Pulsatile Lavage Systems with High Impact Pressure and High Flow Produce Cleaner Cancellous Bone Prior to Cementation in Cemented Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Pressure Pulsatile Lavage Systems
2.2. Cleaning Parameters
2.3. Cancellous Bone/Carbon Foam Cleaning Effect
2.4. Statistic
3. Results
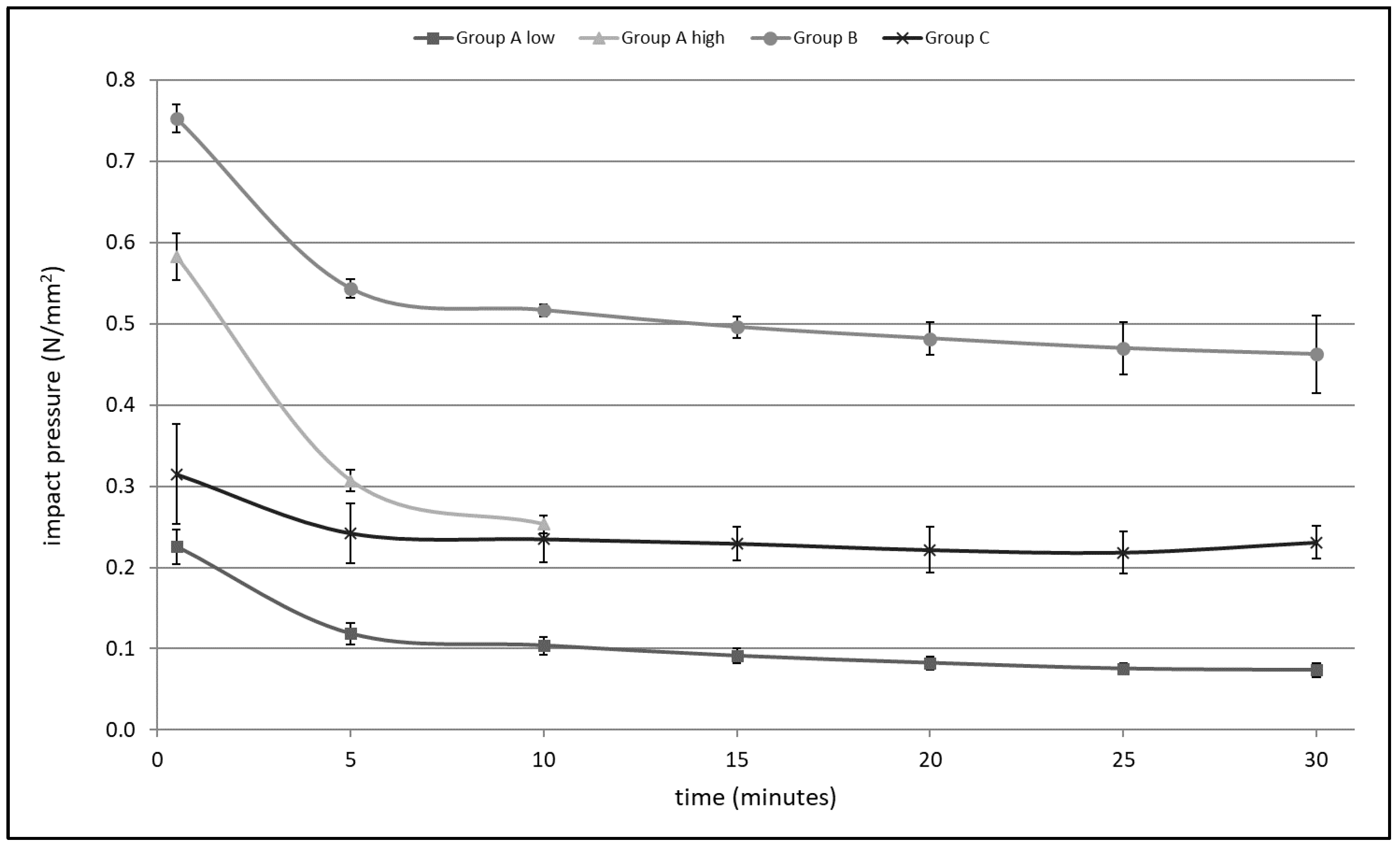
3.1. Impact Pressure
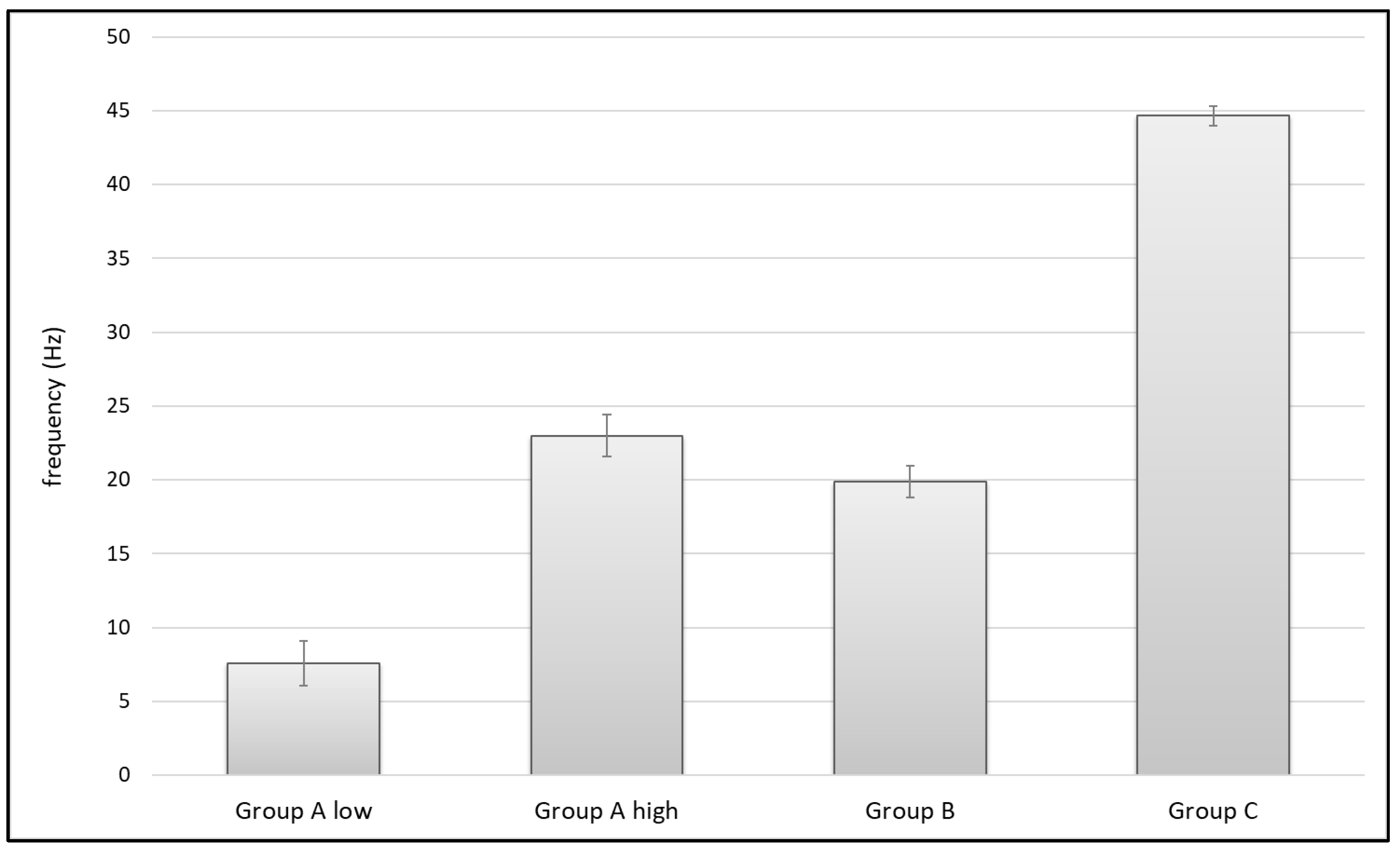
3.2. Pulse Frequency
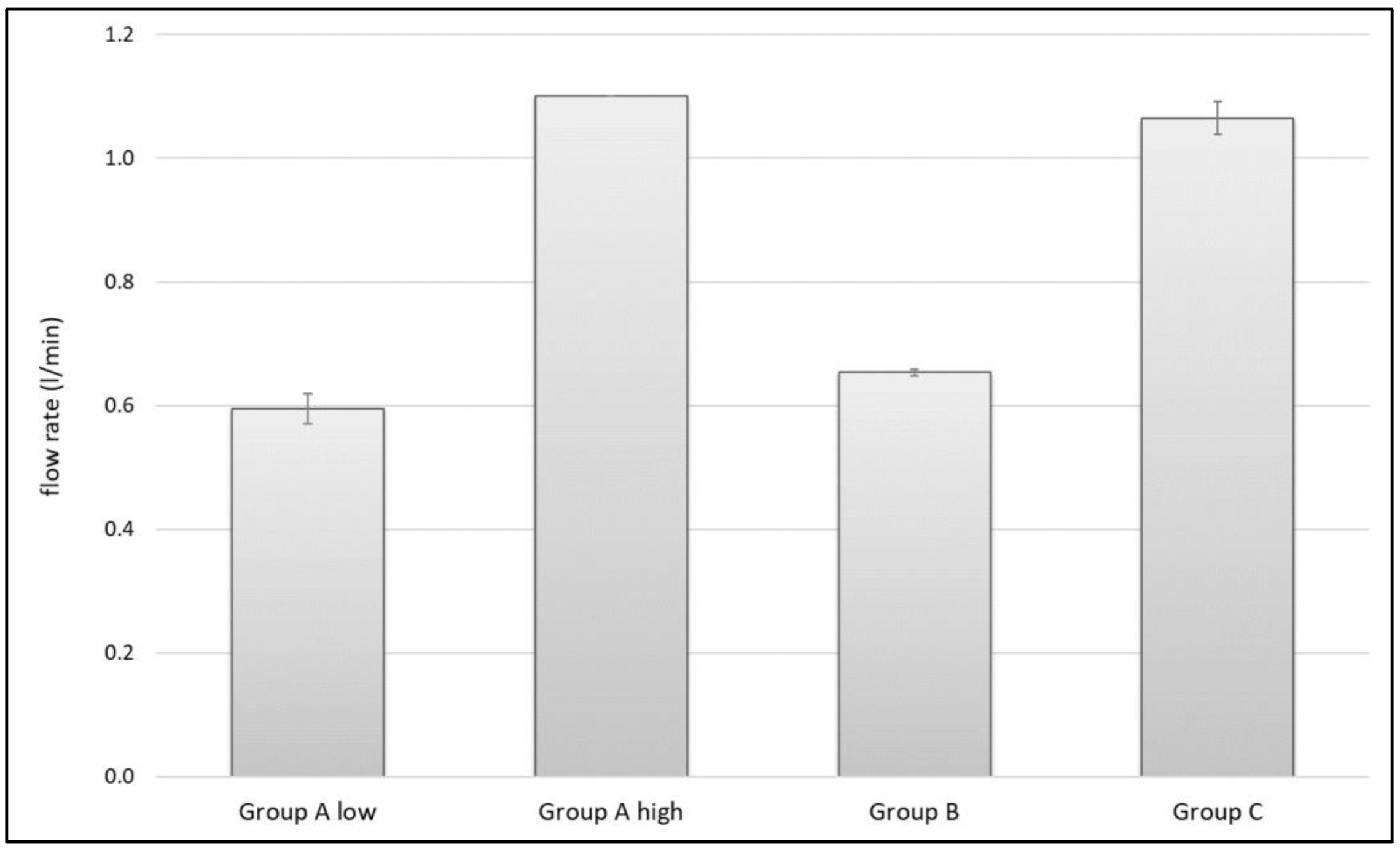
3.3. Flow Rate
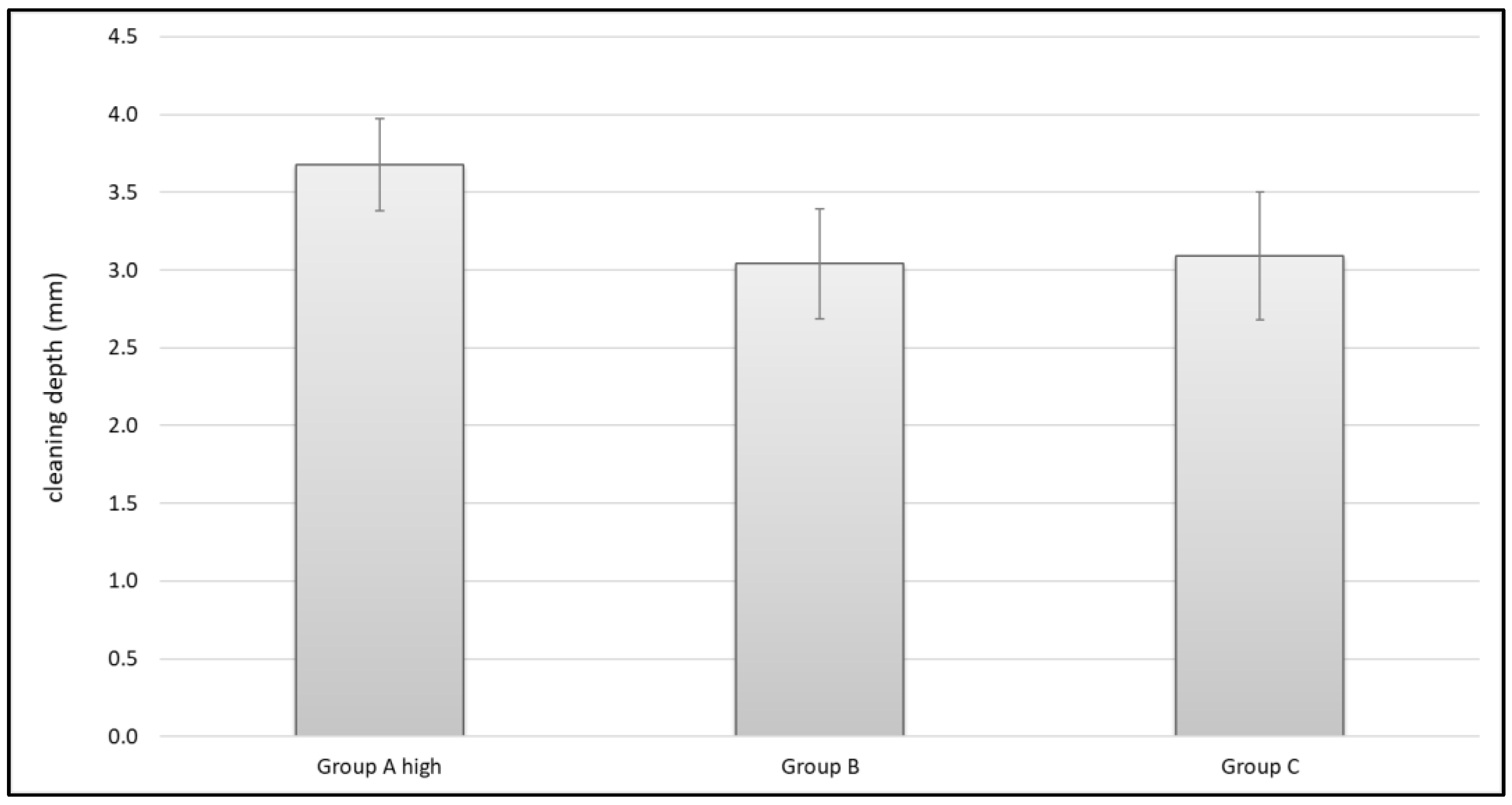
3.4. Cleaning Effects in Carbon Specimens
4. Discussion
4.1. Impact Pressure
4.2. Pulse Frequency
4.3. Flow Rate
4.4. Cleaning Effects in Carbon Specimens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritter, M.A.; Keating, E.M.; Sueyoshi, T.; Davis, K.E.; Barrington, J.W.; Emerson, R.H. Twenty-Five-Years and Greater, Results After Nonmodular Cemented Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Herberts, P.; Malchau, H. Long-term registration has improved the quality of hip replacement: A review of the Swedish THR Register comparing 160,000 cases. Acta Orthop. Scand. 2000, 71, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malchau, H.; Herberts, P.; Eisler, T.; Garellick, G.; Soderman, P. The Swedish Total Hip Replacement Register. J. Bone Jt. Surg. Am. 2002, 84 (Suppl. 2), 2–20. [Google Scholar] [CrossRef]
- Bunyoz, K.I.; Malchau, E.; Malchau, H.; Troelsen, A. Has the Use of Fixation Techniques in THA Changed in This Decade? The Uncemented Paradox Revisited. Clin. Orthop. Relat. Res. 2020, 478, 697–704. [Google Scholar] [CrossRef]
- German Arthroplasty Registry (EPRD)—2019 Annual Report. 2019. Available online: https://www.eprd.de/fileadmin/user_upload/Dateien/Publikationen/Berichte/EPRD_Jahresbericht_2019_EN_doppelseitig_F_Web-Stand-251120.pdf (accessed on 22 December 2021). [CrossRef]
- NZOA. The New Zealand Joint Registry—Fifteen Year Report January 1999 to December 2013; NZOA: Wellington, New Zealand, 2014; Volume 15. [Google Scholar]
- Gademan, M.G.J.; van Steenbergen, L.N.; Cannegieter, S.C.; Nelissen, R.; Marang-van de Mheen, P.J. Population-based 10-year cumulative revision risks after hip and knee arthroplasty for osteoarthritis to inform patients in clinical practice: A competing risk analysis from the Dutch Arthroplasty Register. Acta Orthop. 2021, 92, 280–284. [Google Scholar] [CrossRef]
- Lim, H.A.; Song, E.K.; Seon, J.K.; Park, K.S.; Shin, Y.J.; Yang, H.Y. Causes of Aseptic Persistent Pain after Total Knee Arthroplasty. Clin. Orthop. Surg. 2017, 9, 50–56. [Google Scholar] [CrossRef]
- Refsum, A.M.; Nguyen, U.V.; Gjertsen, J.E.; Espehaug, B.; Fenstad, A.M.; Lein, R.K.; Ellison, P.; Hol, P.J.; Furnes, O. Cementing technique for primary knee arthroplasty: A scoping review. Acta Orthop. 2019, 90, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, M.; Solomon, B.L.; Viscopoleanu, G.; Antoniac, I.V. Evolution of Cementation Techniques and Bone Cements in Hip Arthroplasty. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; pp. 859–899. [Google Scholar] [CrossRef]
- Khan, M.; Osman, K.; Green, G.; Haddad, F.S. The epidemiology of failure in total knee arthroplasty: Avoiding your next revision. Bone Jt. J. 2016, 98, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Kochbati, R.; Rbai, H.; Jlailia, M.; Makhlouf, H.; Bouguira, A.; Daghfous, M.S. Predictive factors of aseptic loosening of cemented total hip prostheses. Pan. Afr. Med. J. 2016, 24, 260. [Google Scholar] [CrossRef]
- Clarius, M.; Seeger, J.B.; Jaeger, S.; Mohr, G.; Bitsch, R.G. The importance of pulsed lavage on interface temperature and ligament tension force in cemented unicompartmental knee arthroplasty. Clin. Biomech. 2012, 27, 372–376. [Google Scholar] [CrossRef]
- Helwig, P.; Konstantinidis, L.; Hirschmuller, A.; Miltenberger, V.; Kuminack, K.; Sudkamp, N.P.; Hauschild, O. Tibial cleaning method for cemented total knee arthroplasty: An experimental study. Indian J. Orthop. 2013, 47, 18–22. [Google Scholar] [CrossRef]
- Breusch, S.J.; Schneider, U.; Reitzel, T.; Kreutzer, J.; Ewerbeck, V.; Lukoschek, M. Significance of jet lavage for in vitro and in vivo cement penetration. Zeitschrift fur Orthopadie und ihre Grenzgebiete 2001, 139, 52–63. [Google Scholar] [CrossRef]
- Jaeger, S.; Seeger, J.B.; Schuld, C.; Bitsch, R.G.; Clarius, M. Tibial cementing in UKA: A three-dimensional analysis of the bone cement implant interface and the effect of bone lavage. J. Arthroplast. 2013, 28, 191–194. [Google Scholar] [CrossRef]
- Maistrelli, G.L.; Antonelli, L.; Fornasier, V.; Mahomed, N. Cement penetration with pulsed lavage versus syringe irrigation in total knee arthroplasty. Clin. Orthop. Relat. Res. 1995, 312, 261–265. [Google Scholar]
- Jaeger, S.; Rieger, J.S.; Bruckner, T.; Kretzer, J.P.; Clarius, M.; Bitsch, R.G. The protective effect of pulsed lavage against implant subsidence and micromotion for cemented tibial unicompartmental knee components: An experimental cadaver study. J. Arthroplast. 2014, 29, 727–732. [Google Scholar] [CrossRef]
- Clarius, M.; Hauck, C.; Seeger, J.B.; James, A.; Murray, D.W.; Aldinger, P.R. Pulsed lavage reduces the incidence of radiolucent lines under the tibial tray of Oxford unicompartmental knee arthroplasty: Pulsed lavage versus syringe lavage. Int. Orthop. 2009, 33, 1585–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waanders, D.; Janssen, D.; Mann, K.A.; Verdonschot, N. The mechanical effects of different levels of cement penetration at the cement-bone interface. J. Biomech. 2010, 43, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, U.J.; Siewe, J.; Delank, K.S.; Eysel, P.; Puschel, K.; Morlock, M.M.; de Uhlenbrock, A.G. Pulsed lavage improves fixation strength of cemented tibial components. Int. Orthop. 2011, 35, 1165–1169. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, U.J.; Puschel, K.; Morlock, M.M.; Nagel, K. An in vitro comparison of tibial tray cementation using gun pressurization or pulsed lavage. Int. Orthop. 2014, 38, 967–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, J.B.; Gie, G.A.; Lee, A.J.; Ling, R.S.; Volz, R.G. Cementing technique and the effects of bleeding. J. Bone Jt. Surg. Br. 1987, 69, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Vanlommel, J.; Luyckx, J.P.; Labey, L.; Innocenti, B.; de Corte, R.; Bellemans, J. Cementing the tibial component in total knee arthroplasty: Which technique is the best? J. Arthroplast. 2011, 26, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Cawley, D.T.; Kelly, N.; McGarry, J.P.; Shannon, F.J. Cementing techniques for the tibial component in primary total knee replacement. Bone Jt. J. 2013, 95, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Hampton, C.B.; Berliner, Z.P.; Nguyen, J.T.; Mendez, L.; Smith, S.S.; Joseph, A.D.; Padgett, D.E.; Rodriguez, J.A. Aseptic Loosening at the Tibia in Total Knee Arthroplasty: A Function of Cement Mantle Quality? J. Arthroplast. 2020, 35, S190–S196. [Google Scholar] [CrossRef]
- Sih, G.C.; Connelly, G.M.; Berman, A.T. The effect of thickness and pressure on the curing of PMMA bone cement for the total hip joint replacement. J. Biomech. 1980, 13, 347–352. [Google Scholar] [CrossRef]
- Gross, A.; Cutright, D.E.; Bhaskar, S.N. Effectiveness of pulsating water jet lavage in treatment of contaminated crushed wounds. Am. J. Surg. 1972, 124, 373–377. [Google Scholar] [CrossRef]
- Wheeler, C.B.; Rodeheaver, G.T.; Thacker, J.G.; Edgerton, M.T.; Edilich, R.F. Side-effects of high pressure irrigation. Surg. Gynecol. Obstet. 1976, 143, 775–778. [Google Scholar] [CrossRef]
- Rodeheaver, G.T.; Pettry, D.; Thacker, J.G.; Edgerton, M.T.; Edlich, R.F. Wound cleansing by high pressure irrigation. Surg. Gynecol. Obstet. 1975, 141, 357–362. [Google Scholar] [CrossRef]
- Gross, A.; Bhaskar, S.N.; Cutright, D.E.; Beasley, J.D., 3rd; Perez, B. The effect of pulsating water jet lavage on experimental contaminated wounds. J. Oral Surg. 1971, 29, 187–190. [Google Scholar]
- Luedtke-Hoffmann, K.A.; Schafer, D.S. Pulsed lavage in wound cleansing. Phys. Ther. 2000, 80, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Kalteis, T.; Pforringer, D.; Herold, T.; Handel, M.; Renkawitz, T.; Plitz, W. An experimental comparison of different devices for pulsatile high-pressure lavage and their relevance to cement intrusion into cancellous bone. Arch. Orthop. Trauma Surg. 2007, 127, 873–877. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bitsch, R.G.; Loidolt, T.; Heisel, C.; Schmalzried, T.P. Cementing techniques for hip resurfacing arthroplasty: Development of a laboratory model. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. 3), 102–110. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, S.; Rieger, J.S.; Obermeyer, B.; Klotz, M.C.; Kretzer, J.P.; Bitsch, R.G. Cement applicator use for hip resurfacing arthroplasty. Med. Eng. Phys. 2015, 37, 447–452. [Google Scholar] [CrossRef]
- Knappe, K.; Stadler, C.; Innmann, M.; Schonhoff, M.; Gotterbarm, T.; Renkawitz, T.; Jaeger, S. Does Pressurized Carbon Dioxide Lavage Improve Bone Cleaning in Cemented Arthroplasty? Appl. Sci. 2021, 11, 6103. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Terbush, M.J.; Goodheart, J.R.; Izant, T.H.; Mann, K.A. Increased initial cement-bone interlock correlates with reduced total knee arthroplasty micro-motion following in vivo service. J. Biomech. 2014, 47, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Buller, L.T.; Rao, V.; Chiu, Y.F.; Nam, D.; McLawhorn, A.S. Primary Total Knee Arthroplasty Performed Using High-Viscosity Cement is Associated with Higher Odds of Revision for Aseptic Loosening. J. Arthroplast. 2020, 35, S182–S189. [Google Scholar] [CrossRef]
- Koh, B.T.; Tan, J.H.; Ramruttun, A.K.; Wang, W. Effect of storage temperature and equilibration time on polymethyl methacrylate (PMMA) bone cement polymerization in joint replacement surgery. J. Orthop. Surg. Res. 2015, 10, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.; Holder, G.; Desoutter, G. The measurement and comparison of jet characteristics of surgical pulse lavage devices. J. Arthroplast. 2003, 18, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.A.; Ayers, D.C.; Werner, F.W.; Nicoletta, R.J.; Fortino, M.D. Tensile strength of the cement-bone interface depends on the amount of bone interdigitated with PMMA cement. J. Biomech. 1997, 30, 339–346. [Google Scholar] [CrossRef]
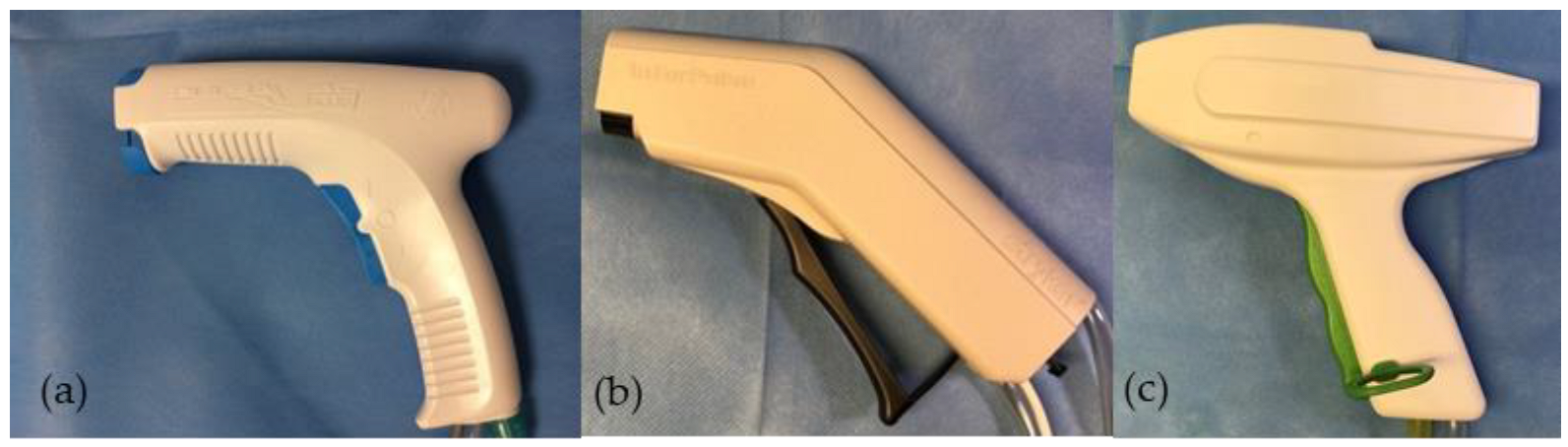

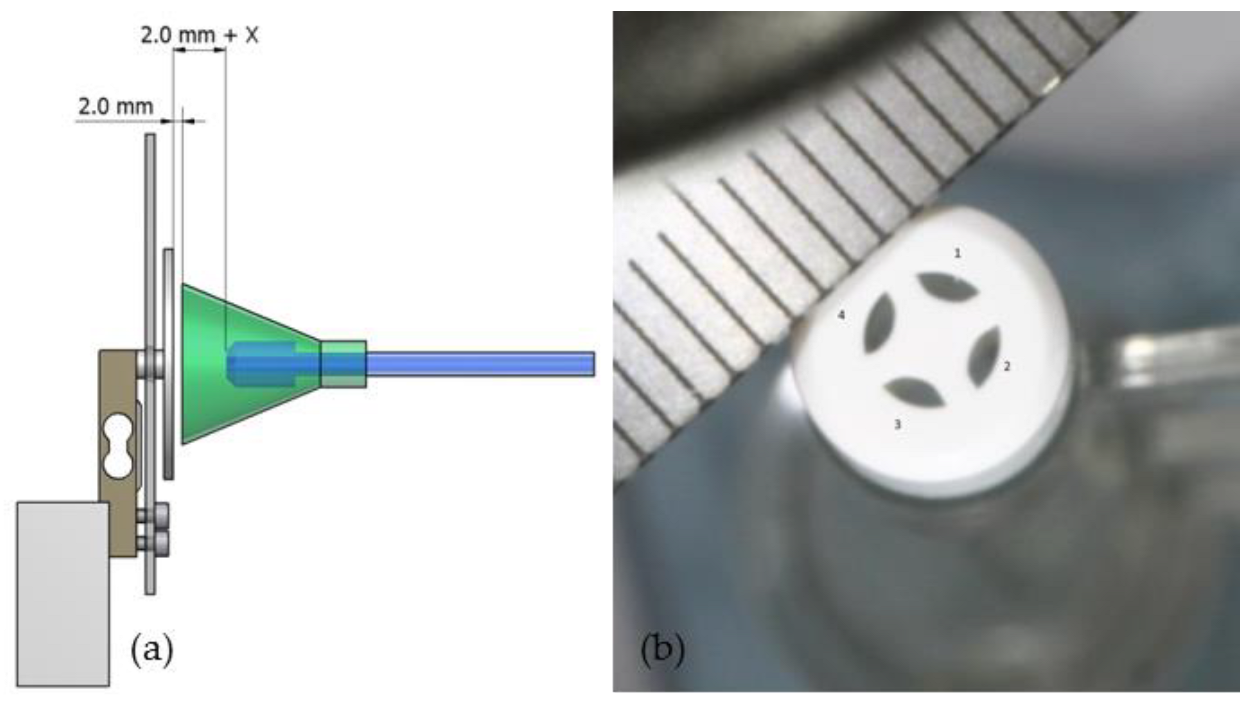



| Lavage System | Distance (2.0 mm + X) | Area Nozzle Orifice |
|---|---|---|
| Group A | 22 mm | 2.1 mm2 |
| Group B | 17 mm | 1.0 mm2 |
| Group C | 18 mm | 4.1 mm2 |
| Lavage System | p-Value 0.5 min | p-Value 5 min | p-Value 10 min | p-Value 15 min | p-Value 20 min | p-Value 25 min | p-Value 30 min |
|---|---|---|---|---|---|---|---|
| Group A low—A high | <0.001 | <0.001 | <0.001 | * | * | * | * |
| Group A low—B | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Group A low—C | <0.037 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Group A high—B | 0.001 | <0.001 | <0.001 | * | * | * | * |
| Group A high—C | <0.001 | 0.009 | 1.0 | * | * | * | * |
| Group B—C | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 30 s | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | Mean | |
|---|---|---|---|---|---|---|---|---|
| Group A low | 0.23 | 0.12 | 0.10 | 0.09 | 0.08 | 0.08 | 0.07 | 0.11 ± 0.01 |
| Group A high | 0.58 | 0.31 | 0.25 | defect | defect | defect | defect | 0.38 ± 0.02 |
| Group B | 0.75 | 0.54 | 0.52 | 0.50 | 0.48 | 0.47 | 0.46 | 0.53 ± 0.02 |
| Group C | 0.31 | 0.24 | 0.23 | 0.23 | 0.22 | 0.22 | 0.23 | 0.24 ± 0.03 |
| 30 s | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | Mean | |
|---|---|---|---|---|---|---|---|---|
| Group A low | 10 | 9 | 8 | 7 | 6 | 7 | 6 | 7.57 ± 1.51 |
| Group A high | 25 | 22 | defect | defect | defect | defect | defect | 23.5 ± 1.53 |
| Group B | 22 | 20 | 20 | 20 | 19 | 19 | 19 | 19.86 ± 1.07 |
| Group C | 46 | 44 | 44.5 | 44.5 | 44 | 44.5 | 45 | 44.64 ± 0.69 |
| 30 s | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | |
|---|---|---|---|---|---|---|---|
| Group A low | 100 | 53 | 46 | 40 | 37 | 33 | 33 |
| Group A high | 100 | 53 | 43 | defect | defect | defect | defect |
| Group B | 100 | 72 | 69 | 66 | 64 | 62 | 61 |
| Group C | 100 | 77 | 75 | 73 | 70 | 69 | 73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knappe, K.; Bitsch, R.G.; Schonhoff, M.; Walker, T.; Renkawitz, T.; Jaeger, S. Pulsatile Lavage Systems with High Impact Pressure and High Flow Produce Cleaner Cancellous Bone Prior to Cementation in Cemented Arthroplasty. J. Clin. Med. 2022, 11, 88. https://doi.org/10.3390/jcm11010088
Knappe K, Bitsch RG, Schonhoff M, Walker T, Renkawitz T, Jaeger S. Pulsatile Lavage Systems with High Impact Pressure and High Flow Produce Cleaner Cancellous Bone Prior to Cementation in Cemented Arthroplasty. Journal of Clinical Medicine. 2022; 11(1):88. https://doi.org/10.3390/jcm11010088
Chicago/Turabian StyleKnappe, Kevin, Rudi G. Bitsch, Mareike Schonhoff, Tilman Walker, Tobias Renkawitz, and Sebastian Jaeger. 2022. "Pulsatile Lavage Systems with High Impact Pressure and High Flow Produce Cleaner Cancellous Bone Prior to Cementation in Cemented Arthroplasty" Journal of Clinical Medicine 11, no. 1: 88. https://doi.org/10.3390/jcm11010088
APA StyleKnappe, K., Bitsch, R. G., Schonhoff, M., Walker, T., Renkawitz, T., & Jaeger, S. (2022). Pulsatile Lavage Systems with High Impact Pressure and High Flow Produce Cleaner Cancellous Bone Prior to Cementation in Cemented Arthroplasty. Journal of Clinical Medicine, 11(1), 88. https://doi.org/10.3390/jcm11010088






