Left Atrial Thrombus in Atrial Fibrillation/Flutter Patients in Relation to Anticoagulation Strategy: LATTEE Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient and Public Involvement
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Population
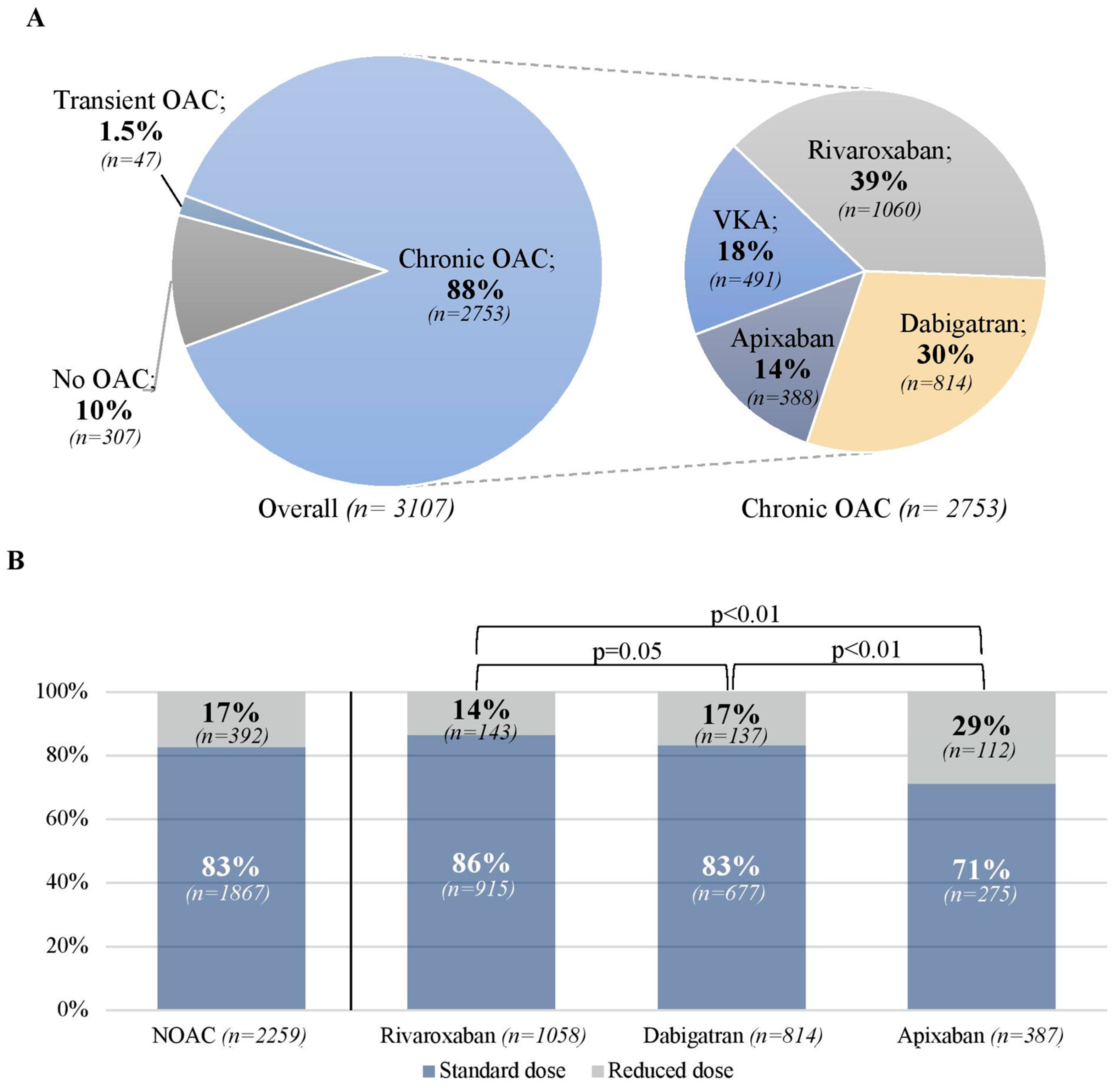
3.2. Oral Anticoagulation
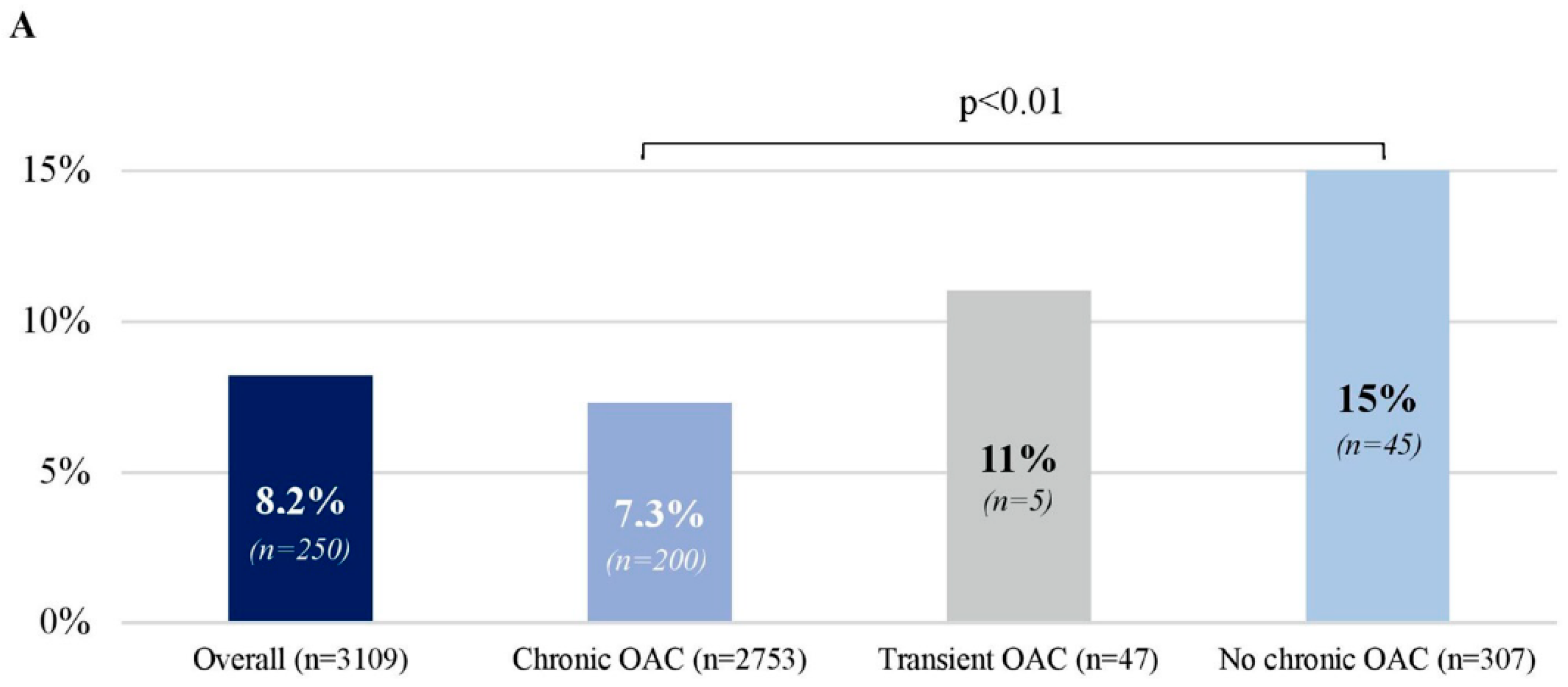
3.3. Left Atrial Thrombus
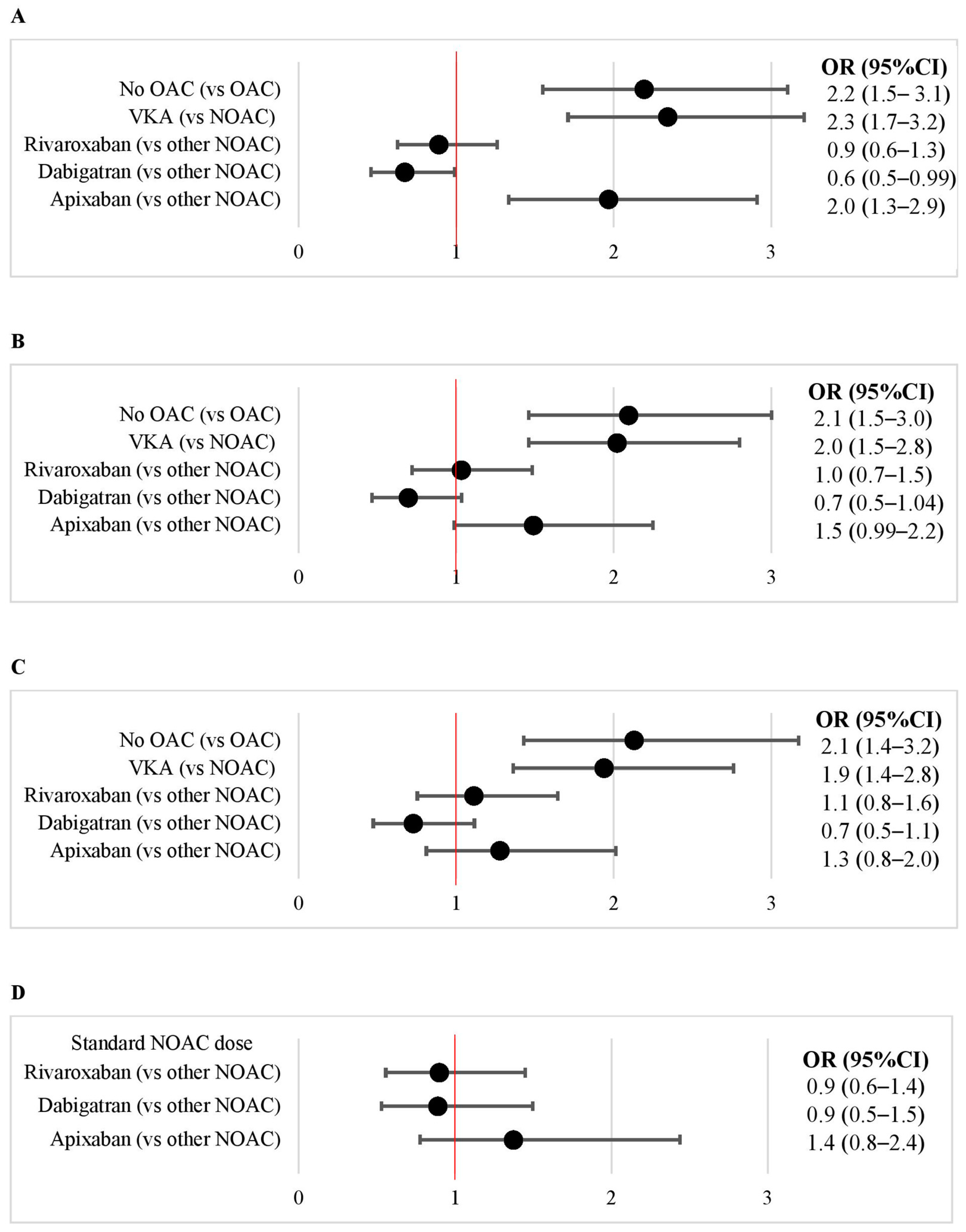
3.4. VKA vs. NOAC
3.5. Apixaban vs. Rivaroxaban vs. Dabigatran
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoddard, M.F.; Singh, P.; Dawn, B.; Longaker, R.A. Left atrial thrombus predicts transient ischemic attack in patients with atrial fibrillation. Am. Heart J. 2003, 145, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Gloekler, S.; Saw, J.; Koskinas, K.C.; Kleinecke, C.; Jung, W.; Nietlispach, F.; Meier, B. Left atrial appendage closure for prevention of death, stroke, and bleeding in patients with nonvalvular atrial fibrillation. Int. J. Cardiol. 2017, 249, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Huber, K.; Whisenant, B.; Kar, S.; Swarup, V.; et al. Percutaneous left atrial appendage closure vs. warfarin for atrial fibrillation: A randomized clinical trial. JAMA 2014, 312, 1988–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Kaplon-Cieslicka, A.; Budnik, M.; Gawalko, M.; Wojcik, M.; Blaszczyk, R.; Uzieblo-Zyczkowska, B.; Krzesinski, P.; Starzyk, K.; Gorczyca, I.; Szymanska, A.; et al. The rationale and design of the LATTEE registry—The first multicenter project on the Scientific Platform of the “Club 30” of the Polish Cardiac Society. Kardiol. Pol. 2019, 77, 1078–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef] [PubMed]
- Fatkin, D.; Kelly, R.P.; Feneley, M.P. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J. Am. Coll. Cardiol. 1994, 23, 961–969. [Google Scholar] [CrossRef] [Green Version]
- Noubiap, J.J.; Agbaedeng, T.A.; Ndoadoumgue, A.L.; Nyaga, U.F.; Kengne, A.P. Atrial thrombus detection on transoesophageal echocardiography in patients with atrial fibrillation undergoing cardioversion or catheter ablation: A pooled analysis of rates and predictors. J. Cardiovasc. Electrophysiol. 2021, 32, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Silverio, A.; Di Maio, M.; Prota, C.; De Angelis, E.; Radano, I.; Citro, R.; Carrizzo, A.; Ciccarelli, M.; Vecchione, C.; Capodanno, D.; et al. Safety and efficacy of non-vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation: Systematic review and meta-analysis of 22 studies and 440 281 patients. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, f20–f29. [Google Scholar] [CrossRef] [PubMed]
- Gawalko, M.; Kaplon-Cieslicka, A.; Budnik, M.; Babiarz, A.; Bodys, A.; Ulinski, R.; Zochowski, M.; Peller, M.; Scislo, P.; Kochanowski, J.; et al. Comparison of different oral anticoagulant regimens in patients with atrial fibrillation undergoing ablation or cardioversion. Pol. Arch. Intern. Med. 2017, 127, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, S.; Ten Cate, H.; Accetta, G.; Angchaisuksiri, P.; Bassand, J.P.; Camm, A.J.; Corbalan, R.; Darius, H.; Fitzmaurice, D.A.; Goldhaber, S.Z.; et al. Quality of Vitamin K Antagonist Control and 1-Year Outcomes in Patients with Atrial Fibrillation: A Global Perspective from the GARFIELD-AF Registry. PLoS ONE 2016, 11, e0164076. [Google Scholar] [CrossRef] [Green Version]
- Reiffel, J.A. Time in the Therapeutic Range for Patients Taking Warfarin in Clinical Trials: Useful, but Also Misleading, Misused, and Overinterpreted. Circulation 2017, 135, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, P.A.; Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; McBane, R.D.; Shah, N.D. Direct Comparison of Dabigatran, Rivaroxaban, and Apixaban for Effectiveness and Safety in Nonvalvular Atrial Fibrillation. Chest 2016, 150, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Larsen, T.B.; Skjoth, F.; Rasmussen, L.H. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J. Am. Coll. Cardiol. 2012, 60, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- Kaplon-Cieslicka, A.; Budnik, M.; Gawalko, M.; Peller, M.; Gorczyca, I.; Michalska, A.; Babiarz, A.; Bodys, A.; Ulinski, R.; Zochowski, M.; et al. Atrial fibrillation type and renal dysfunction as important predictors of left atrial thrombus. Heart 2019, 105, 1310–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budnik, M.; Gawalko, M.; Gorczyca, I.; Uzieblo-Zyczkowska, B.; Krzesinki, P.; Kochanowski, J.; Scislo, P.; Michalska, A.; Jelonek, O.; Starzyk, K.; et al. Risk of left atrial appendage thrombus in patients with atrial fibrillation and chronic kidney disease. Cardiol. J. 2020, 29, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmalska, K.; Rzyman, M.; Miekus, P.; Gilis-Malinowska, N.; Nowak, R.; Fijalkowski, M. Usefulness of transesophageal echocardiography before cardioversion in atrial arrhythmias. Cardiol. J. 2021, 28, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variable | VKA (n = 491) | NOAC (n = 2262) | p 1 | NOAC (n = 2262) | p 2 | ||
|---|---|---|---|---|---|---|---|
| Rivaroxaban (n = 1060) | Dabigatran (n = 814) | Apixaban (n = 388) | |||||
| Demographics | |||||||
| Age (years) | 68 [62–74] n = 490 | 66 [59–73] n = 2259 | <0.01 | 66 [58–73] n = 1059 | 65 [58–72] n = 812 | 71 [63–77] n = 388 | <0.01 |
| Age ≥ 75 years | 108/490 (22%) | 471/2259 (21%) | 0.58 | 200/1059 (19%) | 146/812 (18%) | 125/388 (32%) | <0.01 |
| Female sex | 196/491 (40%) | 826/2262 (37%) | 0.16 | 380/1060 (36%) | 281/814 (35%) | 165/388 (43%) | 0.02 |
| BMI (kg/m2) | 29 [26–33] n = 463 | 29 [26–33] n = 2107 | 0.55 | 29 [26–33] n = 983 | 29 [26–33] n = 767 | 29 [26–32] n = 357 | 0.26 |
| Indications for TEE | |||||||
| Direct current cardioversion for AF/AFl | 282/475 (59%) | 1055/2236 (47%) | <0.01 | 501/1052 (48%) | 321/804 (40%) | 233/380 (61%) | <0.01 |
| AF/AFl ablation | 193/475 (41%) | 1181/2236 (53%) | <0.01 | 551/1052 (52%) | 483/804 (60%) | 147/380 (39%) | <0.01 |
| AF/AFl type | |||||||
| AF | 428/491 (87%) | 2025/2262 (90%) | 0.13 | 956/1060 (90%) | 737/814 (91%) | 332/388 (86%) | 0.02 |
| AFl | 80/491 (16%) | 309/2262 (14%) | 0.13 | 137/1060 (13%) | 102/814 (13%) | 70/388 (18%) | 0.02 |
| AF/AFl paroxysmal | 158/487 (32%) | 969/2259 (43%) | <0.01 | 458/1059 (43%) | 380/813 (47%) | 131/387 (34%) | <0.01 |
| AF/AFl persistent | 262/487 (54%) | 1107/2259 (49%) | 0.06 | 528/1059 (50%) | 360/813 (44%) | 219/387 (57%) | <0.01 |
| AF/AFl long-standing persistent | 67/487 (14%) | 183/2259 (8.1%) | <0.01 | 73/1059 (6.9%) | 73/813 (9.0%) | 37/387 (9.6%) | 0.13 |
| Comorbidities | |||||||
| Hypertension | 385/490 (79%) | 1736/2261 (77%) | 0.41 | 811/1060 (77%) | 618/814 (76%) | 307/387 (79%) | 0.41 |
| Heart failure | 254/491 (52%) | 902/2253 (40%) | <0.01 | 410/1057 (39%) | 306/809 (38%) | 186/387 (48%) | <0.01 |
| Mechanical valve prosthesis | 68/491 (14%) | 3/2258 (0.1%) | <0.01 | 1/1060 (0.1%) | 1/811 (0.1%) | 1/387 (0.3%) | 0.75 |
| Biological valve prosthesis (including TAVI) | 24/491 (4.9%) | 24/2258 (1.1%) | <0.01 | 8/1060 (0.8%) | 10/820 (1.2%) | 6/387 (1.5%) | 0.39 |
| Vascular disease | 193/490 (39%) | 755/2261 (33%) | 0.01 | 333/1060 (31%) | 265/814 (33%) | 157/387 (41%) | <0.01 |
| Previous stroke | 40/490 (8.2%) | 171/2261 (7.6%) | 0.64 | 69/1060 (6.5%) | 63/814 (7.7%) | 39/387 (10%) | 0.07 |
| Ischemic stroke/TIA/systemic embolism | 56/488 (11%) | 229/2258 (10%) | 0.37 | 91/1060 (8.6%) | 87/812 (11%) | 51/386 (13%) | 0.03 |
| Previous bleeding | 17/490 (3.5%) | 82/2261 (3.6%) | 1.00 | 29/1060 (2.7%) | 26/814 (3.2%) | 27/387 (7.0%) | <0.01 |
| Diabetes mellitus | 141/490 (29%) | 538/2261 (24%) | 0.02 | 236/1060 (22%) | 194/814 (24%) | 108/387 (28%) | 0.08 |
| GFR (mL/min) | 80 [60–100] n = 439 | 82 [64–103] n = 2031 | 0.049 | 84 [67–106] n = 946 | 85 [66–105] n = 734 | 73 [57–90] n = 351 | <0.01 |
| GFR < 50 mL/min | 65/439 (15%) | 200/2031 (9.9%) | <0.01 | 76/946 (8.0%) | 60/734 (8.2%) | 64/351 (18%) | <0.01 |
| COPD | 31/490 (6.3%) | 105/2261 (4.6%) | 0.13 | 37/1060 (3.5%) | 44/814 (5.4%) | 24/387 (6.2%) | 0.04 |
| Anemia 3 | 102/472 (22%) | 312/2189 (14%) | <0.01 | 123/1028 (12%) | 116/785 (15%) | 73/376 (19%) | <0.01 |
| Thromboembolic risk and indications to chronic OAC | |||||||
| CHA2DS2-VASc score | 3 [2–5] n = 487 | 3 [2–4] n = 2246 | <0.01 | 3 [2–4] n = 1056 | 3 [1–4] n = 805 | 4 [2–5] n = 385 | <0.01 |
| Class I indications to OAC 4 | 414/488 (85%) | 1632/2246 (73%) | <0.01 | 767/1056 (73%) | 550/805 (68%) | 315/385 (82%) | <0.01 |
| -moderate/severe MS or mechanical valve prosthesis | 77/488 (16%) | 10/2246 (0.4%) | <0.01 | 5/1056 (0.5%) | 2/805 (0.2%) | 3/385 (0.8%) | 0.43 |
| Class IIa indications 5 | 53/488 (11%) | 408/2246 (18%) | <0.01 | 185/1056 (18%) | 175/805 (22%) | 48/385 (12%) | <0.01 |
| No indications to chronic OAC 6 | 21/488 (4.3%) | 206/2246 (9.2%) | <0.01 | 104/1056(9.9%) | 80/805 (9.9%) | 22/385 (5.7%) | 0.04 |
| International normalized ratio (INR) for patients on VKA | |||||||
| Data on INR during hospitalization | 473/491 (96%) | non-applicable | |||||
| INR 2–3 at hospital admission | 197/473 (42%) | ||||||
| Data on INR before hospitalization | 332/491 (68%) | ||||||
| INR 2–3 before hospitalization | 178/332 (46%) | ||||||
| Antithrombotic therapy | |||||||
| Heparin (periprocedural): | 32/489 (6.5%) | 77/2255 (3.4%) | <0.01 | 38/1056 (3.6%) | 27/812 (3.3%) | 12/387 (3.1%) | 0.89 |
| -heparin ≥ 2 days | 14/489 (2.9%) | 9/2253 (0.4%) | <0.01 | 4/1056 (0.4%) | 3/811 (0.4%) | 2/386 (0.5%) | 0.91 |
| Antiplatelets | 69/491 (14%) | 169/2262 (7.5%) | <0.01 | 66/1060 (6.2%) | 60/814 (7.4%) | 43/388 (11%) | <0.01 |
| Transesophageal echocardiography | |||||||
| Left atrial appendage emptying velocity (cm/s) | 30 [20–44] n = 419 | 40 [28–55] n = 1917 | <0.01 | 40 [28–55] n = 897 | 41 [30–60] n = 695 | 36 [24–50] n = 325 | <0.01 |
| SEC | 165/485 (34%) | 523/2236 (23%) | <0.01 | 250/1048 (24%) | 171/805 (21%) | 102/383 (27%) | 0.11 |
| Left atrial thrombus | 64/491 (13%) | 136/2262 (6.0%) | <0.01 | 60/1060 (5.7%) | 38/814 (4.7%) | 38/388 (9.8%) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapłon-Cieślicka, A.; Gawałko, M.; Budnik, M.; Uziębło-Życzkowska, B.; Krzesiński, P.; Starzyk, K.; Gorczyca-Głowacka, I.; Daniłowicz-Szymanowicz, L.; Kaufmann, D.; Wójcik, M.; et al. Left Atrial Thrombus in Atrial Fibrillation/Flutter Patients in Relation to Anticoagulation Strategy: LATTEE Registry. J. Clin. Med. 2022, 11, 2705. https://doi.org/10.3390/jcm11102705
Kapłon-Cieślicka A, Gawałko M, Budnik M, Uziębło-Życzkowska B, Krzesiński P, Starzyk K, Gorczyca-Głowacka I, Daniłowicz-Szymanowicz L, Kaufmann D, Wójcik M, et al. Left Atrial Thrombus in Atrial Fibrillation/Flutter Patients in Relation to Anticoagulation Strategy: LATTEE Registry. Journal of Clinical Medicine. 2022; 11(10):2705. https://doi.org/10.3390/jcm11102705
Chicago/Turabian StyleKapłon-Cieślicka, Agnieszka, Monika Gawałko, Monika Budnik, Beata Uziębło-Życzkowska, Paweł Krzesiński, Katarzyna Starzyk, Iwona Gorczyca-Głowacka, Ludmiła Daniłowicz-Szymanowicz, Damian Kaufmann, Maciej Wójcik, and et al. 2022. "Left Atrial Thrombus in Atrial Fibrillation/Flutter Patients in Relation to Anticoagulation Strategy: LATTEE Registry" Journal of Clinical Medicine 11, no. 10: 2705. https://doi.org/10.3390/jcm11102705
APA StyleKapłon-Cieślicka, A., Gawałko, M., Budnik, M., Uziębło-Życzkowska, B., Krzesiński, P., Starzyk, K., Gorczyca-Głowacka, I., Daniłowicz-Szymanowicz, L., Kaufmann, D., Wójcik, M., Błaszczyk, R., Hiczkiewicz, J., Łojewska, K., Mizia-Stec, K., Wybraniec, M. T., Kosmalska, K., Fijałkowski, M., Szymańska, A., Dłużniewski, M., ... Grabowski, M. (2022). Left Atrial Thrombus in Atrial Fibrillation/Flutter Patients in Relation to Anticoagulation Strategy: LATTEE Registry. Journal of Clinical Medicine, 11(10), 2705. https://doi.org/10.3390/jcm11102705











