Update of PSMA Theranostics in Prostate Cancer: Current Applications and Future Trends
Abstract
:1. Introduction
2. Methods
3. PSMA-Based Imaging
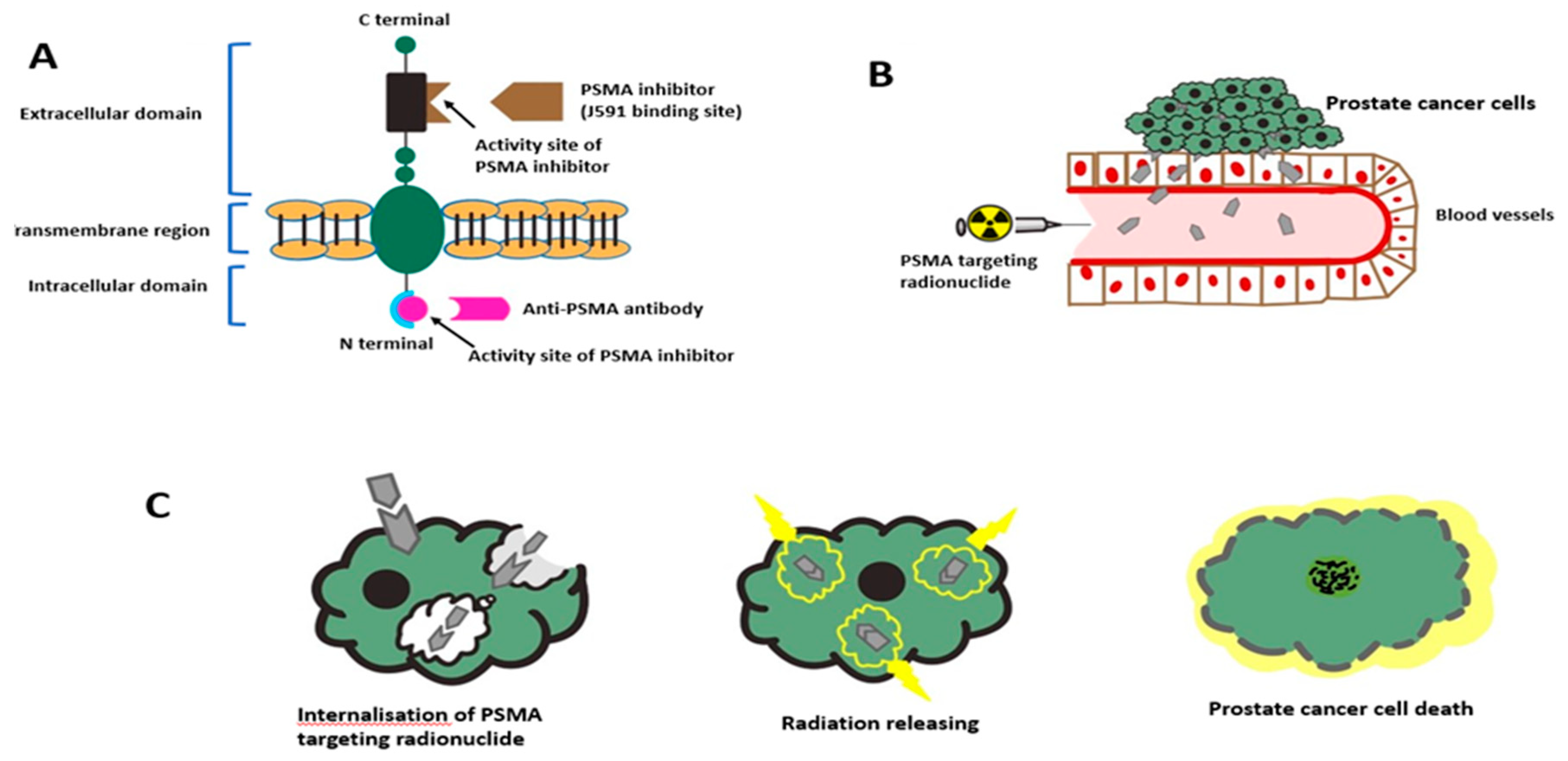
3.1. Anti-PSMA Antibodies
3.2. PSMA Ligands for PET Imaging
3.2.1. Gallium-68 (68Ga)-Labeled PSMA Radiopharmaceuticals
3.2.2. Fluorine (18F)-Labeled PSMA Radiopharmaceuticals
4. Role of PSMA Imaging in PCa
4.1. PSMA Imaging for Initial Staging
4.1.1. T-Staging
4.1.2. N-Staging
4.1.3. M-Staging
4.2. Evaluation of Biochemically Recurrent Disease (BCR)
4.3. Clinical Interpretation and Common Pitfalls in PSMA-Targeted Imaging
4.3.1. Bone Uptake
- (a)
- Benign bone diseases
- (b)
- Nonspecific bone uptake
4.3.2. Uptake in Lymph Nodes
4.3.3. Breast Uptake
5. PSMA-Targeted Radionuclide Therapy
5.1. PSMA-Targeted Radioligand Therapy
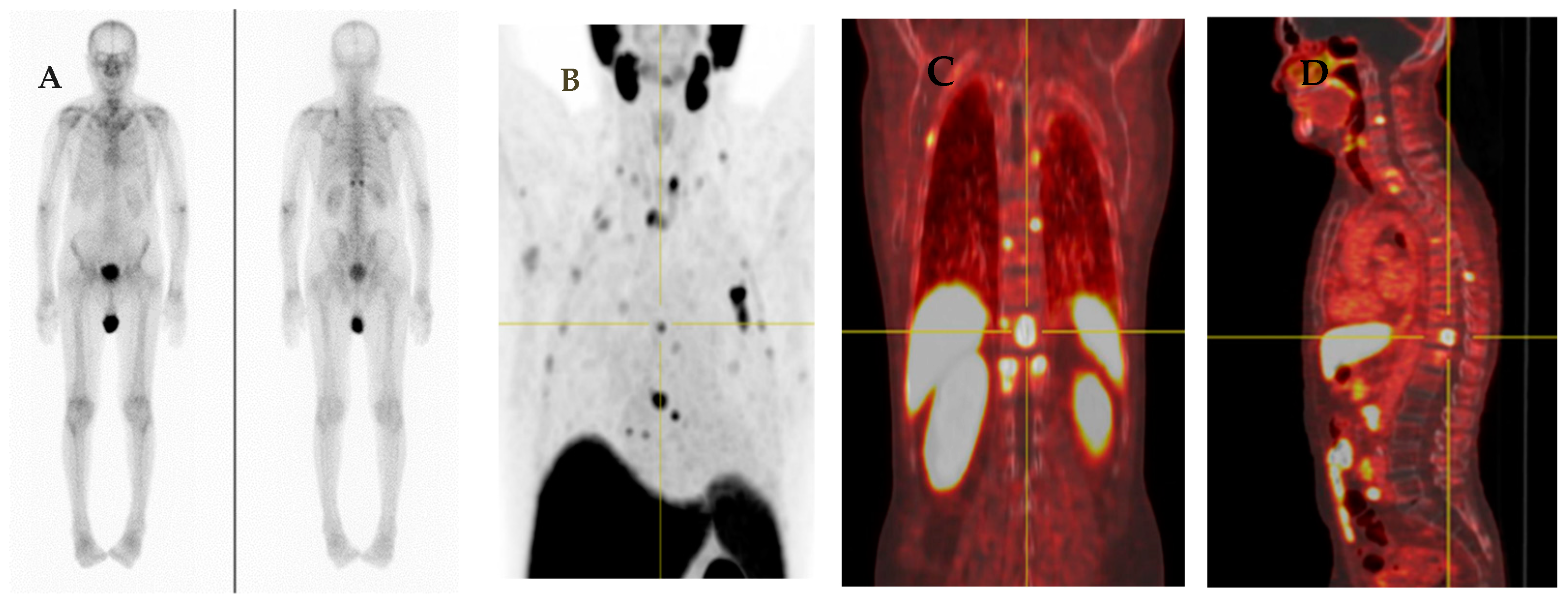
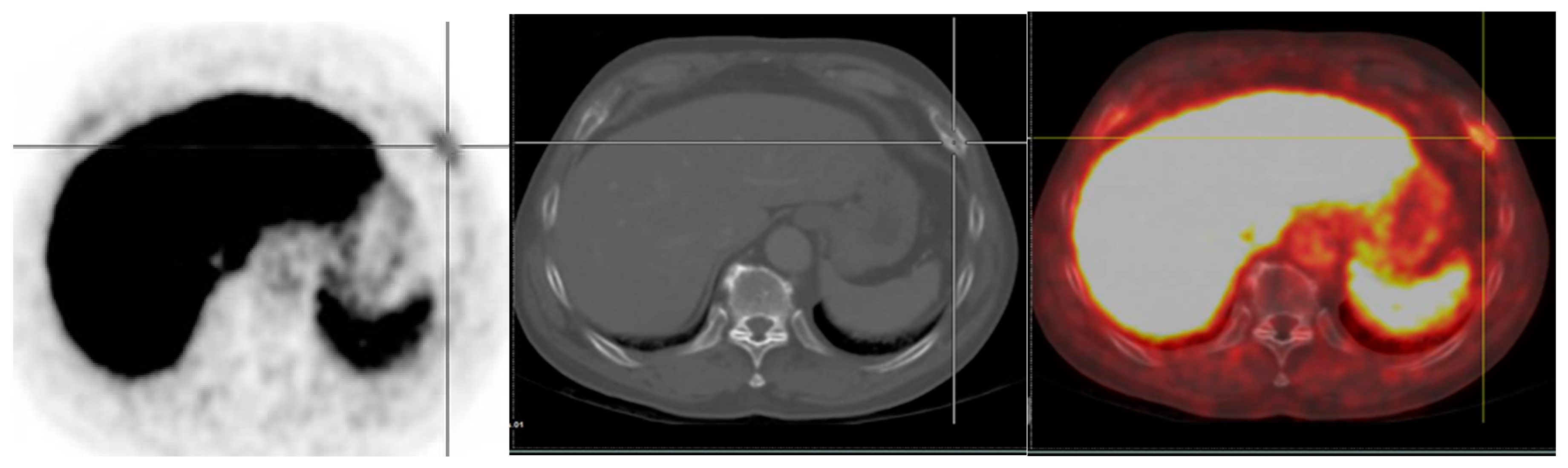
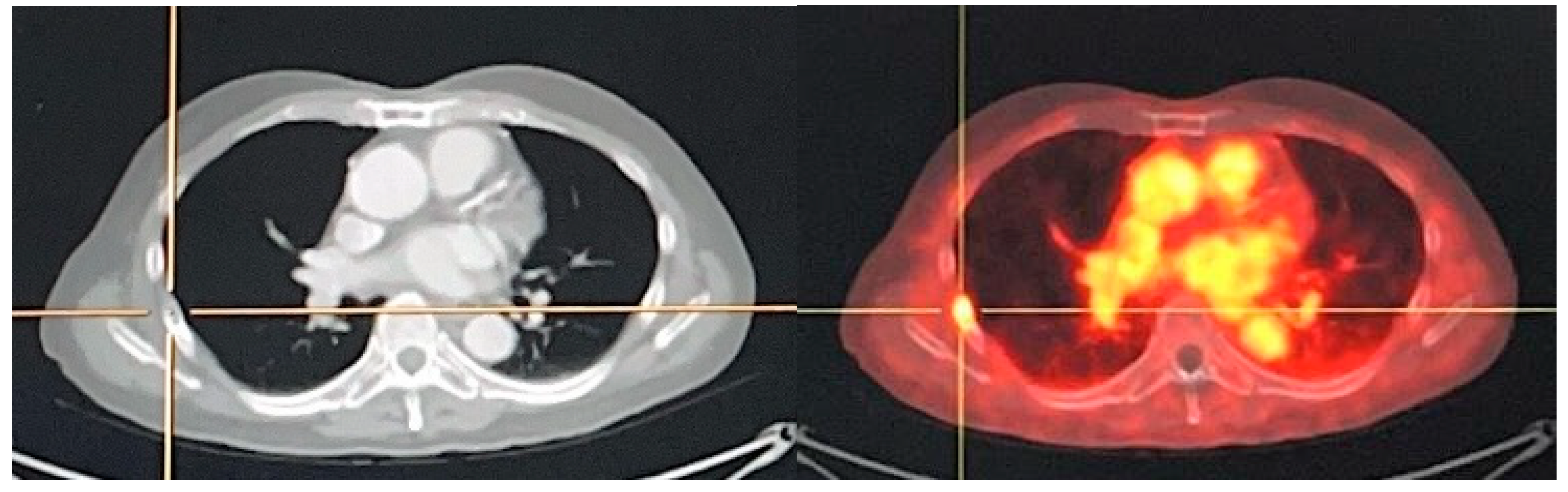
5.1.1. 177Lu-PSMA Radioligand Therapy
- (a)
- 177Lu-PSMA-617
- (b)
- 177Lu-PSMA-I&T
- (c)
- Combination of 177Lu-PSMA 617 with androgen receptor-axis-targeted therapies (ARAT)
- (d)
- Combination of 177Lu PSMA-617 with DNA damage repair inhibitor
- (e)
- Combination of 177Lu PSMA-617 with checkpoint inhibitor immunotherapy
5.1.2. Alpha-Emitting PSMA-Targeted Radioligand Therapy
- (a)
- 225Ac-PSMA-617
- (b)
- 213Bi-labeled PSMA-617
5.2. Anti-PSMA Radioimmunotherapy
5.2.1. 177Lu-J591 Antibody
5.2.2. 225Ac-J591 Antibody
5.2.3. 227TH-PSMA-TTC Antibody
6. Economic Benefits and Cost-Effectiveness
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Survival Rates for Prostate Cancer. Available online: https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 19 September 2021).
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Eklund, M.; Jäderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Grönberg, H.; Nordström, T. MRI-Targeted or Standard Biopsy in Prostate Cancer Screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, M.; Chen, S.; Lei, X.; Zhang, X.; Huan, Y. A meta-analysis of use of Prostate Imaging Reporting and Data System Version 2 (PI-RADS V2) with multiparametric MR imaging for the detection of prostate cancer. Eur. Radiol. 2017, 27, 5204–5214. [Google Scholar] [CrossRef]
- Drost, F.J.H.; Osses, D.F.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Roobol, M.J.; Schoots, I.G. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. 2019, 4, CD012663. [Google Scholar] [CrossRef]
- Hövels, A.; Heesakkers, R.A.M.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Suh, C.H.; Shinagare, A.B.; Westenfield, A.M.; Ramaiya, N.H.; Van den Abbeele, A.D.; Kim, K.W. Yield of bone scintigraphy for the detection of metastatic disease in treatment-naive prostate cancer: A systematic review and meta-analysis. Clin. Radiol. 2018, 73, 158–167. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Santis, M.D.; Gillessen, S.; Henry, A.M.; van der Kwast, T.H.; Lam, T.B.; Mason, M.D.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer 2022. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP_SIOG-Guidelines-on-Prostate-Cancer-2022_2022-04-25-063938_yfos.pdf (accessed on 1 May 2022).
- Zhang, H.; Xin, P.; Zhang, Y. Efficacy and safety of the 177Lu-PSMA-617 therapy in the treatment of metastatic castration-resistant prostate cancer: A meta-analysis. Zhonghua Nan Ke Xue 2021, 27, 63–69. [Google Scholar]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Sahoo, R.K.; Tripathi, M.; Dwivedi, S.N.; Bal, C. 225Ac-PSMA-617-targeted alpha therapy for the treatment of metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. Prostate 2021, 81, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, M.S.; Sheikhbahaei, S.; Werner, R.A.; Pienta, K.J.; Pomper, M.G.; Solnes, L.B.; Gorin, M.A.; Wang, N.-Y.; Rowe, S.P. A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177-labeled Prostate-specific Membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2021, 80, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Sood, A.; Das, C.K.; Mittal, B.R. Evolving role of 225 Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer-a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021, 24, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.; Emmett, L.; Sandhu, S.; Irvani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. 177Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Bauman, G.; von Eyben, R.; Rahbar, K.; Soydal, C.; Haug, A.R.; Virgolini, I.; Kulkarni, H.; Baum, R.; Paganelli, G. Optimizing PSMA Radioligand Therapy for Patients with Metastatic Castration-Resistant Prostate Cancer. A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 9054. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R.; Sood, A. Visceral Metastases as Predictors of Response and Survival Outcomes in Patients of Castration-Resistant Prostate Cancer Treated With 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2020, 45, 935–942. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y. Therapeutic Responses and Survival Effects of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer: A Meta-analysis. Clin. Nucl. Med. 2018, 43, 728–734. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand Therapy with 177Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. AJR 2019, 213, 275–285. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Roviello, G.; Kiljunen, T.; Uprimny, C.; Virgolini, I.; Kairemo, K.; Joensuu, T. Third-line treatment and 177 Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 496–508. [Google Scholar] [CrossRef] [Green Version]
- Calopedos, R.J.S.; Chalasani, V.; Asher, R.; Emmett, L.; Woo, H.H. Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, D.S.; Su, S.L.; Bacich, D.J.; Horiguchi, Y.; Luo, Y.; Powell, C.; Zandvliet, D.; Russell, P.; Molloy, P.; Nowak, N.J.; et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim. Biophys. Acta 1998, 1443, 113–127. [Google Scholar] [CrossRef]
- Israeli, R.S.; Powell, C.T.; Corr, J.G.; Fair, W.R.; Heston, W.D. Expression of the prostate-specific membrane antigen. Cancer Res. 1994, 54, 1807–1811. [Google Scholar] [PubMed]
- Murphy, G.P.; Greene, T.G.; Tino, W.T.; Boynton, A.L.; Holmes, E.H. Isolation and characterization of monoclonal antibodies specific for the extracellular domain of prostate specific membrane antigen. J. Urol. 1998, 160, 2396–2401. [Google Scholar] [CrossRef]
- Tasch, J.; Gong, M.; Sadelain, M.; Heston, W.D. A unique folate hydrolase, prostate-specific membrane antigen (PSMA): A target for immunotherapy? Crit. Rev. Immunol. 2001, 21, 249–261. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Murphy, G.P.; Elgamal, A.A.; Su, S.L.; Bostwick, D.G.; Holmes, E.H. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer 1998, 83, 2259–2269. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Grauer, L.S.; Lawler, K.D.; Marignac, J.L.; Kumar, A.; Goel, A.S.; Wolfert, R.L. Identification, purification, and subcellular localization of prostate-specific membrane antigen PSMʹ protein in the LNCaP prostatic carcinoma cell line. Cancer Res. 1998, 58, 4787–4789. [Google Scholar]
- Heston, W.D. Characterization and glutamyl preferring carboxypeptidase function of prostate specific membrane antigen: A novel folate hydrolase. Urology 1997, 49, 104–112. [Google Scholar] [CrossRef]
- Huang, E.; Teh, B.S.; Mody, D.R.; Carpenter, L.S.; Butler, E.B. Prostate adenocarcinoma presenting with inguinal lymphadenopathy. Urology 2003, 61, 463. [Google Scholar] [CrossRef]
- Wu, L.M.; Xu, J.R.; Ye, Y.Q.; Lu, Q.; Hu, J.N. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: A systematic review and meta-analysis. AJR Am. J. Roentgenol. 2012, 199, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birtle, A.J.; Freeman, A.; Masters, J.R.W.; Payne, H.A.; Harland, S.H.; BAUS Section of Oncology Cancer Registry. Tumour markers for managing men who present with metastatic prostate cancer and serum prostate-specific antigen levels of < 10 ng/mL. BJU Int. 2005, 96, 303–307. [Google Scholar] [CrossRef]
- Evans, M.J.; Smith-Jones, P.M.; Wongvipat, J.; Navarro, V.; Kim, S.; Bander, N.H.; Larson, S.M.; Sawyers, C.L. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl. Acad. Sci. USA 2011, 108, 9578–9582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Möller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef]
- Ross, J.S.; Sheehan, C.E.; Fisher, H.A.; Kaufman, R.P., Jr.; Kaur, P.; Gray, K.; Webb, I.; Gray, G.S.; Mosher, R.; Kallakury, B.V.S. Correlation of primary tumour prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 6357–6362. [Google Scholar] [PubMed]
- Kiess, A.P.; Banerjee, S.R.; Mease, R.C.; Rowe, S.P.; Rao, A.; Foss, C.A.; Chen, Y.; Yang, X.; Cho, S.Y.; Nimmagadda, S.; et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 241–268. [Google Scholar]
- Rosenthal, S.A.; Haseman, M.K.; Polascik, T.J. Utility of capromab-pendetide (ProstaScint) imaging in the management of prostate cancer. Tech. Urol. 2001, 7, 27–37. [Google Scholar]
- Wilkinson, S.; Chodak, G. The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. J. Urol. 2004, 172, 133–136. [Google Scholar] [CrossRef]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Rischpler, C.; Beck, T.I.; Okamoto, S.; Schlitter, A.M.; Knorr, K.; Schwaiger, M.; Gschwend, J.; Maurer, T.; Meyer, P.T.; Eiber, E.M. 68Ga-PSMA-HBED-CC Uptake in Cervical, Celiac, and Sacral Ganglia as an Important Pitfall in Prostate Cancer PET Imaging. J. Nucl. Med. 2018, 59, 1406–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a 68Ga-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C.; et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.-B.; Kübler, H.; Haberhorn, U.; Eisenhut, M.; et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Matthias Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective comparison of 18F-Fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. 68Ga-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef]
- Dewes, S.; Schiller, K.; Sauter, K.; Eiber, M.; Maurer, T.; Schwaiger, M.; Gschwend, J.E.; Combs, S.E.; Habl, G. Integration of 68Ga-PSMA-PET imaging in planning of primary definitive radiotherapy in prostate cancer: A retrospective study. Radiat. Oncol. 2016, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.-J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef]
- Ling, S.W.; de Jong, A.C.; Schoots, I.G.; Nasserinejad, K.; Busstra, M.B.; van der Veldt, A.A.M.; Brabander, T. Comparison of 68Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography for Primary Staging of Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2021, 33, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; Wieler, H.J.; Baues, C.; Kuntz, N.J.; Richardsen, I.; Schreckenberger, M. The Impact of 68Ga-PSMA PET/CT and PET/MRI on the Management of Prostate Cancer. Urology 2019, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mease, R.C.; Dusich, C.L.; Foss, C.A.; Ravert, H.T.; Dannals, R.F.; Seidelet, J.; Prideaux, A.; Fox, J.J.; Sgouros, G.; Kozikowski, A.P.; et al. N-[N-[(S)-1,3-Dicarboxypropyl] carbamoyl]-4-18F-fluorobenzyl-l-cysteine, 18F-DCFBC: A new imaging probe for prostate cancer. Clin. Cancer Res. 2008, 14, 3036–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.Y.; Gage, K.L.; Mease, R.C.; Senthamizhchelvan, S.; Holt, D.P.; Jeffrey-Kwanisai, A.; Endres, C.J.; Dannals, R.F.; Sgouros, S.G.; Lodge, M.; et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J. Nucl. Med. 2012, 53, 1883–1891. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pullambhatla, M.; Foss, C.A.; Byun, Y.; Nimmagadda, S.; Senthamizhchelvan, S.; Sgouros, G.; Mease, R.C.; Pomper, M.G. 2-(3-{1-Carboxy-5-[(6-[18F] fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyl, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011, 17, 7645–7653. [Google Scholar] [CrossRef] [Green Version]
- Dietlein, M.; Kobe, C.; Kuhnert, G.; Stockter, S.; Fischer, T.; Schomäcker, K.; Schmidt, M.; Dietlein, F.; Zlatopolskiy, B.D.; Krapf, P.; et al. Comparison of 18F-DCFPyL and 68Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol. Imaging Biol. 2015, 17, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [Green Version]
- FDA Approves 68Gallium PSMA-11 for PSMA-Targeted PET Imaging in Prostate Cancer. Available online: https://ascopost.com/issues/december-10-2020/fda-approves-gallium-68-psma-11-for-psma-targeted-pet-imaging-in-prostate-cancer/?bc_md5=7b5c61365d6220a9cca0c0ba65753923&utm_source=TAP-EN-120120&utm_medium=email (accessed on 19 September 2021).
- FDA Approves Second PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 19 September 2021).
- Mokoala, K.; Lawal, I.; Lengana, T.; Kgatle, M.; Giesel, F.; Vorster, M.; Sathekge, M. PSMA Theranostics: Science and Practice. Cancers 2021, 13, 3904. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent—Update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef]
- Bratan, F.; Niaf, E.; Melodelima, C.; Chesnais, A.L.; Souchon, R.; Mège-Lechevallier, F.; Colombel, M.; Rouvière, O. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: A prospective study. Eur. Radiol. 2013, 23, 2019–2029. [Google Scholar] [CrossRef]
- Heesakkers, R.; Hövels, A.M.; Jager, G.J.; Bosch, H.; Witjes, J.A.; Raat, H.; Severens, J.L.; Adang, E.M.M.; van der Kaa, C.H.; Fütterer, J.J.; et al. MRI with a lymph-node specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: A prospective multicohort study. Lancet Oncol. 2008, 9, 850–856. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga–Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, T.; Wang, X.; Yu, Y.B.; Fan, Z.Y.; Li, D.X.; Luo, L.; Yang, X.C.; Jiao, W.; Niu, H.T. Diagnostic Performance of 68Ga Labelled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging for Staging the Prostate Cancer with Intermediate or High Risk Prior to Radical Prostatectomy: A Systematic Review and Meta-analysis. World J. Mens. Health 2020, 38, 208–219. [Google Scholar] [PubMed]
- Gorin, M.A.; Rowe, S.P.; Patel, H.D.; Vidal, I.; Ay, M.M.; Javadi, M.S.; Solnes, L.B.; Ross, A.E.; Schaeffer, E.M.; Bivalacqua, T.J.; et al. Prostate Specific Membrane Antigen Targeted 18F-DCFPyL Positron Emission Tomography/Computerized Tomography for the Preoperative Staging of High Risk Prostate Cancer: Results of a Prospective, Phase II, Single Center Study. J. Urol. 2018, 199, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.J.; Nielsen, J.B.; Langkilde, N.C.; Petersen, A.; Afshar-Oromieh, A.; De Souza, N.M.; De Paepe, K.; Fisker, R.V.; Arp, D.T.; Carl, J.; et al. 68Ga-PSMA PET/CT compared with MRI/CT and diffusion-weighted MRI for primary lymph node staging prior to definitive radiotherapy in prostate cancer: A prospective diagnostic test accuracy study. World J. Urol. 2020, 38, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET/CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Chakraborty, P.S.; Kumar, R.; Tripathi, M.; Das, C.J.; Bal, C. Detection of brain metastasis with 68Ga-labeled PSMA ligand PET/CT: A novel radiotracer for imaging of prostate carcinoma. Clin. Nucl. Med. 2015, 40, 328–329. [Google Scholar] [CrossRef]
- Kabasakal, L.; Demirci, E.; Ocak, M.; Akyel, R.; Nematyazar, J.; Aygun, A.; Halac, M.; Talat, Z.; Araman, A. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl. Med. Commun. 2015, 36, 582–587. [Google Scholar] [CrossRef]
- Shen, G.; Deng, H.; Hu, S.; Jia, Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A meta-analysis. Skelet. Radiol. 2014, 43, 1503–1513. [Google Scholar] [CrossRef]
- Pyka, T.; Okamoto, S.; Dahlbender, M.; Tauber, R.; Retz, M.; Heck, M.; Tamaki, N.; Schwaiger, M.; Maurer, T.; Eiber, M. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2114–2121. [Google Scholar] [CrossRef]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Death in patients with recurrent prostate cancer after radical prostatectomy: Prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J. Clin. Oncol. 2007, 25, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Toussi, A.; Stewart-Merrill, S.B.; Boorjian, S.A.; Psutka, S.P.; Thompson, R.H.; Frank, I.; Tollefson, M.K.; Gettman, M.T.; Carlson, R.E.; Rangel, L.J.; et al. Standardizing the Definition of Biochemical Recurrence after Radical Prostatectomy-What Prostate Specific Antigen Cut Point Best Predicts a Durable Increase and Subsequent Systemic Progression? J. Urol. 2016, 195, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, T.; van den Bergh, R.C.; Briers, E.; Cornford, P.; Cumberbatch, M.; Tilki, D.; De Santis, M.; Fanti, S.; Fossati, N.; Gillessen, S.; et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur. Urol. Focus 2020, 6, 231–234. [Google Scholar] [CrossRef]
- Calais, J.; Fendler, W.P.; Eiber, M.; Gartmann, J.; Chu, F.I.; Nickols, N.G.; Reiter, R.E.; Rettig, M.B.; Marks, L.S.; Ahlering, T.E.; et al. Impact of 68Ga-PSMA-11 PET/CT on the management of prostate cancer patients with biochemical recurrence. J. Nucl. Med. 2018, 59, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.J.; Carroll, P.R.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.; Pantel, A.R.; Cho, S.Y.; Gage, K.; Piert, M.; et al. Impact of PSMA-targeted imaging with 18F-DCFPyL-PET/CT on clinical management of patients (pts) with biochemically recurrent (BCR) prostate cancer (PCa): Results from a phase III, prospective, multicenter study (CONDOR). J. Clin. Oncol. 2020, 38 (Suppl. S15), 5501. [Google Scholar]
- Amin, A.; Blazevski, A.; Thompson, J.; Scheltema, M.J.; Hofman, M.S.; Murphy, D.; Lawrentschuk, N.; Sathianathen, N.; Kapoor, J.; Woo, H.H.; et al. Protocol for the PRIMARY clinical trial, a prospective, multicentre, cross-sectional study of the additive diagnostic value of gallium-68 prostate-specific membrane antigen positron-emission tomography/computed tomography to multiparametric magnetic resonance imaging in the diagnostic setting for men being investigated for prostate cancer. BJU Int. 2020, 125, 515–524. [Google Scholar]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen-Targeted PET Imaging: PSMA-RADS Version 1.0. Nucl. Med. 2018, 59, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Afshar-Oromieh, A.; Eiber, M.; Solnes, L.B.; Javadi, M.S.; Ross, A.E.; Pienta, K.J.; Allaf, M.E.; Haberkorn, U.; Pomper, M.G.; et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2117–2136. [Google Scholar] [CrossRef]
- Plouznikof, N.; Garcia, C.; Artigas, C.; Entezari, K.; Flamen, P. Heterogeneity of 68Ga-PSMA PET/CT uptake in fibrous dysplasia. Clin. Nucl. Med. 2019, 44, E593–E594. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Murphey, M.D.; Motamedi, K.; Mulligan, M.E.; Resnik, C.S.; Gannon, F.H. From the archives of the AFIP. Radiologic spectrum of Paget disease of bone and its complications with pathologic correlation. Radiographics 2002, 22, 1191–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gykiere, P.; Goethals, L.; Everaert, H. Healing Sacral Fracture Masquerading as Metastatic Bone Disease on a 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2016, 41, e346–e347. [Google Scholar] [CrossRef] [PubMed]
- Vamadevan, S.; Le, K.; Bui, C.; Mansberg, R. Incidental PSMA Uptake in an Undisplaced Fracture of a Vertebral Body. Clin. Nucl. Med. 2017, 42, 465–466. [Google Scholar] [CrossRef]
- Jochumsen, M.R.; Dias, A.H.; Bouchelouche, K. Benign Traumatic Rib Fracture: A Potential Pitfall on 68Ga-Prostate-Specific Membrane Antigen PET/CT for Prostate Cancer. Clin. Nucl. Med. 2018, 43, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, E.; Paschali, A.; Giannoula, E.; Chatzipavlidou, V. Rib Fractures Mimicking Bone Metastases in 18F-PSMA-1007 PET/CT for Prostate Cancer. Clin. Nucl. Med. 2019, 44, e46–e48. [Google Scholar] [CrossRef]
- Kroenke, M.; Mirzoyan, L.; Horn, T.; Peeken, J.C.; Wurzer, A.; Wester, H.J.; Makowski, M.; Weber, W.A.; Eiber, M.; Rauscher, I. Matched-Pair Comparison of 68 Ga-PSMA-11 and 18 F-rhPSMA-7 PET/CT in Patients with Primary and Biochemical Recurrence of Prostate Cancer: Frequency of Non-Tumor-Related Uptake and Tumor Positivity. J. Nucl. Med. 2021, 62, 1082–1088. [Google Scholar] [CrossRef]
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: A multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4483–4494. [Google Scholar] [CrossRef]
- Wang, C.; Shen, Y. Study on the distribution features of bone metastases in prostate cancer. Nucl. Med. Commun. 2012, 33, 379–383. [Google Scholar] [CrossRef]
- Chen, M.Y.; Franklin, A.; Yaxley, J.; Gianduzzo, T.; McBean, R.; Wong, D.; Tatkovic, A.; McEwan, L.; Walters, J.; Kua, B. Solitary rib lesions showing prostate-specific membrane antigen (PSMA) uptake in pre-treatment staging 68 Ga-PSMA-11 positron emission tomography scans for men with prostate cancer: Benign or malignant? BJU Int. 2020, 126, 396–401. [Google Scholar] [CrossRef]
- Arnfield, E.G.; Thomas, P.A.; Roberts, M.J.; Pelecanos, A.M.; Ramsay, S.C.; Lin, C.Y.; Latter, M.J.; Garcia, P.L.; Pattison, D.A. Clinical insignifcance of [18F] PSMA-1007 avid non-specifc bone lesions: A retrospective evaluation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4495–4507. [Google Scholar] [CrossRef] [PubMed]
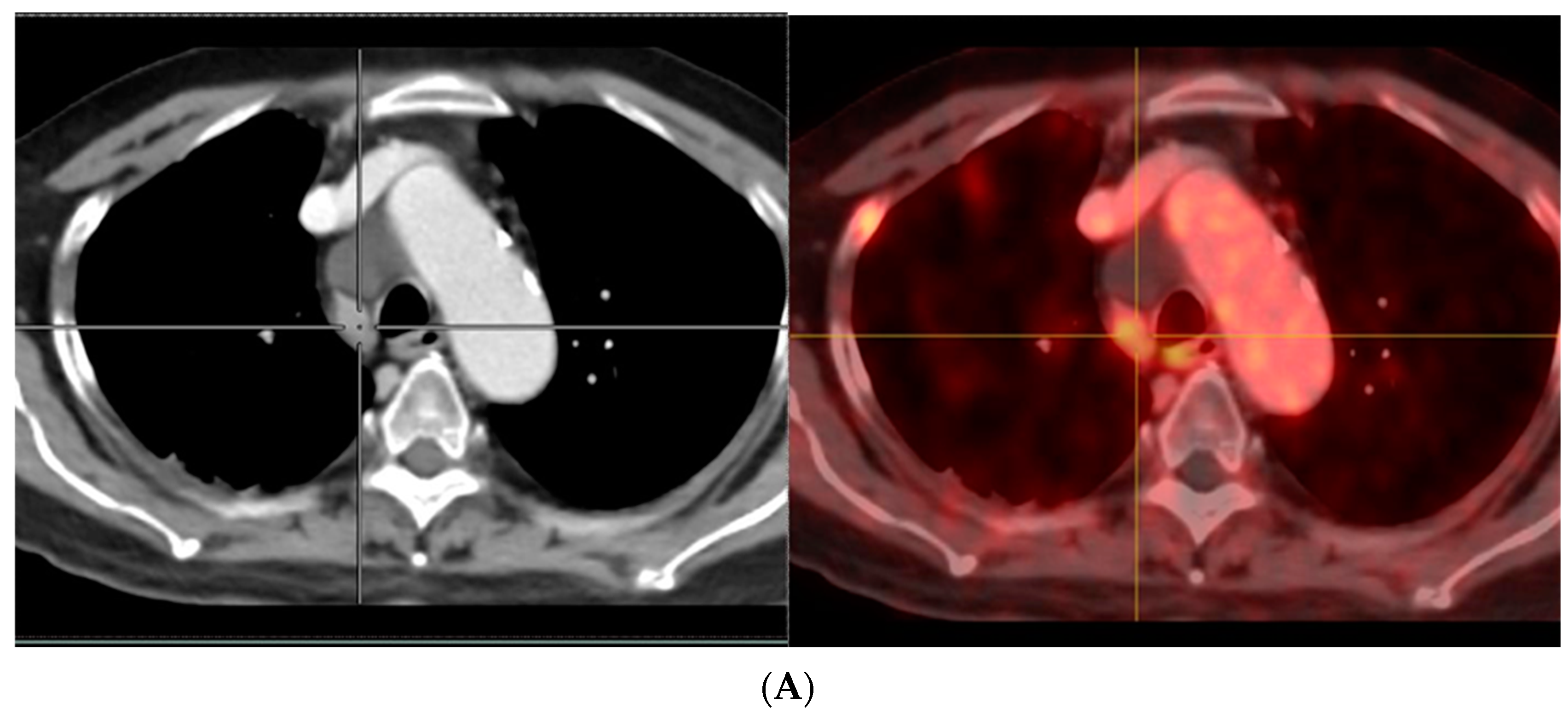
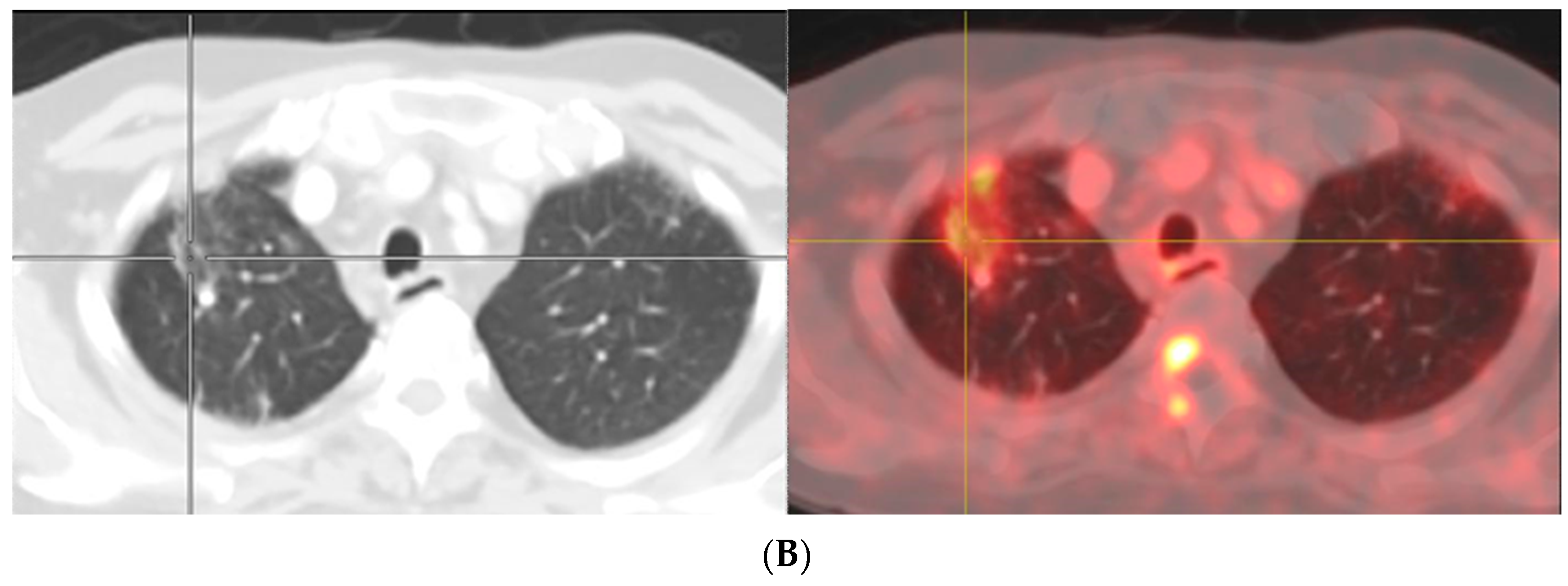
- Afshar-Oromieh, A.; Sattler, L.P.; Steiger, K.; Holland-Letz, T.; Da Cunha, M.L.; Mier, W.; Neels, O.; Kopka, K.; Weichert, W.; Haberkorn, U. Tracer uptake in mediastinal and paraaortal thoracic lymph nodes as a potential pitfall in image interpretation of PSMA ligand PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1179–1187. [Google Scholar] [CrossRef]
- Dobs, A.; Darkes, M.J.M. Incidence and management of gynecomastia in men treated for prostate cancer. J. Urol. 2005, 174, 1737–1742. [Google Scholar] [CrossRef]
- Alesini, D.; Iacovelli, R.; Palazzo, A.; Altavilla, A.; Risi, E.; Urbano, F.; Manai, C.; Passaro, A.; Magri, V.; Cortesi, E. Multimodality treatment of gynecomastia in patients receiving antiandrogen therapy for prostate cancer in the era of abiraterone acetate and new antiandrogen molecules. Oncology 2013, 84, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.; Joy, A.; Nair, B.P.; Pillai, M.R.A.; Madhavan, J. False Positive Uptake in Bilateral Gynecomastia on 68Ga-PSMA PET/CT Scan. Clin. Nucl. Med. 2017, 42, e412–e414. [Google Scholar] [CrossRef] [PubMed]
- Gorur, G.D.; Hekimsoy, T.; Isgoren, S. Re: False Positive Uptake in Bilateral Gynecomastia on 68Ga-PSMA PET/CT Scan. Clin. Nucl. Med. 2018, 43, 785. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Koumna, S.; Pouliot, F.; Beauregard, J.M.; Kolinsky, M. PSMA Theranostics: Current Landscape and Future Outlook. Cancers 2021, 13, 4023. [Google Scholar] [CrossRef]
- Ruigrok, E.A.M.; van Weerden, W.M.; Nonnekens, J.; de Jong, M. The future of PSMA-targeted radionuclide therapy: An overview of recent preclinical research. Pharmaceutics 2019, 11, 560. [Google Scholar] [CrossRef] [Green Version]
- Ruigrok, E.A.; van Vliet, N.; Dalm, S.U.; de Blois, E.; van Gent, D.C.; Haeck, J.; de Ridder, C.; Stuurman, D.; Konijnenberg, M.W.; van Weerden, W.M.; et al. Extensive preclinical evaluation of lutetium-177-labeled PSMA-specific tracers for prostate cancer radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1339–1350. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Dosimetry, Safety and Potential Benefit of 177Lu-PSMA-617 Prior to Prostatectomy (LuTectomy). Available online: https://clinicaltrials.gov/ct2/show/NCT04430192?term=Lutectomy&draw=2&rank=1 (accessed on 1 May 2022).
- In Men With Metastatic Prostate Cancer, What Is the Safety and Benefit of Lutetium-177 PSMA Radionuclide Treatment in Addition to Chemotherapy (UpFrontPSMA). Available online: https://clinicaltrials.gov/ct2/show/NCT04343885?term=UpFrontPSMA&draw=2&rank=1 (accessed on 1 May 2022).
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [PubMed] [Green Version]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Study Evaluating mCRPC Treatment Using PSMA [Lu-177]-PNT2002 Therapy after Second-Line Hormonal Treatment (SPLASH). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04647526 (accessed on 19 September 2021).
- Shah, H.; Vaishampayan, U. Therapy of Advanced Prostate Cancer: Targeting the Androgen Receptor Axis in Earlier Lines of Treatment. Target. Oncol. 2018, 13, 679–689. [Google Scholar] [CrossRef]
- An International Prospective Open-Label, Randomized, Phase III Study Comparing 177Lu-PSMA-617 in Combination With Soc, Versus SoC Alone, in Adult Male Patients With mHSPC (PSMAddition). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04720157 (accessed on 21 October 2021).
- Enzalutamide with Lu PSMA-617 versus Enzalutamide Alone in Men with Metastatic Castration-Resistant Prostate Cancer (ENZA-p). Available online: https://clinicaltrials.gov/ct2/show/NCT04419402?term=NCT04419402&draw=2&rank=1 (accessed on 1 May 2022).
- Wengner, A.M.; Scholz, A.; Haendler, B. Targeting DNA Damage Response in Prostate and Breast Cancer. Int. J. Mol. Sci. 2020, 21, 8273. [Google Scholar] [CrossRef]
- 177Lu-PSMA-617 Therapy and Olaparib in Patients with Metastatic Castration Resistant Prostate Cancer (LuPARP). Available online: https://clinicaltrials.gov/ct2/show/NCT03874884 (accessed on 21 October 2021).
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, A.; Mollica, V.; Cimadamore, A.; Santoni, M.; Scarpelli, M.; Giunchi, F.; Cheng, L.; Lopez-Beltran, A.; Fiorentino, M.; Montironi, R.; et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells 2020, 9, 2051. [Google Scholar] [CrossRef]
- PRINCE (PSMA-Lutetium Radionuclide Therapy and ImmuNotherapy in Prostate CancEr). Available online: https://clinicaltrials.gov/ct2/show/NCT03658447 (accessed on 21 October 2021).
- Delker, A.; Fendler, W.P.; Kratochwil, C.; Brunegraf, A.; Gosewisch, A.; Gildehaus, F.J.; Tritschler, S.; Stief, C.G.; Kopka, K.; Haberkorn, U.; et al. Dosimetry for 177Lu-DKFZ-PSMA-617: A new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 42–51. [Google Scholar] [CrossRef]
- Kabasakal, L.; AbuQbeitah, M.; Aygün, A.; Yeyin, N.; Ocak, M.; Demirci, E.; Toklu, T. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol Imaging 2015, 42, 1976–1983. [Google Scholar] [CrossRef]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Pommé, S.; Marouli, M.; Suliman, G.; Dikmen, H.; Van Ammel, R.; Jobbágy, V.; Dirican, A.; Stroh, H.; Paepen, J.; Bruchertseifer, F.; et al. Measurement of the 225Ac half-life. Appl. Radiat. Isot. 2012, 70, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted alpha therapy of mCRPC with 225Actinium-PSMA-617: Dosimetry estimate and empirical dose finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advancedprostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [Green Version]
- McDevitt, M.R.; Barendswaard, E.; Ma, D.; Lai, L.; Curcio, M.J.; Sgouros, G.; Ballangrud, Å.M.; Yang, W.H.; Finn, R.D.; Pellegrini, V.; et al. An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000, 60, 6095–6100. [Google Scholar] [PubMed]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in mCRPC. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1099–1100. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Schmidt, K.; Afshar-Oromieh, A.; Bruchertseifer, F.; Rathke, H.; Morgenstern, A.; Haberkorn, U.; Giesel, F.L. Targeted alpha therapy of mCRPC: Dosimetry estimate of 213Bismuth-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar]
- Smith-Jones, P.M.; Vallabahajosula, S.; Goldsmith, S.J.; Navarro, V.; Hunter, C.J.; Bastidas, D.; Bander, N.H. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000, 60, 5237–5243. [Google Scholar]
- Tagawa, S. AUA 2020: Comparison of Prostate-Specific Membrane Antigen-Targeted Radionuclide Therapy with Lutetium-177 via Antibody J591 vs. Small Molecule Ligand PSMA-617. Available online: https://www.urotoday.com/conference-highlights/aua-2020/aua-2020-prostate-cancer/122691-aua-2020-comparison-of-prostate-specific-membrane-antigen-targeted-radionuclide-therapy-with-lutetium-177-via-antibody-j591-vs-small-molecule-ligand-psma-617.html (accessed on 19 September 2021).
- Bander, N.; Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J. Clin. Oncol. 2005, 23, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagawa, S.T.; Vallabhajosula, S.; Christos, P.J.; Jhanwar, Y.S.; Batra, J.S.; Lam, L.; Osborne, J.; Beltran, H.; Molina, A.M.; Goldsmith, S.J.; et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer 2019, 125, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Osborne, J.; Fernandez, E.; Thomas, C.; Niaz, M.J.; Ciriaco, A.; Vallabhajosula, S.; Vlachostergios, P.J.; Molina, A.M.; Sternberg, C.N.; et al. Phase I dose-escalation study of PSMA-targeted alpha emitter 225Ac-J591 in men with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38 (Suppl. S15), 5560. [Google Scholar]
- Hammer, S.; Hagemann, U.B.; Zitzmann-Kolbe, S.; Larsen, A.; Ellingsen, C.; Geraudie, S.; Grant, D.; Indrevoll, B.; Smeets, R.; von Ahsen, O.; et al. Preclinical Efficacy of a PSMA-Targeted Thorium-227 Conjugate (PSMA-TTC), a Targeted Alpha Therapy for Prostate Cancer. Clin. Cancer Res. 2020, 26, 1985–1996. [Google Scholar] [CrossRef] [Green Version]
- Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Antitumor Activity of a Thorium-227 Labeled Antibody-chelator Conjugate Alone and in Combination with Darolutamide, in Patients with Metastatic Castration Resistant Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03724747 (accessed on 19 September 2021).
- Scholte, M.; Barentsz, J.O.; Sedelaar, J.M.; Gotthardt, M.; Grutters, J.P.; Rovers, M.M. Modelling Study with an Interactive Model Assessing the Cost-effectiveness of 68Ga Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography and Nano Magnetic Resonance Imaging for the Detection of Pelvic Lymph Node Metastases in Patients with Primary Prostate Cancer. Eur. Urol. Focus 2020, 6, 967–974. [Google Scholar]
- Gordon, L.G.; Elliott, T.H.; Joshi, A.; Williams, E.D.; Vela, I. Exploratory cost-effectiveness analysis of 68Gallium-PSMA PET/MRI-based imaging in patients with biochemical recurrence of prostate cancer. Clin. Exp. Metastasis 2020, 37, 305–312. [Google Scholar] [CrossRef]
- de Feria Cardet, R.E.; Hofman, M.S.; Segard, T.; Yim, J.; Williams, S.; Francis, R.J.; Frydenberg, M.; Lawrentschuk, N.; Murphy, D.G.; Lourenco, R.D.A. Is Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Imaging Cost-effective in Prostate Cancer: An Analysis Informed by the proPSMA Trial. Eur. Urol. 2021, 79, 413–418. [Google Scholar] [CrossRef]
- Schwenck, J.; Olthof, S.C.; Pfannenberg, C.; Reischl, G.; Wegener, D.; Marzec, J.; Bedke, J.; Stenzl, A.; Nikolaou, K.; la Fougère, C.; et al. Intention-to-Treat Analysis of 68Ga-PSMA and 11C-Choline PET/CT Versus CT for Prostate Cancer Recurrence After Surgery. J. Nucl. Med. 2019, 60, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
| Definition | |||
|---|---|---|---|
| Low Risk | Intermediate Risk | High Risk | |
| PSA < 10 ng/mL GS < 7 or cT1-T2a | PSA 10–20 ng/mL GS = 7 or cT2b | PSA > 20 ng/mL GS > 7 or cT2c | Any PSA, any GS cT3-4, or cN+ Locally advanced |
| Authors | Year | Type of Study | Objectives | Number of Studies and/or Patients | Results |
|---|---|---|---|---|---|
| Zhang et al. [12] | 2021 | Meta-analysis | To evaluate the clinical efficacy and safety of the 177Lu-PSMA-617 therapy in the treatment of metastatic castration-resistant prostate cancer (mCRPC). | 12 studies, 508 patients | After the first cycle of treatment, the pooled rate of PSA decline was 69.30%, and that of >50% PSA decline was 35.90% without significant adverse events. |
| Sartor O, et al. [13] | 2021 | Prospective, open-label, randomized, international, phase 3 trial (VISION trial) | To compare efficacy of 177Lu-PSMA-617 (7.4 GBq every 6 weeks × 6 cycles) combined standard of care (SOC) compared to SOC alone | 831 patients | Significant improvement in OS by median of 4.0 months and significantly longer PFS based on imaging. |
| Ballal et al. [14] | 2021 | Systematic Review | To evaluate the role of 225Ac-PSMA as a salvage treatment in mCRPC | 3 studies, 141 patients | 225Ac-PSMA-617 revealed biochemical response, improved survival, caused low treatment-related toxicity proving a promising salvage treatment option in mCRPC patients. |
| Sadaghiani MS et al. [15] | 2021 | Systematic Review | To evaluate the efficacy and toxicity of 177Lu-PSMA-targeted radioligand therapy (PRLT) | 24 studies, 1192 patients | PRLT is associated with ≥50% reduction in PSA level in a large number of patients and a low rate of toxicity |
| Satapathy S et al. [16] | 2021 | Systematic Review | To evaluate the role of 225Ac-PSMA RLT in mCRPC. | 10 studies, 256 patients | 225Ac-PSMA RLT is an efficacious and safe treatment option for mCRPC. |
| Hofman MS et al. [17] | 2021 | Randomized, open-label, phase 2 trial (TheraP trial) | To compare 177Lu-PSMA-617 with cabazitaxel in patients with mCRPC. | 291 patients | 177Lu-PSMA-617 compared with cabazitaxel in Mcrpc led to a higher PSA response and fewer grade 3 or 4 adverse events. |
| von Eyben FE et al. [18] | 2020 | Systematic Review | To evaluate treatment outcome of 177Lu-PSMA RLT in mCRPC | 36 studies, 2346 patients | Half of all patients obtained a PSA decline of ≥50% and lived longer than those with less PSA decline. 10% of developed hematologic toxicity (anemia grade 3) |
| Satapathy S et al. [19] | 2020 | Systematic review and meta-analysis | To evaluate the impact of visceral metastases on biochemical response and survival outcomes in mCRPC treated with 177Lu-PSMA RLT. | 12 studies, 1504 patients | Presence of visceral metastases was associated with poor response and survival outcomes in patients of mCRPC treated with 177Lu-PSMA RLT |
| Kim YJ [20] | 2020 | Meta-analysis | To evaluate treatment responses after the 1st cycle of 177Lu-PSMA-617 RLT | 10 studies, 455 patients | Two-thirds of any PSA decline and one-third of >50% PSA decline after the 1st cycle of 177Lu-PSMA-617 RLT in mCRPC. Any PSA decline showed survival prolongation after the 1st cycle of the 177Lu-PSMA-617. |
| Yadav MP et al. [21] | 2019 | Systematic Review and meta-analysis | To evaluate efficacy and safety data on 177Lu-PSMA RLT for mCRPC | 17 studies, 744 patients | 177Lu-PSMA RLT is an effective treatment of advanced-stage mCRPC refractory to SOC with low toxicity. |
| von Eyben FE et al. [22] | 2017 | Systematic Review | To compare efficacy of 177Lu PSMA RLT and third-line treatment for mCRPC | 12 studies, 669 patients | 177Lu-PSMA-617 RTL and 177Lu-PSMA I&T gave better effects and caused fewer adverse effects than third-line treatment |
| Calopedos RJS et al. [23] | 2017 | Systematic review and meta-analysis | To assess treatment response of 177Lu-PSMA in mCRPC | 10 studies, 369 patients | Two-thirds of patients had biochemical response (any PSA decline was 68%, >50% PSA decline was 37%) |
| Role | Information |
|---|---|
| Diagnosis |
|
| Primary staging |
|
| Recurrent detection (re-staging) |
|
| Selection for radionuclide therapy |
|
| Ligand (PSMA 617) | mAb (J591) |
|---|---|
| Small (mw 1400) | Large (mw 150,000) |
| Short circulation time | Long circulation time (days) |
|
|
| Rapidly diffuse to all sites of expression | Mostly target via vasculature |
| Toxicities | Toxicities |
|
|
| Clinical Trial | Status | Phase | Patients | Interventions |
|---|---|---|---|---|
| ACTRN12615000912583 (LuPSMA) [105] | completed | 2 | 40 | 177Lu-PSMA-617 in progressive mCRPC |
| NCT03392428 (TheraP) [17] | active, not recruiting | 2 | 200 | 177Lu-PSMA-617 vs. cabazitaxel in progressive mCRPC |
| NCT03511664(VISION) [13] | active, not recruiting | 3 | 831 | 177Lu-PSMA-617 + SOC vs. SOC in progressive mCRPC |
| NCT04430192 (LuTectomy) [106] | recruiting | 1/2 | 20 | 177Lu-PSMA-617 followed by prostatectomy |
| NCT04343885 (UpFrontPSMA) [107] | recruiting | 2 | 140 | Sequential 177Lu-PSMA-617 + docetaxel vs. docetaxel in metastatic hormone-naive PCa |
| NCT04419402 (ENZA-P) [113] | recruiting | 2 | 160 | Enzalutamide + 177Lu-PSMA-617 vs. Enzalutamide alone in mCRPC |
| NCT04647526 (SPLASH) [110] | recruiting | 3 | 415 | 177Lu-PSMA-I&T vs. ARAT in progressive mCRPC |
| NCT04720157 (PSMAddition) [112] | recruiting | 3 | 1126 | 177Lu-PSMA-617 + SOC vs. SOC alone in mHSPC |
| NCT03874884 (LuPARP) [115] | recruiting | 1 | 52 | 177Lu-PSMA-617 + olaparib in progressive mCRPC |
| NCT03658447 (PRINCE) [118] | active, not recruiting | 1/2 | 37 | 177Lutetium-PSMA-617 + pembrolizumab (mCRPC) |
| NCT03276572 [137] | active, not recruiting | 1 | 31 | 225Ac-J591 in mCRPC |
| NCT03724747 [139] | recruiting | 1 | 198 | 227Th-PSMA-TTC in progressive mCRPC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewput, C.; Vinjamuri, S. Update of PSMA Theranostics in Prostate Cancer: Current Applications and Future Trends. J. Clin. Med. 2022, 11, 2738. https://doi.org/10.3390/jcm11102738
Kaewput C, Vinjamuri S. Update of PSMA Theranostics in Prostate Cancer: Current Applications and Future Trends. Journal of Clinical Medicine. 2022; 11(10):2738. https://doi.org/10.3390/jcm11102738
Chicago/Turabian StyleKaewput, Chalermrat, and Sobhan Vinjamuri. 2022. "Update of PSMA Theranostics in Prostate Cancer: Current Applications and Future Trends" Journal of Clinical Medicine 11, no. 10: 2738. https://doi.org/10.3390/jcm11102738
APA StyleKaewput, C., & Vinjamuri, S. (2022). Update of PSMA Theranostics in Prostate Cancer: Current Applications and Future Trends. Journal of Clinical Medicine, 11(10), 2738. https://doi.org/10.3390/jcm11102738






