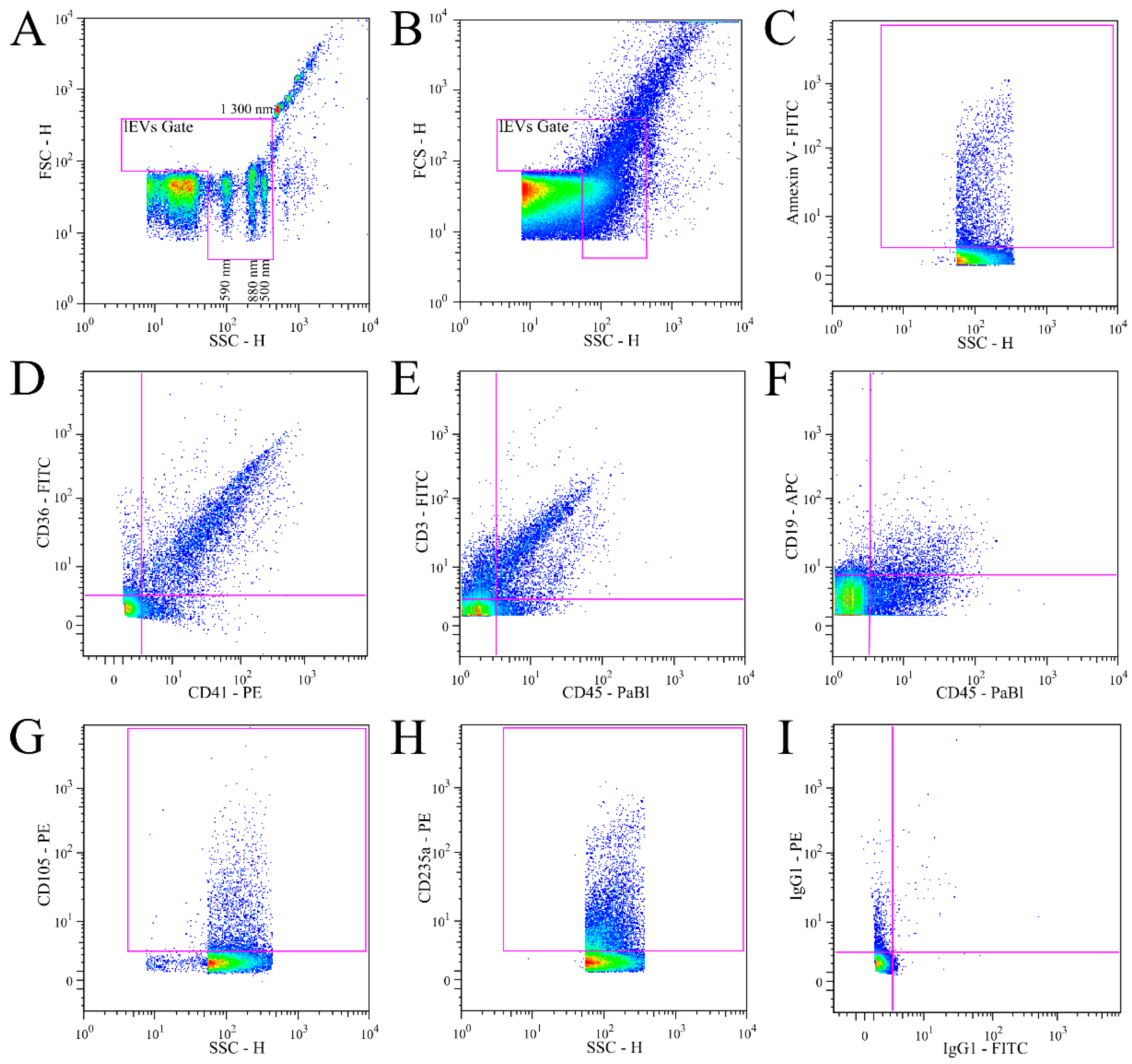
2.5. Flow Cytometry Analysis
2.6. FACS Light Scatter Calibration
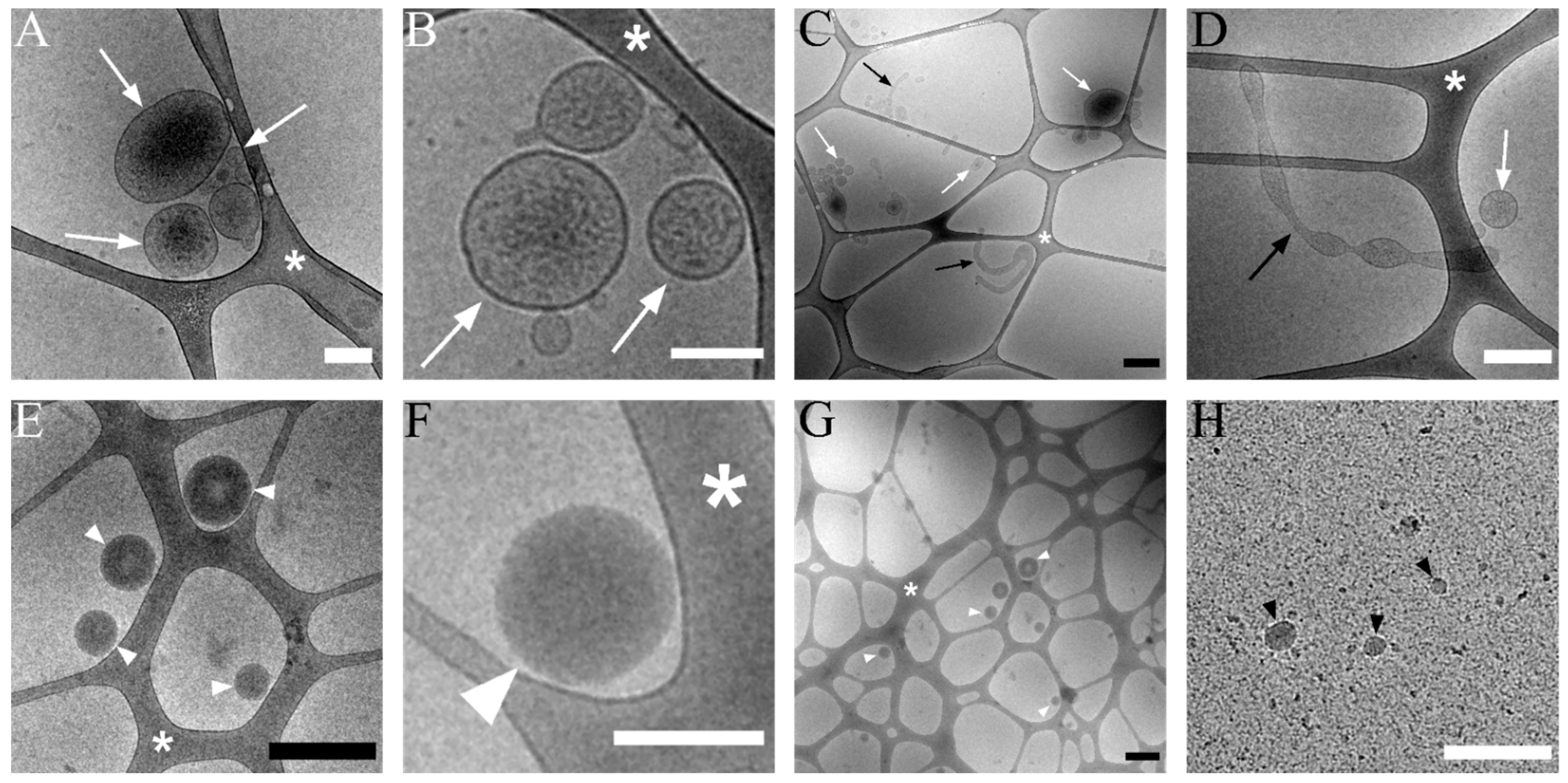
2.7. Transmission Cryo-Electron Microscopy (Cryo-TEM)
2.8. Data Evaluation and Statistics
3. Results
3.1. Validation of lEVs Flow Cytometry Analysis
3.2. Transmission Cryo-Electron Microscopy of Isolated lEVs
3.3. Analysis of Isolated Fresh Plasma lEVs of MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Saposnik, G.; Sempere, A.P.; Raptis, R.; Prefasi, D.; Selchen, D.; Maurino, J. Decision making under uncertainty, therapeutic inertia, and physicians’ risk preferences in the management of multiple sclerosis (DIScUTIR MS). BMC Neurol. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- Nylander, A.; Hafler, D.A. Multiple sclerosis. J. Clin. Investig. 2012, 122, 1180–1188. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Porro, C.; Trotta, T.; Panaro, M.A. Microvesicles in the brain: Biomarker, messenger or mediator? J. Neuroimmunol. 2015, 288, 70–78. [Google Scholar] [CrossRef]
- Carandini, T.; Colombo, F.; Finardi, A.; Casella, G.; Garzetti, L.; Verderio, C.; Furlan, R. Microvesicles: What is the role in multiple sclerosis? Front. Neurol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sáenz-Cuesta, M.; Osorio-Querejeta, I.; Otaegui, D. Extracellular Vesicles in Multiple Sclerosis: What are They Telling Us? Front. Cell. Neurosci. 2014, 8, 100. [Google Scholar] [CrossRef]
- Burnouf, T.; Chou, M.-L.; Goubran, H.; Cognasse, F.; Garraud, O.; Seghatchian, J. An overview of the role of microparticles/microvesicles in blood components: Are they clinically beneficial or harmful? Transfus. Apher. Sci. 2015, 53, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Ishikawa, A.; Kuroda, M. Roles of exosomes and microvesicles in disease pathogenesis. Adv. Drug Deliv. Rev. 2013, 65, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Julich, H.; Mims, A.; Lukacs-Kornek, V.; Kornek, M. Extracellular vesicle profiling and their use as potential disease specific biomarker. Front. Immunol. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Słomka, A.; Urban, S.K.; Lukacs-Kornek, V.; Żekanowska, E.; Kornek, M. Large Extracellular Vesicles: Have We Found the Holy Grail of Inflammation? Front. Immunol. 2018, 9, 2723. [Google Scholar] [CrossRef]
- Croese, T.; Furlan, R. Extracellular vesicles in neurodegenerative diseases. Mol. Asp. Med. 2018, 60, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Blonda, M.; Amoruso, A.; Martino, T.; Avolio, C. New Insights Into Immune Cell-Derived Extracellular Vesicles in Multiple Sclerosis. Front. Neurol. 2018, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Pieragostino, D.; Lanuti, P.; Cicalini, I.; Cufaro, M.C.; Ciccocioppo, F.; Ronci, M.; Simeone, P.; Onofrj, M.; van der Pol, E.; Fontana, A.; et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J. Proteom. 2019, 204, 10. [Google Scholar] [CrossRef]
- Minagar, A.; Jy, W.; Jimenez, J.J.; Sheremata, W.A.; Mauro, L.M.; Mao, W.W.; Horstman, L.L.; Ahn, Y.S. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 2001, 56, 1319–1324. [Google Scholar] [CrossRef]
- Sheremata, W.A.; Jy, W.; Delgado, S.; Minagar, A.; McLarty, J.; Ahn, Y. Interferon-β1a reduces plasma CD31+ endothelial microparticles (CD31+EMP) in multiple sclerosis. J. Neuroinflamm. 2006, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Lowery-Nordberg, M.; Eaton, E.; Gonzalez-Toledo, E.; Harris, M.K.; Chalamidas, K.; McGee-Brown, J.; Ganta, C.V.; Minagar, A.; Cousineau, D.; Alexander, J.S. The effects of high dose interferon-beta 1a on plasma microparticles: Correlation with MRI parameters. J. Neuroinflamm. 2011, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Ramiro, B.; Nacarino, P.O.; Serrano-Pertierra, E.; Blanco-Gelaz, M.A.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Tunon, A.; Lopez-Larrea, C.; Millan, J.; et al. Microparticles in multiple sclerosis and clinically isolated syndrome: Effect on endothelial barrier function. BMC Neurosci. 2014, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Sheremata, W.A.; Jy, W.; Horstman, L.L.; Ahn, Y.S.; Alexander, S.; Minagar, A. Evidence of platelet activation in multiple sclerosis. J. Neuroinflamm. 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenz-Cuesta, M.; Irizar, H.; Castillo-Trivino, T.; Munoz-Culla, M.; Osorio-Querejeta, I.; Prada, A.; Sepulveda, L.; Lopez-Mato, M.P.; de Munain, A.L.; Comabella, M.; et al. Circulating microparticles reflect treatment effects and clinical status in multiple sclerosis. Biomark. Med. 2014, 8, 653–661. [Google Scholar] [CrossRef]
- Alexander, J.S.; Chervenak, R.; Weinstock-Guainan, B.; Tsunoda, I.; Ramanathan, M.; Martinez, N.; Omura, S.; Sato, F.; Chaitanya, G.V.; Minagar, A.; et al. Blood circulating microparticle species in relapsing-remitting and secondary progressive multiple sclerosis. A case-control, cross sectional study with conventional MRI and advanced iron content imaging outcomes. J. Neurol. Sci. 2015, 355, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Trachtenberg, A.J.; Hochberg, F.H.; Skog, J.; Kuo, W.P. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front. Physiol. 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, J.A.; Horak, P.; Wilkinson, J.S.; Ford, V.J.; Jones, J.C.; Smith, D.; Holloway, J.A.; Englyst, N.A. FCMPASS Software Aids Extracellular Vesicle Light Scatter Standardization. Cytom. Part A 2020, 97, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Dubochet, J.; Adrian, M.; Chang, J.J.; Homo, J.C.; Lepault, J.; McDowall, A.W.; Schultz, P. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 1988, 21, 129–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Pol, E.; van Gemert, M.J.C.; Sturk, A.; Nieuwland, R.; Van Leeuwen, T.G. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J. Thromb. Haemost. 2012, 10, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Borgiani, B.; Verderio, C.; Furlan, R. Microvesicles: Novel biomarkers for neurological disorders. Front. Physiol. 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Properzi, F.; Logozzi, M.; Fais, S. Exosomes: The future of biomarkers in medicine. Biomark. Med. 2013, 7, 769–778. [Google Scholar] [CrossRef]
- Jy, W.; Minagar, A.; Jimenez, J.J.; Sheremata, W.A.; Mauro, L.M.; Horstman, L.L.; Bidot, C.; Ahn, Y.S. Endothelial microparticles (EMP) bind and activate monocytes: Elevated empmonocyte conjugates in multiple sclerosis. Front. Biosci. Landmark 2004, 9, 3137–3144. [Google Scholar] [CrossRef] [Green Version]
- Simak, J.; Holada, K.; Risitano, A.M.; Zivny, J.H.; Young, N.S.; Vostal, J.G. Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2004, 125, 804–813. [Google Scholar] [CrossRef]
- Ojeda-Fernandez, L.; Recio-Poveda, L.; Aristorena, M.; Lastres, P.; Blanco, F.J.; Sanz-Rodriguez, F.; Gallardo-Vara, E.; de las Casas-Engel, M.; Corbi, A.; Arthur, H.M.; et al. Mice Lacking Endoglin in Macrophages Show an Impaired Immune Response. PLoS Genet. 2016, 12, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, E.; Pericacho, M.; Bachelot-Loza, C.; Pidard, D.; Gaussem, P.; Poirault-Chassac, S.; Blanco, F.J.; Langa, C.; Gonzalez-Manchon, C.; Novoa, J.M.L.; et al. Human endoglin as a potential new partner involved in platelet-endothelium interactions. Cell. Mol. Life Sci. 2018, 75, 1269–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suades, R.; Padro, T.; Vilahur, G.; Martin-Yuste, V.; Sabate, M.; Sans-Rosello, J.; Sionis, A.; Badimon, L. Growing thrombi release increased levels of CD235a(+) microparticles and decreased levels of activated platelet-derived microparticles. Validation in ST-elevation myocardial infarction patients. J. Thromb. Haemost. 2015, 13, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A.; Spanbroek, R.; Lottaz, C.; Gautier, E.L.; Frankenberger, M.; Hoffmann, R.; Lang, R.; Haniffa, M.; Collin, M.; Tacke, F.; et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010, 115, E10–E19. [Google Scholar] [CrossRef]
- Swerlick, R.A.; Lee, K.H.; Wick, T.M.; Lawley, T.J. Human Dermal Microvascular Endothelial but not Human Umbilical Vein Endothelial-Cells Express Cd36 in vivo and in vitro. J. Immunol. 1992, 148, 78–83. [Google Scholar]
- Mitjavila-Garcia, M.T.; Cailleret, M.; Godin, I.; Nogueira, M.M.; Cohen-Solal, K.; Schiavon, V.; Lecluse, Y.; Le Pesteur, F.; Lagrue, A.N.; Vainchenker, W. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development 2002, 129, 2003–2013. [Google Scholar] [CrossRef]
- Rheinlander, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunol. Lett. 2018, 196, 22–32. [Google Scholar] [CrossRef]
- Wang, K.M.; Wei, G.Q.; Liu, D.L. CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Thematic Review Series: Exosomes and Microvesicles: Lipids as Key Components of their Biogenesis and Functions Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodar, B.W.; Kittel, A.; Paloczi, K.; Vukman, K.V.; Osteikoetxea, X.; Szabo-Taylor, K.; Nemeth, A.; Sperlagh, B.; Baranyai, T.; Giricz, Z.; et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci. Rep. 2016, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, E.S.; Maranhao, R.C. Rapid, simple laser-light-scattering method for HDL particle sizing in whole plasma. Clin. Chem. 2004, 50, 1086–1088. [Google Scholar] [CrossRef]
- Jamaly, S.; Ramberg, C.; Olsen, R.; Latysheva, N.; Webster, P.; Sovershaev, T.; Braekkan, S.K.; Hansen, J.B. Impact of preanalytical conditions on plasma concentration and size distribution of extracellular vesicles using Nanoparticle Tracking Analysis. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, M.; Miller, V.M.; Heit, J.A.; Owen, W.G. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J. Immunol. Methods 2012, 375, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.C.; Zhang, L.M.; Wang, H.X.; Yuan, G.L.; Guo, A.Y.; Li, Q.B.; Chen, Z.C. Impact of collection, isolation and storage methodology of circulating microvesicles on flow cytometric analysis. Exp. Ther. Med. 2015, 10, 2093–2101. [Google Scholar] [CrossRef]
- Hujacova, A.; Brozova, T.; Mosko, T.; Kostelanska, M.; Stranak, Z.; Holada, K. Platelet Extracellular Vesicles in Cord Blood of Term and Preterm Newborns Assayed by Flow Cytometry: The Effect of Delay in Sample Preparation and of Sample Freezing. Folia Biol. 2020, 66, 204–211. [Google Scholar]
- De Rond, L.; Libregts, S.; Rikkert, L.G.; Hau, C.M.; van der Pol, E.; Nieuwland, R.; van Leeuwen, T.G.; Coumans, F.A.W. Refractive index to evaluate staining specificity of extracellular vesicles by flow cytometry. J. Extracell. Vesicles 2019, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Erdbrugger, U.; Rudy, C.K.; Etter, M.E.; Dryden, K.A.; Yeager, M.; Klibanov, A.L.; Lannigan, J. Imaging Flow Cytometry Elucidates Limitations of Microparticle Analysis by Conventional Flow Cytometry. Cytom. Part A 2014, 85, 756–770. [Google Scholar] [CrossRef]
- Lucchetti, D.; Battaglia, A.; Ricciardi-Tenore, C.; Colella, F.; Perelli, L.; de Maria, R.; Scambia, G.; Sgambato, A.; Fattorossi, A. Measuring Extracellular Vesicles by Conventional Flow Cytometry: Dream or Reality? Int. J. Mol. Sci. 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Holcar, M.; Kanduser, M.; Lenassi, M. Blood Nanoparticles—Influence on Extracellular Vesicle Isolation and Characterization. Front. Pharmacol. 2021, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Chandler, W.L. Measurement of Microvesicle Levels in Human Blood Using Flow Cytometry. Cytom. Part B Clin. Cytom. 2016, 90, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.N.; Koster, A.J.; Bertina, R.M.; Osanto, S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell. Vesicles 2013, 2, 7. [Google Scholar] [CrossRef]
- Masvekar, R.; Mizrahi, J.; Park, J.; Williamson, P.R.; Bielekova, B. Quantifications of CSF Apoptotic Bodies Do not Provide Clinical Value in Multiple Sclerosis. Front. Neurol. 2019, 10, 11. [Google Scholar] [CrossRef]
- de Rond, L.; van der Pol, E.; Bloemen, P.R.; Van Den Broeck, T.; Monheim, L.; Nieuwland, R.; van Leeuwen, T.G.; Coumans, F.A.W. A Systematic Approach to Improve Scatter Sensitivity of a Flow Cytometer for Detection of Extracellular Vesicles. Cytom. Part A 2020, 97, 582–591. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 43. [Google Scholar] [CrossRef] [Green Version]
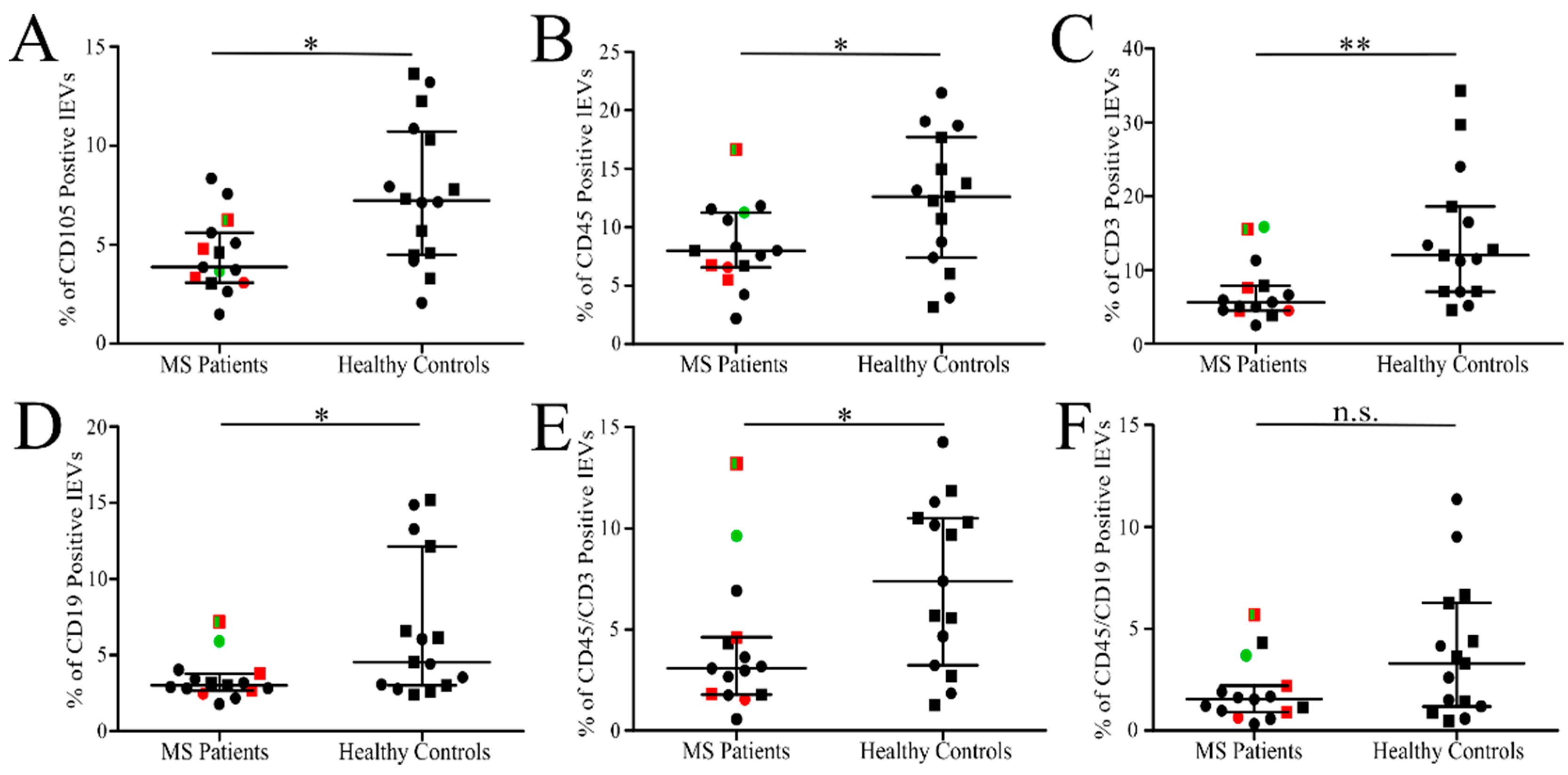
| Analysis of Fresh lEVs Isolated from Blood Plasma of MS Patients and Healthy Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Count of Positive lEVs/µL of Plasma | % of Positive lEVs | |||||||||
| Labelling | Patient | 95% CI | Control | 95% CI | p < 0.05 | Patient | 95% CI | Control | 95% CI | p < 0.05 |
| All events | 9783 | 4884–14,683 | 7659 | 5554–9764 | no | --- | --- | no | ||
| Events in lEVs gate | 1620 | 576–2663 | 1116 | 789–1443 | no | 15.1 | 12.9–17.4 | 15.0 | 12.6–17.4 | no |
| Annexin V | 212 | 115–308 | 203 | 132–275 | no | 17.0 | 12.4–21.5 | 20.9 | 17.5–24.2 | no |
| CD105 | 54 | 34–74 | 72 | 41–103 | no | 4.5 | 3.4–5.5 | 7.6 | 5.7–9.5 | yes |
| CD235a | 250 | 178–323 | 244 | 160–328 | no | 24.6 | 19.2–30.1 | 26.4 | 19.8–33.1 | no |
| CD36 | 226 | 178–274 | 236 | 168–304 | no | 19.3 | 14.2–24.4 | 25.1 | 19.0–31.3 | no |
| CD41 | 171 | 132–211 | 180 | 124–235 | no | 14.3 | 10.3–18.2 | 18.6 | 14.7–22.5 | no |
| CD36 + CD41 | 131 | 101–160 | 146 | 98–193 | no | 11.4 | 7.8–14.9 | 15.4 | 11.6–19.1 | no |
| CD45 * | 108 | 75–140 | 126 | 93–159 | no | 8.4 | 6.4–10.4 | 12.3 | 9.1–15.4 | yes |
| CD19 * | 50 | 27–74 | 81 | 36–126 | no | 3.4 | 2.6–4.2 | 6.7 | 4.1–9.3 | yes |
| CD3 * | 95 | 57–133 | 166 | 87–245 | no | 7.1 | 4.9–9.3 | 14.3 | 9.4–19.3 | yes |
| CD45 + CD19 * | 23 | 13–34 | 45 | 16–74 | no | 1.9 | 1.1–2.7 | 3.9 | 2.0–5.7 | no |
| CD45 + CD3 * | 49 | 28–70 | 79 | 46–112 | no | 4.1 | 2.2–6.0 | 7.4 | 5.1–9.6 | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soukup, J.; Kostelanská, M.; Kereïche, S.; Hujacová, A.; Pavelcová, M.; Petrák, J.; Kubala Havrdová, E.; Holada, K. Flow Cytometry Analysis of Blood Large Extracellular Vesicles in Patients with Multiple Sclerosis Experiencing Relapse of the Disease. J. Clin. Med. 2022, 11, 2832. https://doi.org/10.3390/jcm11102832
Soukup J, Kostelanská M, Kereïche S, Hujacová A, Pavelcová M, Petrák J, Kubala Havrdová E, Holada K. Flow Cytometry Analysis of Blood Large Extracellular Vesicles in Patients with Multiple Sclerosis Experiencing Relapse of the Disease. Journal of Clinical Medicine. 2022; 11(10):2832. https://doi.org/10.3390/jcm11102832
Chicago/Turabian StyleSoukup, Jakub, Marie Kostelanská, Sami Kereïche, Andrea Hujacová, Miluše Pavelcová, Jiří Petrák, Eva Kubala Havrdová, and Karel Holada. 2022. "Flow Cytometry Analysis of Blood Large Extracellular Vesicles in Patients with Multiple Sclerosis Experiencing Relapse of the Disease" Journal of Clinical Medicine 11, no. 10: 2832. https://doi.org/10.3390/jcm11102832
APA StyleSoukup, J., Kostelanská, M., Kereïche, S., Hujacová, A., Pavelcová, M., Petrák, J., Kubala Havrdová, E., & Holada, K. (2022). Flow Cytometry Analysis of Blood Large Extracellular Vesicles in Patients with Multiple Sclerosis Experiencing Relapse of the Disease. Journal of Clinical Medicine, 11(10), 2832. https://doi.org/10.3390/jcm11102832






