Implementing and Evaluating the Impact of BoneRx: A Healthy Bone Prescription for Men with Prostate Cancer Initiating Androgen Deprivation Therapy
Abstract
1. Introduction
2. Materials and Methods
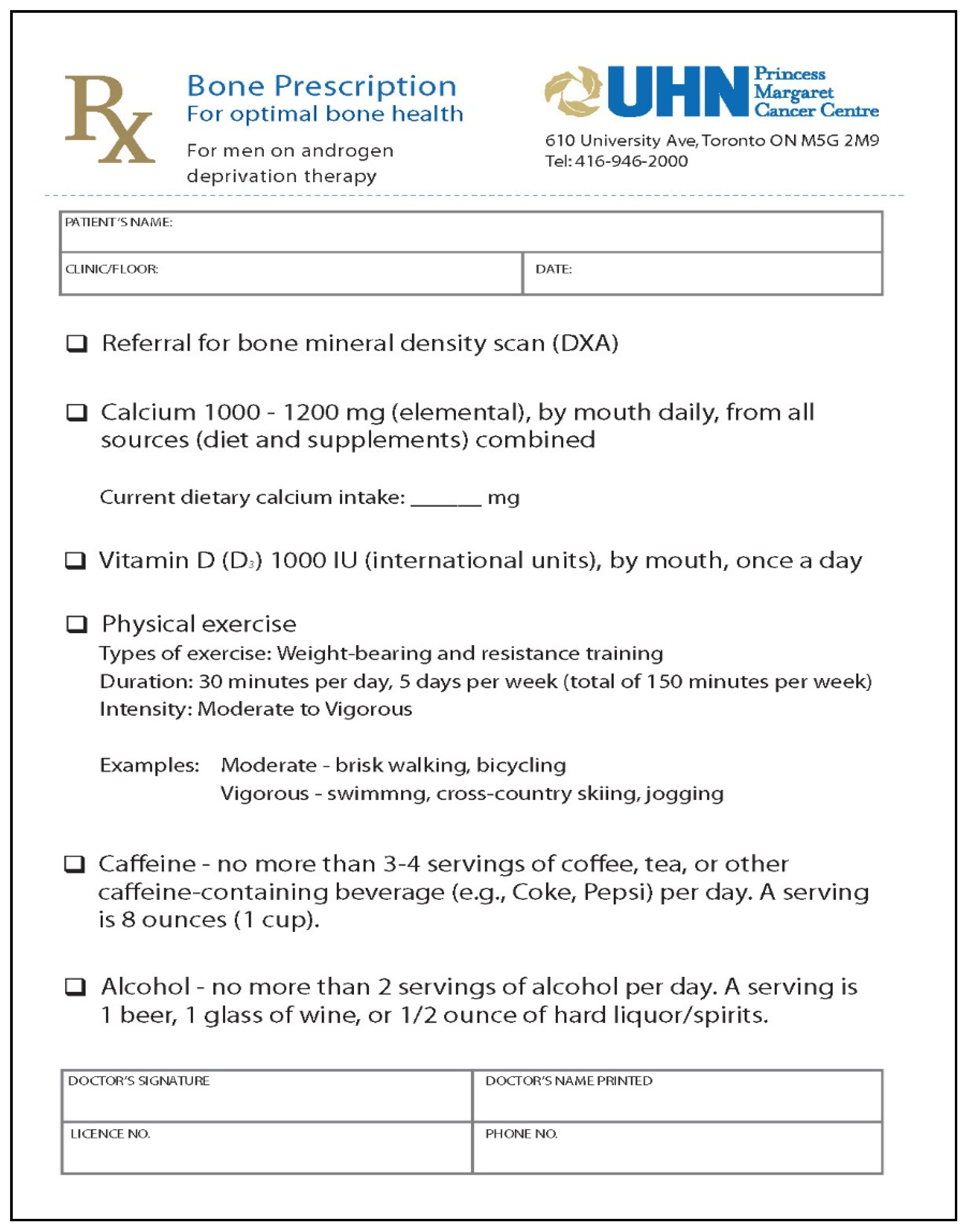
2.1. BoneRx Intervention
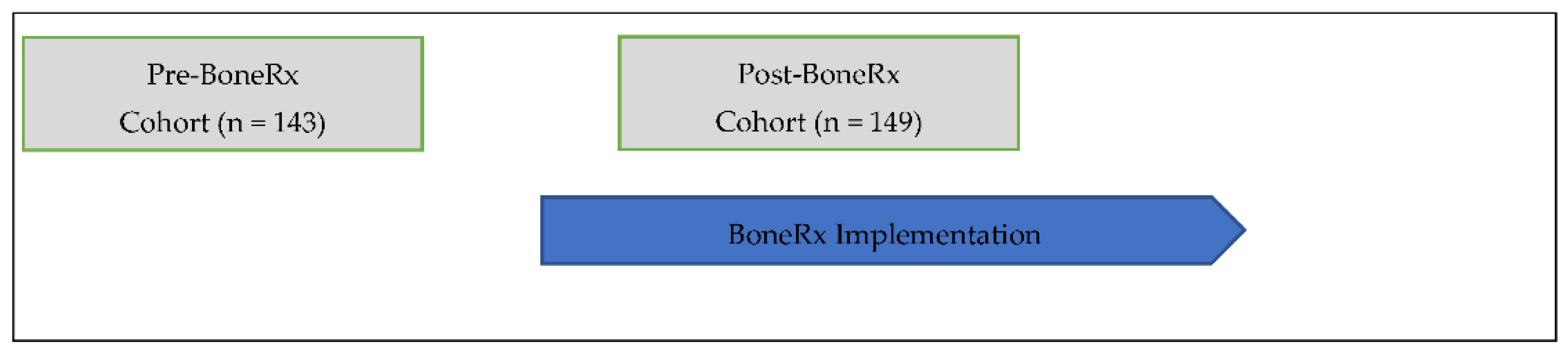
2.2. Procedure and Participants
2.3. Outcome Measures
2.4. Statistical Analyses
3. Results
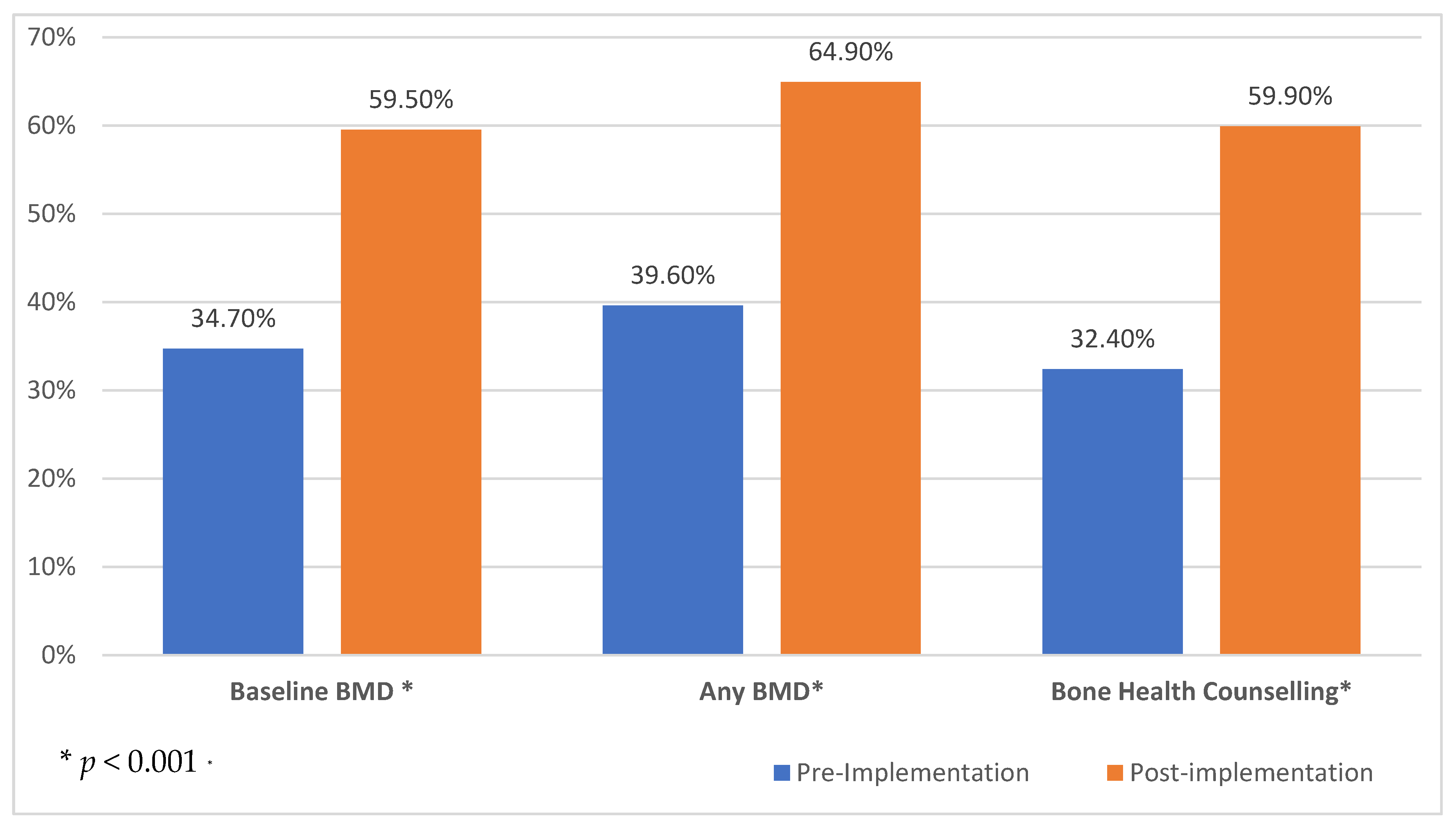
3.1. Bone Health Care
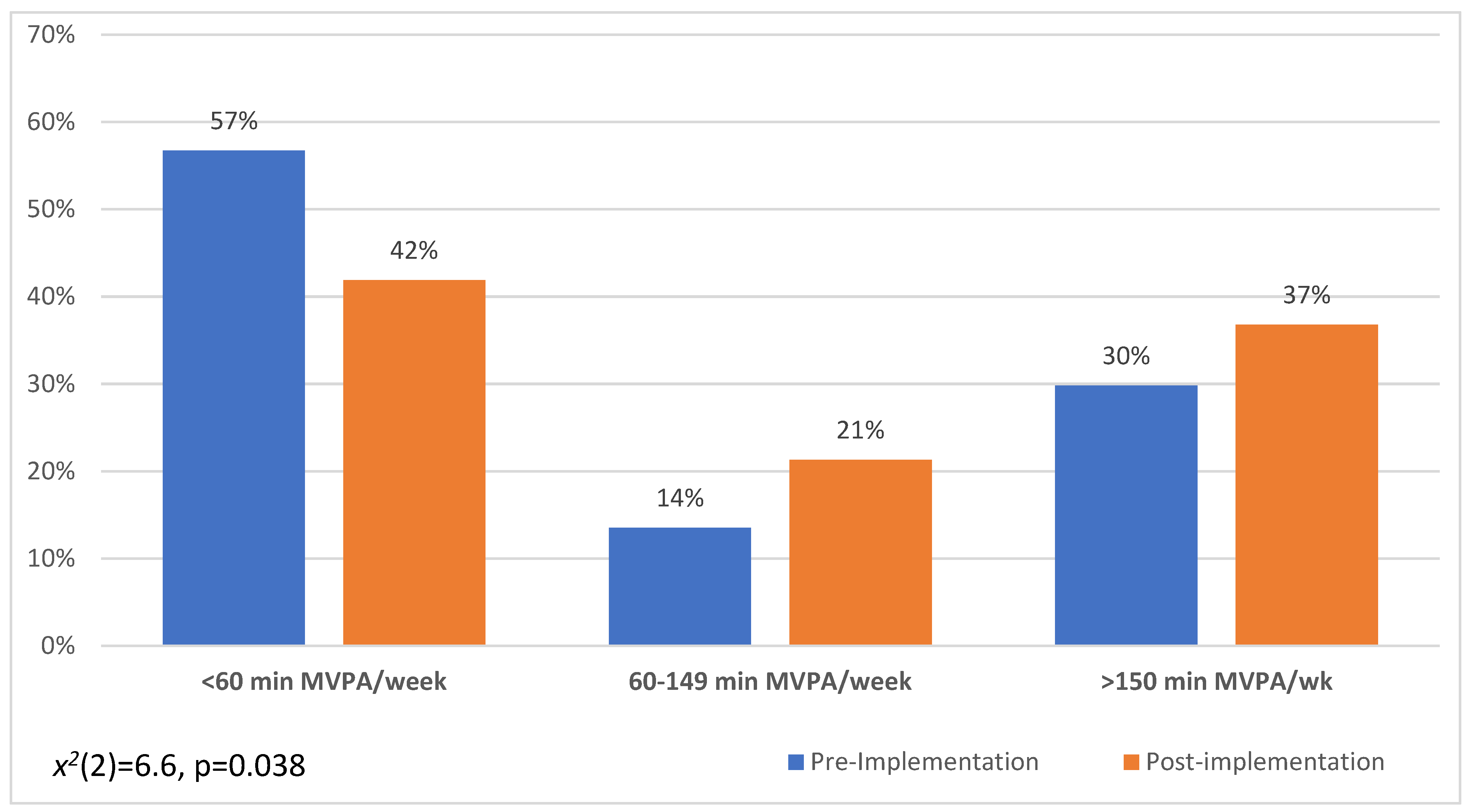
3.2. Healthy Bone Behaviours
3.3. Osteoporosis Knowledge and Health Beliefs
3.4. Satisfaction with BoneRx
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, M.V.; Grossfeld, G.D.; Sadetsky, N.; Mehta, S.S.; Lubeck, D.P.; Carroll, P.R. Contemporary patterns of androgen deprivation therapy use for newly diagnosed prostate cancer. Urology 2002, 60, 7–11. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.-F.; Freeman, J.L.; Orihuela, E.; Goodwin, J.S. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer 2005, 103, 1615–1624. [Google Scholar] [CrossRef]
- Gilbert, S.; Kuo, Y.; Shahinian, V. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol. Oncol. 2011, 29, 647–653. [Google Scholar] [CrossRef]
- Schröder, F.H.; Kurth, K.-H.; Fossa, S.D.; Hoekstra, W.; Karthaus, P.P.; De Prijck, L.; Collette, L. Early versus Delayed Endocrine Treatment of T2-T3 pN1-3 M0 Prostate Cancer without Local Treatment of the Primary Tumour: Final Results of European Organisation for the Research and Treatment of Cancer Protocol 30846 after 13 Years of Follow-up (a Randomised Controlled Trial). Eur. Urol. 2009, 55, 14–22. [Google Scholar] [CrossRef]
- Kawakami, J.; Cowan, J.E.; Elkin, E.P.; Latini, D.M.; DuChane, J.; Carroll, P.R. Androgen-deprivation therapy as primary treatment for localized prostate cancer: Data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE). Cancer 2006, 106, 1708–1714. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Bae, K.; Hanks, G.E.; Porter, A.; Grignon, D.J.; Brereton, H.D.; Venkatesan, V.; Lawton, C.A.; Rosenthal, S.A.; Sandler, H.M.; et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J. Clin. Oncol. 2008, 26, 2497–2504. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Chen, M.H.; Renshaw, A.A.; Loffredo, M.; Kantoff, P.W. Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA 2008, 299, 289–295. [Google Scholar] [CrossRef]
- DiBlasio, C.J.; Malcolm, J.B.; Hammett, J.; Wan, J.Y.; Aleman, M.A.; Patterson, A.L.; Wake, R.W.; Derweesh, I.H. Survival outcomes in men receiving androgen-deprivation therapy as primary or salvage treatment for localized or advanced prostate cancer: 20-year single-centre experience. BJU Int. 2009, 104, 1208–1214. [Google Scholar] [CrossRef]
- Pagliarulo, V.; Bracarda, S.; Eisenberger, M.A.; Mottet, N.; Schröder, F.H.; Sternberg, C.N.; Studer, U.E. Contemporary role of androgen deprivation therapy for prostate cancer. Eur. Urol. 2012, 61, 11–25. [Google Scholar] [CrossRef]
- Wilt, T.J.; MacDonald, R.; Rutks, I.; Shamliyan, T.A.; Taylor, B.; Kane, R.L. Systematic review: Comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann. Intern. Med. 2008, 148, 435–448. [Google Scholar] [CrossRef]
- Taylor, L.G.; Canfield, S.E.; Du, X.L. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer 2009, 115, 2388–2399. [Google Scholar] [CrossRef]
- Hershman, D.L.; Unger, J.M.; Wright, J.D.; Ramsey, S.D.; Till, C.; Tangen, C.M.; Barlow, W.E.; Blanke, C.D.; Thompson, I.M.; Hussain, M. Adverse Health Events Following Intermittent and Continuous Androgen Deprivation in Patients with Metastatic Prostate Cancer. JAMA Oncol. 2016, 2, 453–461. [Google Scholar] [CrossRef]
- Morgans, A.K.; Higano, C.S. Back to Basics: Addressing Bone Health in Men with Prostate Cancer on Androgen Deprivation Therapy. Eur. Urol. Oncol. 2019, 2, 562–564. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.Y.; Kim, K.J.; Hong, N.; Kim, J.W.; Hah, Y.S.; Koo, K.C.; Kim, J.H.; Cho, K.S. Effect of Androgen-Deprivation Therapy on Bone Mineral Density in Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 113. [Google Scholar] [CrossRef]
- Gralow, J.R.; Biermann, J.S.; Farooki, A.; Fornier, M.N.; Gagel, R.F.; Kumar, R.; Litsas, G.; McKay, R.; Podoloff, D.A.; Srinivas, S.; et al. NCCN Task Force Report: Bone Health in Cancer Care. J. Natl. Compr. Cancer Netw. 2013, 11 (Suppl. 3), S1–S50; quiz S51. [Google Scholar] [CrossRef]
- Thorstenson, A.; Bratt, O.; Akre, O.; Hellborg, H.; Holmberg, L.; Lambe, M.; Bill-Axelson, A.; Stattin, P.; Adolfsson, J. Incidence of fractures causing hospitalisation in prostate cancer patients: Results from the population-based PCBaSe Sweden. Eur. J. Cancer 2012, 48, 1672–1681. [Google Scholar] [CrossRef]
- Greenspan, S.L.; Nelson, J.B.; Trump, D.L.; Wagner, J.M.; Miller, M.E.; Perera, S.; Resnick, N.M. Skeletal Health After Continuation, Withdrawal, or Delay of Alendronate in Men with Prostate Cancer Undergoing Androgen-Deprivation Therapy. J. Clin. Oncol. 2008, 26, 4426–4434. [Google Scholar] [CrossRef]
- Lattouf, J.B.; Saad, F. Bone complications of androgen deprivation therapy: Screening, prevention, and treatment. Curr. Opin. Urol. 2010, 20, 247–252. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.F.; Freeman, J.L.; Goodwin, J.S. Risk of fracture after androgen deprivation for prostate cancer. N. Engl. J. Med. 2005, 352, 154–164. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Duong-Hua, M.; Cheung, A.M.; Sutradhar, R.; Warde, P.; Fleshner, N.E.; Paszat, L. Fracture types and risk factors in men with prostate cancer on androgen deprivation therapy: A matched cohort study of 19,079 men. J. Urol. 2010, 184, 918–923. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Mohamedali, H.Z.; Gulamhusein, H.; Panju, A.H.; Breunis, H.; Timilshina, N.; Fleshner, N.; Krahn, M.D.; Naglie, G.; Tannock, I.F.; et al. Changes in bone mineral density in men starting androgen deprivation therapy and the protective role of vitamin D. Osteoporos. Int. 2013, 24, 2571–2579. [Google Scholar] [CrossRef]
- Wang, A.; Obertová, Z.; Brown, C.; Karunasinghe, N.; Bishop, K.; Ferguson, L.; Lawrenson, R. Risk of fracture in men with prostate cancer on androgen deprivation therapy: A population-based cohort study in New Zealand. BMC Cancer 2015, 15, 837. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, P.Y.; Wang, S.W.; Tsai, M.H.; Wang, Y.C.L.; Tai, C.L.; Luo, H.L.; Wang, H.-J.; Chen, C.Y. Risk of Fracture During Androgen Deprivation Therapy Among Patients with Prostate Cancer: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Pharmacol. 2021, 12, 652979. [Google Scholar] [CrossRef]
- Shao, Y.H.; Moore, D.F.; Shih, W.; Lin, Y.; Jang, T.L.; Lu-Yao, G.L. Fracture after androgen deprivation therapy among men with a high baseline risk of skeletal complications. BJU Int. 2013, 111, 745. [Google Scholar] [CrossRef]
- Fink, H.A.; Ensrud, K.E.; Nelson, D.B.; Schreiner, P.J.; Zhao, Y.; Cummings, S.R.; Nevitt, M.C. Disability after clinical fracture in postmenopausal women with low bone density: The fracture intervention trial (FIT). Osteoporos. Int. 2003, 14, 69. [Google Scholar] [CrossRef]
- Borhan, S.; Papaioannou, A.; Gajic-Veljanoski, O.; Kennedy, C.; Ioannidis, G.; Berger, C.; Goltzman, D.; Josse, R.; Kovacs, C.S.; Hanley, D.A.; et al. Incident Fragility Fractures Have a Long-Term Negative Impact on Health-Related Quality of Life of Older People: The Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 2019, 34, 838–848. [Google Scholar] [CrossRef]
- Oefelein, M.G.; Ricchiuti, V.; Conrad, W.; Resnick, M.I. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J. Urol. 2002, 168, 1005–1007. [Google Scholar] [CrossRef]
- Lavallée, L.T.; McLarty, R.; Tran, C.; Breauj, R.H.; Richard, P.; Shayegan, B.; Danielson, B.; Jammal, M.-P.; Saad, F. Canadian Urologic Association best practice report: Bone health in prostate cancer. Can. Urol. Assoc. J. 2021, 15, 375. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Brown, J.E.; Handforth, C.; Compston, J.E.; Cross, W.; Parr, N.; Selby, P.; Wood, S.; Drudge-Coates, L.; Walsh, J.S.; Mitchell, C.; et al. Guidance for the assessment and management of prostate cancer treatment-induced bone loss. A consensus position statement from an expert group. J. Bone Oncol. 2020, 25, 100311. [Google Scholar] [CrossRef]
- Saylor, P.J.; Rumble, R.B.; Tagawa, S.; Eastham, A.; Finelli, A.; Reddy, P.S. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a Cancer Care Ontario Guideline. J. Clin. Oncol. 2020, 38, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Berruti, A.; Di Maio, M.; Procopio, G.; Bracarda, S.; Ibrahim, T.; Bertoldo, F. Bone health management in the continuum of prostate cancer disease: A review of the evidence with an expert panel opinion. ESMO Open 2020, 5, e000652, Erratum in ESMO Open 2020, 5, e000652corr1. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Staehelin, H.B.; Orav, J.E.; Stuck, A.E.; Theiler, R.O.; Wong, J.B.; Egli, A.N.; Kiel, D.P.; Henschkowski, J. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. BMJ 2009, 339, b3692. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Whitney, J.C.; Lord, S.R.; Herbert, R.D.; Cumming, R.G.; Close, J.C. Effective exercise for the prevention of falls: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2008, 56, 2234. [Google Scholar] [CrossRef]
- Lewiecki, E.; Binkley, N.; Clark, P.; Kim, S.; Leslie, W.; Morin, S. Core principles for fracture prevention: North American Consensus from the National Osteoporosis Foundation, Osteoporosis Canada, and Academia Nacional de Medicina de Mexico. Osteoporos. Int. 2020, 31, 2073–2076. [Google Scholar] [CrossRef] [PubMed]
- Owen, P.; Daly, R.M.; Livingston, P.M.; Fraser, S.F. Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: An update. Prostate Cancer Prostatic Dis. 2017, 20, 137–145. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Yun, L.; Cheung, A.M.; Paszat, L. Screening for osteoporosis in men receiving androgen deprivation therapy. JAMA 2012, 307, 255–256. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.-F. Patterns of Bone Mineral Density Testing in Men Receiving Androgen Deprivation for Prostate Cancer. J. Gen. Intern. Med. 2013, 28, 1440–1446. [Google Scholar] [CrossRef]
- Morgans, A.K.; Smith, M.R.; O’Malley, A.J.; Keating, N.L. Bone density testing among prostate cancer survivors treated with androgen-deprivation therapy. Cancer 2012, 119, 863–870. [Google Scholar] [CrossRef]
- Suarez-Almazor, M.E.; Peddi, P.; Luo, R.; Nguyen, H.T.; Elting, L.S. Low rates of bone mineral density measurement in Medicare beneficiaries with prostate cancer initiating androgen deprivation therapy. Support. Care Cancer 2013, 22, 537–544. [Google Scholar] [CrossRef]
- Kirk, P.S.; Borza, T.; Shahinian, V.B.; Caram, M.E.; Makarov, D.V.; Shelton, J.B.; Leppert, J.T.; Blake, R.M.; Davis, J.A.; Hollenbeck, B.K.; et al. The implications of baseline bone-health assessment at initiation of androgen-deprivation therapy for prostate cancer. Br. J. Urol. 2017, 121, 558–564. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Rahman, S.; Warde, P.R.; Jewett, M.A.; Jaffer, T.; Cheung, A.M. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: A survey of urologists and radiation oncologists. Urology 2006, 68, 126–131. [Google Scholar] [CrossRef]
- Damji, A.N.; Bies, K.; Alibhai, S.M.; Jones, J.M. Bone health management in men undergoing ADT: Examining enablers and barriers to care. Osteoporos. Int. 2014, 26, 951–959. [Google Scholar] [CrossRef]
- Nadler, M.; Alibhai, S.M.; Catton, P.; Catton, C.; To, M.J.; Jones, J.M. Osteoporosis knowledge, health beliefs, and healthy bone behaviours in patients on androgen-deprivation therapy (ADT) for prostate cancer. BJU Int. 2013, 111, 1301–1309. [Google Scholar] [CrossRef]
- Lassemillante, A.-C.M.; Skinner, T.L.; Hooper, J.D.; Prins, J.B.; Wright, O.R.L. Osteoporosis-Related Health Behaviors in Men with Prostate Cancer and Survivors: Exploring Osteoporosis Knowledge, Health Beliefs, and Self-Efficacy. Am. J. Men’s Health 2016, 11, 13–23. [Google Scholar] [CrossRef]
- Walker, L.M.; Tran, S.; Wassersug, R.J.; Thomas, B.; Robinson, J.W. Patients and partners lack knowledge of androgen deprivation therapy side effects. Urol. Oncol. 2012, 31, 1098–1105. [Google Scholar] [CrossRef]
- Giguère, A.; Zomahoun, H.T.V.; Carmichael, P.-H.; Uwizeye, C.B.; Légaré, F.; Grimshaw, J.M.; Gagnon, M.-P.; Auguste, D.U.; Massougbodji, J. Printed educational materials: Effects on professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2020, 2020, CD004398. [Google Scholar] [CrossRef]
- Pantoja, T.; Grimshaw, J.M.; Colomer, N.; Castañon, C.; Martelli, J.L. Manually-generated reminders delivered on paper: Effects on professional practice and patient outcomes. Cochrane Database Syst. Rev. 2019, 12, CD001174. [Google Scholar] [CrossRef]
- Fønhus, M.S.; Dalsbø, T.K.; Johansen, M.; Fretheim, A.; Skirbekk, H.; Flottorp, S.A. Patient-mediated interventions to improve professional practice. Cochrane Database Syst. Rev. 2018, 2018, CD012472. [Google Scholar] [CrossRef]
- Kastner, M.; Straus, S. Clinical decision support tools for osteoporosis disease management: A systematic review of randomized controlled trials. J. Gen. Intern. Med. 2008, 23, 2095–2105. [Google Scholar] [CrossRef]
- Nadler, M.; Alibhai, S.; Catton, P.; Catton, C.; Jones, J. The impact of bone mineral density testing, fracture assessment, and osteoporosis education in men treated by androgen deprivation for prostate cancer: A pilot study. Support. Care Cancer 2014, 22, 2409–2415. [Google Scholar] [CrossRef]
- Logan, J.; Graham, I.D. Toward a Comprehensive Interdisciplinary Model of Health Care Research Use. Sci. Commun. 1998, 20, 227–246. [Google Scholar] [CrossRef]
- Logan, J.; Graham, I. The Ottawa Model of Research Use. In Models and Frameworks for Implementing Evidence-Based Practice: Linking Evidence to Action; Rycroft-Malone, J., Bucknall, T., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 83–108. [Google Scholar]
- Ailinger, R.; Lasus, H.; Braun, M. Revision of the Facts on Osteoporosis Quiz. Nurs. Res. 2003, 52, 198–201. [Google Scholar] [CrossRef]
- Gaines, J.M.; Marx, K.A.; Narrett, M.; Caudill, J.; Landsman, J.; Parrish, J.M. Validation of the Male Osteoporosis Knowledge Quiz. Am. J. Men’s Health 2010, 5, 78–83. [Google Scholar] [CrossRef]
- Kim, K.K.; Horan, M.L.; Gendler, P.; Patel, M.K. Development and evaluation of the Osteoporosis Health Belief Scale. Res. Nurs. Health 1991, 14, 155–163. [Google Scholar] [CrossRef]
- Magkos, F.; Manios, Y.; Babaroutsi, E.; Sidossis, L.S. Development and validation of a food frequency questionnaire for assessing dietary calcium intake in the general population. Osteoporos. Int. 2006, 17, 304–312. [Google Scholar] [CrossRef]
- Hung, A.; Hamidi, M.; Riazantseva, E.; Thompson, L.; Tile, L.; Tomlinson, G.; Stewart, B.; Cheung, A.M. Validation of a calcium assessment tool in postmenopausal Canadian women. Maturitas 2011, 69, 168–172. [Google Scholar] [CrossRef]
- Godin, G. Godin Leisure-Time Exercise Questionnaire. Med. Sci. Sports Exerc. 1997, 29, S36–S38. [Google Scholar]
- Alibhai, S.M.; Breunis, H.; Mph, N.T.; Hamidi, M.S.; Cheung, A.M.; Tomlinson, G.; Manokumar, T.; Samadi, O.; Sandoval, J.; Durbano, S.; et al. Improving bone health in men with prostate cancer receiving androgen deprivation therapy: Results of a randomized phase 2 trial. Cancer 2017, 124, 1132–1140. [Google Scholar] [CrossRef]
- Tsang, D.S.; Jones, J.M.; Samadi, O.; Shah, S.; Mitsakakis, N.; Catton, C.N.; Jeon, W.; To, J.; Breunis, H.; Alibhai, S.M.H. Healthy Bones Study: Can a prescription coupled with education improve bone health for patients receiving androgen deprivation therapy?—A before/after study. Support. Care Cancer 2018, 26, 2861–2869. [Google Scholar] [CrossRef]
- Yuksel, N.; Majumdar, S.R.; Biggs, C.; Tsuyuki, R.T. Community pharmacist-initiated screening program for osteoporosis: Randomized controlled trial. Osteoporos. Int. 2010, 21, 391–398. [Google Scholar] [CrossRef]
- Grimshaw, J.M.; Thomas, R.E.; MacLennan, G.; Fraser, C.; Ramsay, C.R.; Vale, L.; Whitty, P.; Eccles, M.P.; Matowe, L.; Shirran, L.; et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol. Assess. 2004, 8, 1–7. [Google Scholar] [CrossRef]
- Beaudart, C.; Hiligsmann, M.; Li, N.; Lewiecki, E.M.; Silverman, S. Effective communication regarding risk of fracture for individuals at risk of fragility fracture: A scoping review. Osteoporos. Int. 2021, 33, 13–26. [Google Scholar] [CrossRef]
- Davison, B.J.; Wiens, K.; Cushing, M. Promoting calcium and vitamin D intake to reduce the risk of osteoporosis in men on androgen deprivation therapy for recurrent prostate cancer. Support. Care Cancer 2011, 20, 2287–2294. [Google Scholar] [CrossRef]
- Stull, V.B.; Snyder, D.C.; Demark-Wahnefried, W. Lifestyle Interventions in Cancer Survivors: Designing Programs That Meet the Needs of This Vulnerable and Growing Population. J. Nutr. 2007, 137 (Suppl. 1), 243S–248S. [Google Scholar] [CrossRef]
- Lawson, P.J.; Flocke, S.A. Teachable moments for health behavior change: A concept analysis. Patient Educ. Couns. 2009, 76, 25–30. [Google Scholar] [CrossRef]
- Ganz, P.A. A Teachable Moment for Oncologists: Cancer Survivors, 10 Million Strong and Growing! J. Clin. Oncol. 2005, 23, 5458–5460. [Google Scholar] [CrossRef]
- Ottenbacher, A.J.; Day, R.S.; Taylor, W.C.; Sharma, S.V.; Sloane, R.; Snyder, D.C.; Kraus, W.E.; Demark-Wahnefried, W. Exercise among breast and prostate cancer survivors—what are their barriers? J. Cancer Surviv. 2011, 5, 413–419. [Google Scholar] [CrossRef]
- Keogh, J.; Patel, A.; MacLeod, R.; Masters, J. Perceived barriers and facilitators to physical activity in men with prostate cancer: Possible influence of androgen deprivation therapy. Eur. J. Cancer Care 2013, 23, 263–273. [Google Scholar] [CrossRef]
- Craike, M.J.; Livingston, P.M.; Botti, M. An exploratory study of the factors that influence physical activity for prostate cancer survivors. Support. Care Cancer 2010, 19, 1019–1028. [Google Scholar] [CrossRef]
- Jones, L.W.; Courneya, K.S. Exercise Counseling and Programming Preferences of Cancer Survivors. Cancer Pract. 2002, 10, 208–215. [Google Scholar] [CrossRef]
- Park, K.-S.; Yoo, J.-I.; Kim, H.-Y.; Jang, S.; Park, Y.; Ha, Y.-C. Education and exercise program improves osteoporosis knowledge and changes calcium and vitamin D dietary intake in community dwelling elderly. BMC Public Health 2017, 17, 966. [Google Scholar] [CrossRef]
- Francis, K.; Matthews, B.L.; Van Mechelen, W.; Bennell, K.; Osborne, R.H. Effectiveness of a community-based osteoporosis education and self-management course: A wait list controlled trial. Osteoporos. Int. 2009, 20, 1563–1570. [Google Scholar] [CrossRef][Green Version]
- Nielsen, D.; Ryg, J.; Nielsen, W.; Knold, B.; Nissen, N.; Brixen, K. Patient education in groups increases knowledge of osteoporosis and adherence to treatment: A two-year randomized controlled trial. Patient Educ. Couns. 2010, 81, 155–160. [Google Scholar] [CrossRef]
- Gai, Q.Y.; Lv, H.; Li, Y.P.; Fu, Q.M.; Li, P. Education intervention for older adults with osteoporosis: A systematic review. Osteoporos. Int. 2019, 31, 625–635. [Google Scholar] [CrossRef]
- Andersen, R.E.; Blair, S.N.; Cheskin, L.J.; Bartlett, S. Encouraging Patients To Become More Physically Active: The Physician’s Role. Ann. Intern. Med. 1997, 127, 395–400. [Google Scholar] [CrossRef]
- Jepson, R.G.; Harris, F.M.; Platt, S.; Tannahill, C. The effectiveness of interventions to change six health behaviours: A review of reviews. BMC Public Health 2010, 10, 538. [Google Scholar] [CrossRef]
- Sedlak, C.A.; Doheny, M.O.; Estok, P.J.; Zeller, R.A.; Winchell, J. DXA, Health Beliefs, and Osteoporosis Prevention Behaviors. J. Aging Health 2007, 19, 742–756. [Google Scholar] [CrossRef]
| Pre-BoneRx Cohort (n = 143) | Post BoneRx Cohort (n = 149) | p-Value | |
|---|---|---|---|
| Age (mean/SD), y | 70.7 (±9.1) | 70.4 (±8.5) | 0.83 |
| Marital Status | |||
| Married/Common Law | 102 (71%) | 115 (77%) | |
| Single/Divorced/Widowed | 42 (29%) | 34 (23%) | 0.22 |
| Language | |||
| English | 127 (92%) | 140 (94%) | |
| Other | 11 (8%) | 9 (6%) | 0.52 |
| Employment | |||
| Employed (full, part, self) | 42 (29%) | 53 (35%) | |
| Retired | 94 (65%) | 92 (62%) | |
| Disability-leave, unemployed | 8 (6%) | 4 (3%) | 0.28 |
| Education | |||
| High school | 58 (41%) | 39 (26%) | |
| College/University | 57 (40%) | 71 (48%) | |
| Post-graduate/Professional | 27 (19%) | 39 (26%) | 0.03 |
| Treatment received a | |||
| Hormone therapy | 143 (100%) | 149 (100%) | |
| Surgery | 53 (39%) | 61 (41%) | 0.69 |
| Radiotherapy | 75 (55%) | 93 (62%) | 0.19 |
| Chemotherapy | 2 (2%) | 5 (3%) | 0.30 |
| Fracture Risk | |||
| Fracture after age 50 b | 15 (11%) | 13 (9%) | 0.62 |
| Fall in last 12 months | 31 (22%) | 21 (14%) | 0.05 |
| Taken oral steroid medication | 11 (8%) | 20 (14%) | 0.09 |
| Outcome | Pre-Intervention (Mean ± sd) | Post-Intervention (Mean ± sd) | p-Value |
|---|---|---|---|
| Osteoporosis Knowledge Score a | 10.9 ± 3.6 (n = 142) | 10.7 ± 3.8 (n = 147) | 0.52 |
| Osteoporosis Health Belief Scale b (OHBS) Score | |||
| Susceptibility | 17.1 ± 4.6 (n = 138) | 16.7 ± 4.6 (n = 133) | 0.41 |
| Seriousness | 17.5 ± 4.5 (n = 137) | 17.4 ± 4.1 (n = 134) | 0.79 |
| Motivation | 23.9 ± 4.0 (n = 136) | 23.5 ± 3.2 (n = 141) | 0.40 |
| Total | 58.6 ± 7.8 (n = 135) | 57.4 ± 7.9 (n = 126) | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, J.M.; Tsang, D.S.; Zheng, S.; Yeheskel, A.; Catton, C.N.; Cheung, A.M.; Hamilton, R.; Alibhai, S.M.H. Implementing and Evaluating the Impact of BoneRx: A Healthy Bone Prescription for Men with Prostate Cancer Initiating Androgen Deprivation Therapy. J. Clin. Med. 2022, 11, 2703. https://doi.org/10.3390/jcm11102703
Jones JM, Tsang DS, Zheng S, Yeheskel A, Catton CN, Cheung AM, Hamilton R, Alibhai SMH. Implementing and Evaluating the Impact of BoneRx: A Healthy Bone Prescription for Men with Prostate Cancer Initiating Androgen Deprivation Therapy. Journal of Clinical Medicine. 2022; 11(10):2703. https://doi.org/10.3390/jcm11102703
Chicago/Turabian StyleJones, Jennifer M., Derek S. Tsang, Shiyu Zheng, Ariel Yeheskel, Charles N. Catton, Angela M. Cheung, Robert Hamilton, and Shabbir M. H. Alibhai. 2022. "Implementing and Evaluating the Impact of BoneRx: A Healthy Bone Prescription for Men with Prostate Cancer Initiating Androgen Deprivation Therapy" Journal of Clinical Medicine 11, no. 10: 2703. https://doi.org/10.3390/jcm11102703
APA StyleJones, J. M., Tsang, D. S., Zheng, S., Yeheskel, A., Catton, C. N., Cheung, A. M., Hamilton, R., & Alibhai, S. M. H. (2022). Implementing and Evaluating the Impact of BoneRx: A Healthy Bone Prescription for Men with Prostate Cancer Initiating Androgen Deprivation Therapy. Journal of Clinical Medicine, 11(10), 2703. https://doi.org/10.3390/jcm11102703







