Survival of Women Previously Diagnosed of Melanoma with Subsequent Pregnancy: A Systematic Review and Meta-Analysis and a Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
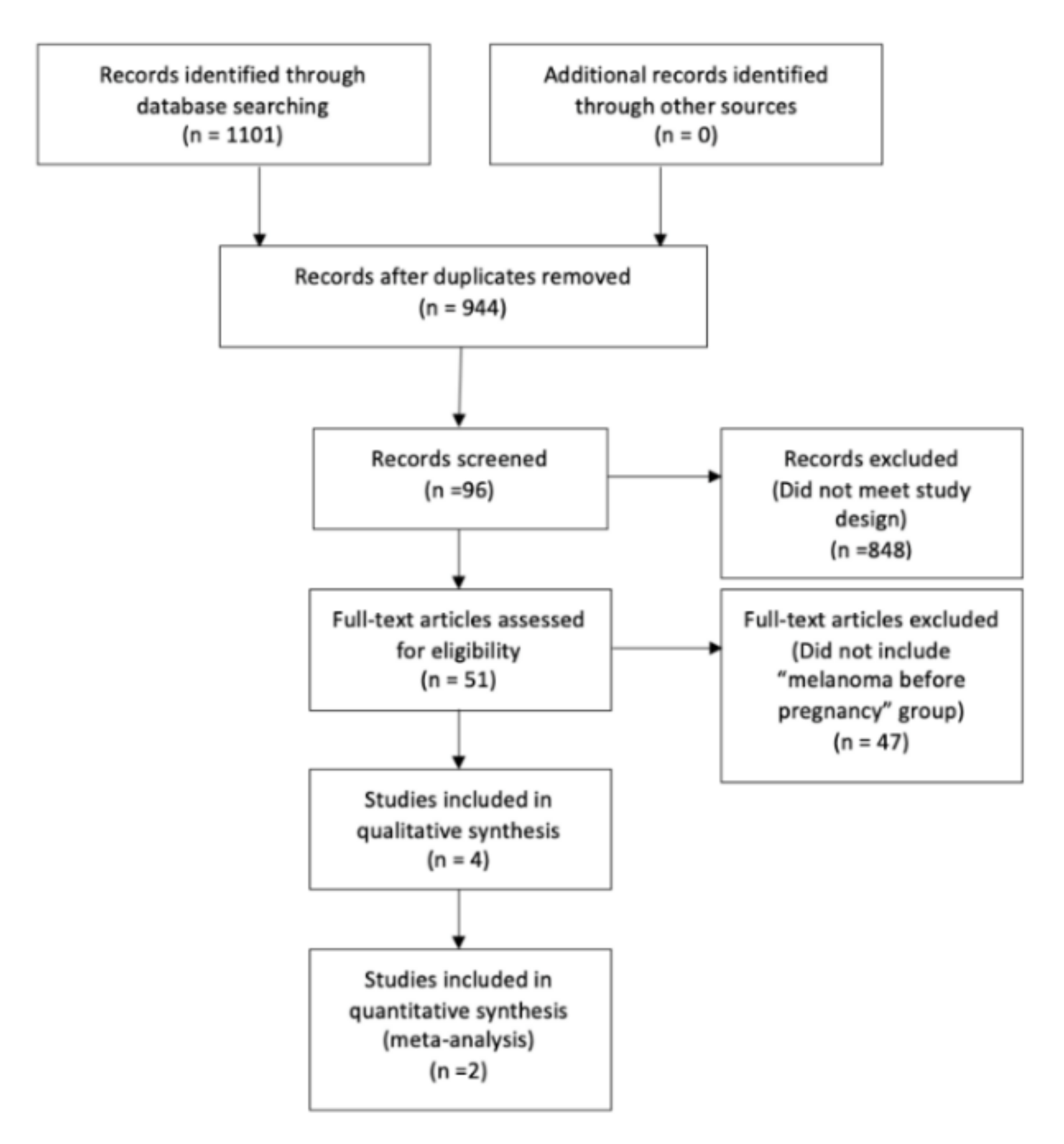
2.2. Selection of Relevant Studies
2.3. Data Extraction
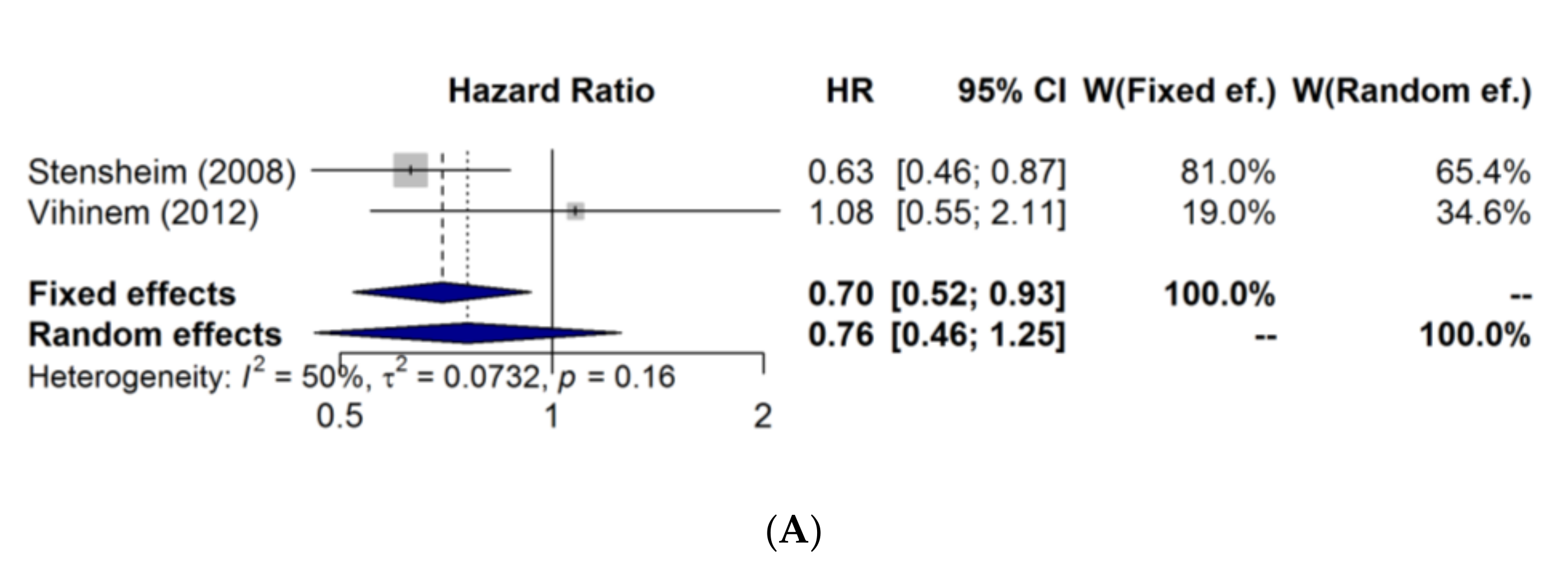
3. Results
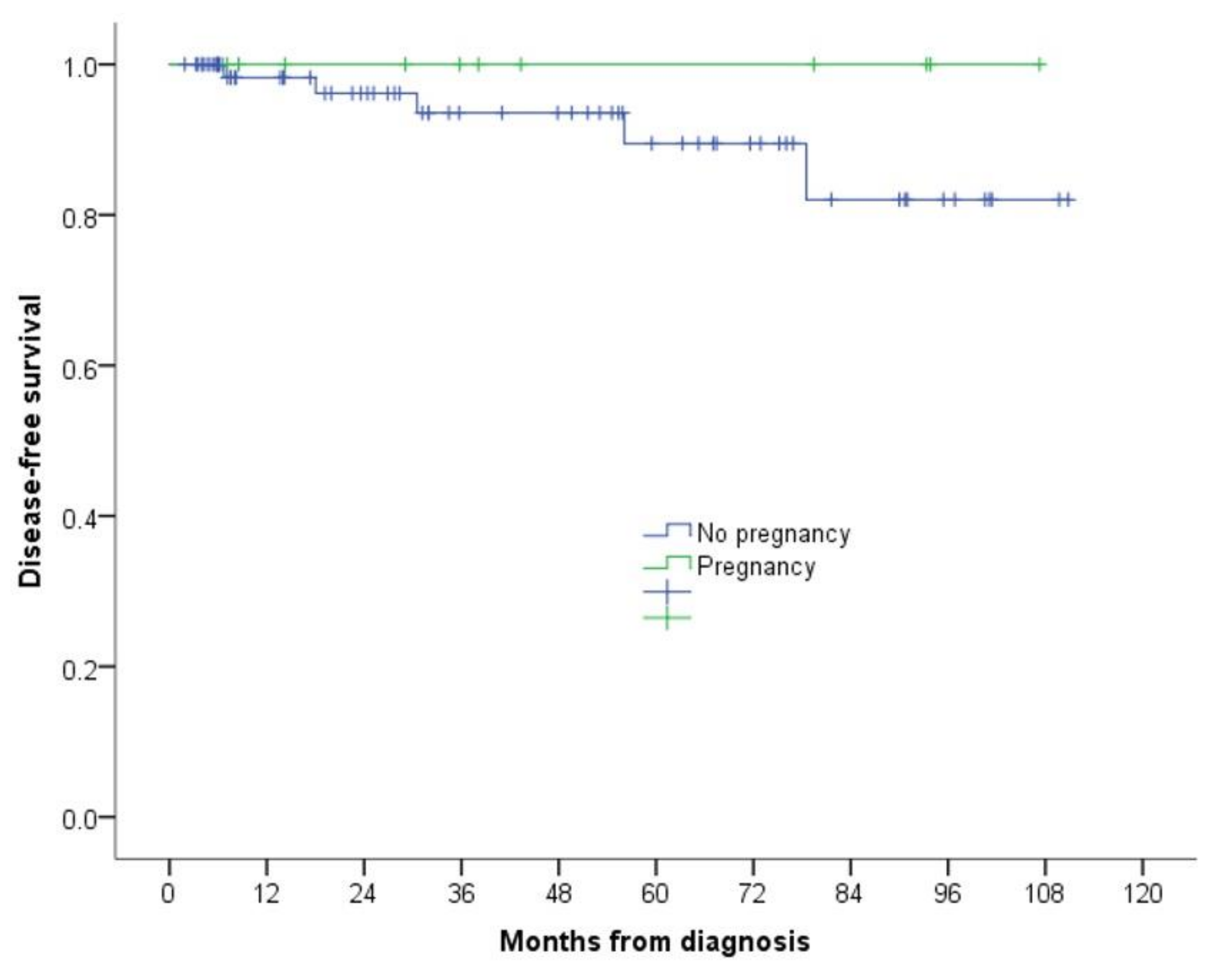
3.1. Our Series
3.2. Study Limitations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Search Strategy
Appendix A.1.1. *Pubmed
Appendix A.1.2. *Embase
Appendix A.1.3. *Cochrane
References
- Greenlee, R.T.; Hill-Harmon, M.B.; Murray, T.; Thun, M. Cancer Statistics. CA Cancer J. Clin. 2001, 51, 15–36. [Google Scholar] [CrossRef]
- Ríos, L.; Nagore, E.; López, J.; Redondo, P.; Marti, R.M.; Fernández-De-Misa, R.; Soler, B. Melanoma Characteristics at Diagnosis From The Spanish National Cutaneous Melanoma Registry: 15 Years of Experience. Actas Dermo-Sifiliogr. 2013, 104, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Lens, M.; Rosdahl, I.; Newton-Bishop, J. Cutaneous Melanoma During Pregnancy: Is the Controversy Over? J. Clin. Oncol. 2009, 27, e11–e12. [Google Scholar] [CrossRef] [PubMed]
- Stensheim, H.; Møller, B.; van Dijk, T.; Fosså, S.D. Cause-Specific Survival for Women Diagnosed with Cancer During Pregnancy or Lactation: A Registry-Based Cohort Study. J. Clin. Oncol. 2009, 27, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pack, G.T.; Scharnagel, I.M. The prognosis for malignant melanoma in the pregnant woman. Cancer 1951, 4, 324–334. [Google Scholar] [CrossRef]
- Guinee, F.; Hess, K.; Taylor, S.; Olsson, H.; Möller, T.; Fahey, T.; Gladikov, J.; Blink, J.V.D.; Bonichon, F.; Dische, S.; et al. Effect of pregnancy on prognosis for young women with breast cancer. Lancet 1994, 343, 1587–1589. [Google Scholar] [CrossRef]
- Katz, V.L.; Farmer, R.M.; Dotters, D. Focus on Primary Care: From Nevus to Neoplasm: Myths of Melanoma in Pregnancy. Obstet. Gynecol. Surv. 2002, 57, 112–119. [Google Scholar] [CrossRef]
- Albrektsen, G. Clinical Stage of Breast Cancer by Parity, Age at Birth, and Time Since Birth: A Progressive Effect of Preg-nancy Hormones? Cancer Epidemiol. Biomark. Prev. 2006, 15, 65–69. [Google Scholar] [CrossRef]
- Byrom, L.; Olsen, C.M.; Knight, L.; Khosrotehrani, K.; Green, A.C. Does Pregnancy After a Diagnosis of Melanoma Affect Progno-sis? Systematic Review and Meta-analysis. Dermatol. Surg. 2015, 41, 875–882. [Google Scholar] [CrossRef]
- Berk-Krauss, J.; Liebman, T.N.; Stein, J.A. Pregnancy and Melanoma: Recommendations for Clinical Scenarios. Int. J. Womens Dermatol. 2018, 4, 113–115. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Lens, M.B.; Rosdahl, I.; Ahlbom, A.; Farahmand, B.Y.; Synnerstad, I.; Boeryd, B.; Bishop, J.A.N. Effect of Pregnancy on Survival in Women With Cutaneous Malignant Melanoma. J. Clin. Oncol. 2004, 22, 4369–4375. [Google Scholar] [CrossRef] [PubMed]
- Savoia, P.; Ortoncelli, M.; Osella-Abate, S.; Quaglino, P.; Mola, F.; Fierro, M.T.; Bernengo, M.G. Melanoma and pregnancy: A 30-year experi-ence at the Turin Melanoma Centre and a review of the literature data. G. Ital. Dermatol. Venereol. 2007, 142, 1–7. [Google Scholar]
- Vihinen, P.; Vainio-Kaila, M.; Talve, L.; Koskivuo, I.; Syrjänen, K.; Pyrhönen, S. Previous pregnancy is a favourable prognostic factor in women with localised cutaneous melanoma. Acta Oncol. 2012, 51, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Gleiss, A.; Oberbauer, R.; Heinze, G. An unjustified benefit: Inmortal time bias in the analysis of time-dependent events. Transpl. Int. 2018, 31, 125–130. [Google Scholar] [CrossRef]
- Wolkewitz, M.; Allignol, A.; Schumacher, M.; Beyersmann, J. Two Pitfalls in Survival Analyses of Time-Dependent Exposure: A Case Study in a Cohort of Oscar Nominees. Am. Stat. 2010, 64, 205–211. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics–Update 2019. Eur. J. Cancer 2020, 126, 141–158. [Google Scholar] [CrossRef]
- Lens, M.; Bataille, V. Melanoma in relation to reproductive and hormonal factors in women: Current review on controversial issues. Cancer Causes Control. 2008, 19, 437–442. [Google Scholar] [CrossRef]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef]
- Driscoll, M.S.; Martires, K.; Bieber, A.K.; Pomeranz, M.K.; Grant-Kels, J.M.; Stein, J.A. Pregnancy and melanoma. J. Am. Acad. Dermatol. 2016, 75, 669–678. [Google Scholar] [CrossRef]
- Still, R.; Brennecke, S. Melanoma in pregnancy. Obs. Med. 2017, 10, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Støer, N.C.; Weiderpass, E.; Pukkala, E.; Ylikorkala, O.; Lyytinen, H. Menopausal Hormone Therapy and Risk of Mela-noma: A Nationwide Register-Based Study in Finland. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1857–1860. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.K.; Halpern, A.C.; Johnson, T.M.; Hood, A.F.; Grichnik, J.M.; Swetter, S.M.; Tsao, H.; Barbosa, V.H.; Chuang, T.-Y.; Duvic, M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2011, 65, 1032–1047. [Google Scholar] [CrossRef] [PubMed]

| Reference | Country | Time Period | Study Design | N Cases | N Controls | Age Cases (Years) | Age Controls (Years) | Follow Up (Months) |
|---|---|---|---|---|---|---|---|---|
| Lens/2004 [12] (1) | Sweden | 1958–1999 | Retrospective cohort study | 966 | 4567 | 26.5 | 36.60 | 154.80 |
| Savoia/2007 [13] (2) | Italy | 1975–2005 | Retrospective cohort study | 54 | 609 | 29.00 | 38.00 | 360.00 |
| Stensheim/2008 [4] (3) | Norway | 1967–2002 | Retrospective cohort study | 797 | 3949 | 26.00 | 40.00 | 182.40 |
| Vihinen/2012 [14] (4) | Finland | 1990–2009 | Retrospective cohort study | 10 | 55 | 29.00 | 38.30 | 53.20 |
| Reference | Breslow (mm) | p | |
|---|---|---|---|
| Cases | Controls | ||
| Lens/2004 [12] (1) | 0.93 | 1.11 | 1.000 |
| Savoia/2007 [13] (2) | 1.80 | 1.01 | 0.000 |
| Stensheim/2008 [4] (3) | missing data | missing data | missing data |
| Vihinen/2012 [14] (4) | 1.39 | 1.39 | missing data |
| Reference | Measure | Cases | Controls | All Groups | Adjusted HR | Crude HR | p |
|---|---|---|---|---|---|---|---|
| Lens/2004 [12] (1) | Global mortality | MD | MD | MD | 0.58 (0.32–1.05) * | MD | 1.000 |
| Savoia/2007 [13] (2) | Global mortality | 9.20 | 119.80 | MD | MD | MD | MD |
| Disease-free survival 5 years | 72% | 92% | MD | MD | MD | 0.000 | |
| Global survival 5 years | 85% | 98% | MD | MD | MD | 0.000 | |
| Stensheim/2008 [4] (3) | Melanoma-specific death | MD | MD | MD | 0.86 (0.60–1.22) ** | 0.63 (0.46–0.88) ** | MD |
| Vihinen/2012 [14] (4) | Disease-free survival | 54.8 | 29.0 | 46.9 | MD | MD | MD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Campayo, N.; Paradela de la Morena, S.; Pértega-Díaz, S.; Iglesias Pena, L.; Vihinen, P.; Mattila, K.; Lens, M.B.; Tejera-Vaquerizo, A.; Fonseca, E. Survival of Women Previously Diagnosed of Melanoma with Subsequent Pregnancy: A Systematic Review and Meta-Analysis and a Single-Center Experience. J. Clin. Med. 2022, 11, 83. https://doi.org/10.3390/jcm11010083
Martínez-Campayo N, Paradela de la Morena S, Pértega-Díaz S, Iglesias Pena L, Vihinen P, Mattila K, Lens MB, Tejera-Vaquerizo A, Fonseca E. Survival of Women Previously Diagnosed of Melanoma with Subsequent Pregnancy: A Systematic Review and Meta-Analysis and a Single-Center Experience. Journal of Clinical Medicine. 2022; 11(1):83. https://doi.org/10.3390/jcm11010083
Chicago/Turabian StyleMartínez-Campayo, Nieves, Sabela Paradela de la Morena, Sonia Pértega-Díaz, Luisa Iglesias Pena, Pia Vihinen, Kalle Mattila, Marko B. Lens, Antonio Tejera-Vaquerizo, and Eduardo Fonseca. 2022. "Survival of Women Previously Diagnosed of Melanoma with Subsequent Pregnancy: A Systematic Review and Meta-Analysis and a Single-Center Experience" Journal of Clinical Medicine 11, no. 1: 83. https://doi.org/10.3390/jcm11010083
APA StyleMartínez-Campayo, N., Paradela de la Morena, S., Pértega-Díaz, S., Iglesias Pena, L., Vihinen, P., Mattila, K., Lens, M. B., Tejera-Vaquerizo, A., & Fonseca, E. (2022). Survival of Women Previously Diagnosed of Melanoma with Subsequent Pregnancy: A Systematic Review and Meta-Analysis and a Single-Center Experience. Journal of Clinical Medicine, 11(1), 83. https://doi.org/10.3390/jcm11010083






