Reduced Sympathetic Reserve Detectable by Heart Rate Response after Dipyridamole in Anginal Patients with Normal Coronary Arteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Comparison with Previous Studies
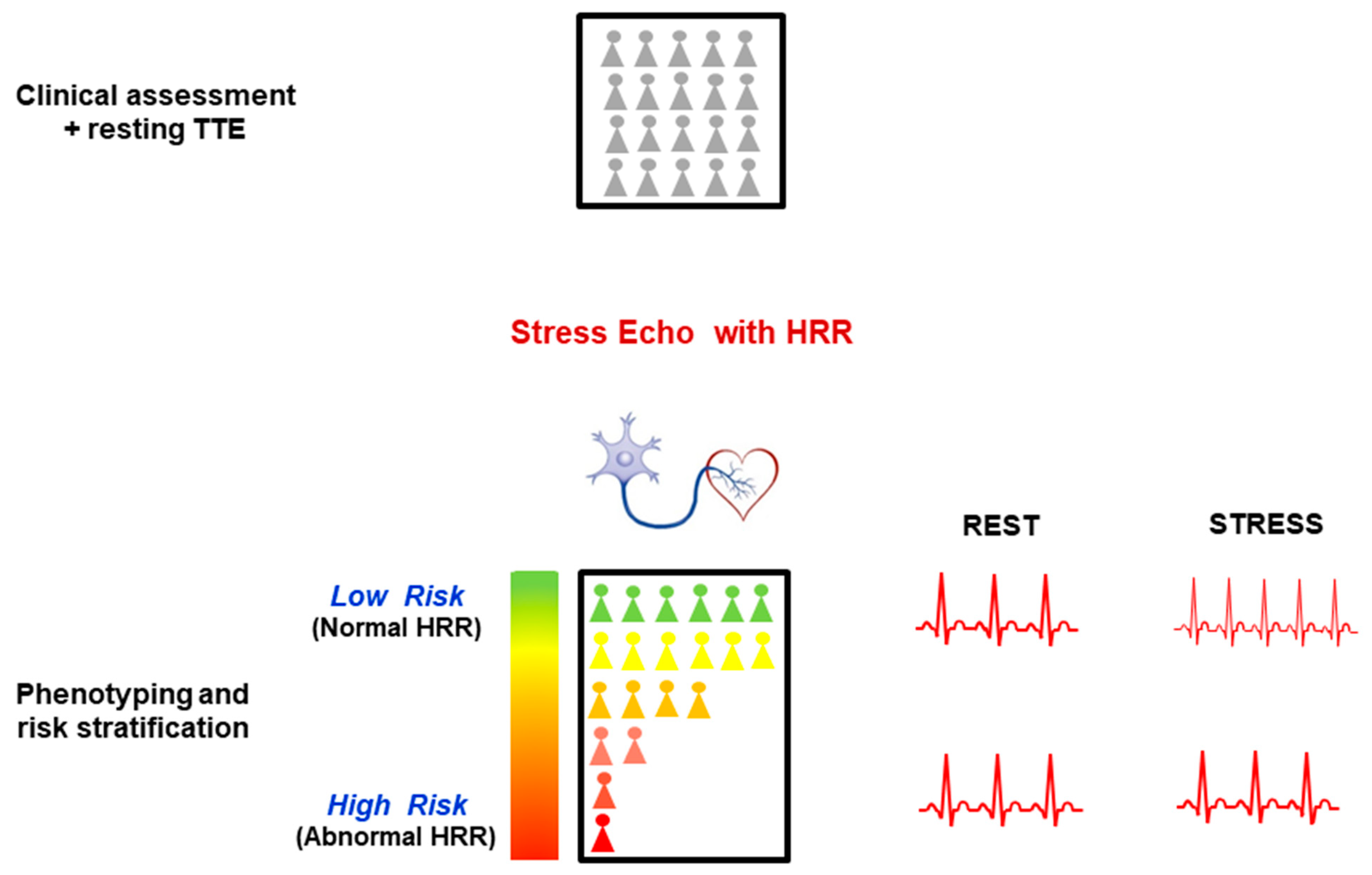
4.2. Clinical Implications
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HRR | heart rate reserve |
| INOCA | ischemia with normal coronary arteries |
| RWMA | regional wall motion abnormality |
| SE | stress echocardiography |
References
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Pepine, C.J.; Shimokawa, H.; Berry, C. Treatment of coronary microvascular dysfunction. Cardiovasc. Res. 2020, 116, 856–870. [Google Scholar] [CrossRef]
- Brubaker, P.H.; Kitzman, D.W. Chronotropic incompetence. Causes, consequences and management. Circulation 2011, 123, 1010–1020. [Google Scholar] [CrossRef] [Green Version]
- Crea, F.; Camici, P.G.; Bairey Merz, C.N. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477, Erratum in: Eur. Heart J. 2020, 41, 4242. [Google Scholar] [CrossRef] [PubMed]
- Hage, F.G.; Iskandrian, A.E. Heart rate response during vasodilator stress myocardial perfusion imaging: Mechanisms and implications. J. Nucl. Cardiol. 2010, 17, 536–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortigiani, L.; Carpeggiani, C.; Landi, P.; Raciti, M.; Bovenzi, F.; Picano, E. Usefulness of blunted heart rate reserve as an imaging-independent prognostic predictor during dipyridamole-echocardiography test. Am. J. Cardiol. 2019, 124, 972–977. [Google Scholar] [CrossRef]
- Biaggioni, I.; Killian, T.J.; Mosqueda-Garcia, R.; Robertson, R.M.; Robertson, D. Adenosine increases sympathetic nerve traffic in humans. Circulation 1991, 83, 1668–1675. [Google Scholar] [CrossRef] [Green Version]
- Dhalla, A.K.; Wong, M.Y.; Wang, W.Q.; Biaggioni, I.; Belardinelli, L. Tachycardia caused by A2A adenosine receptor agonists is mediated by direct sympathoexcitation in awake rats. J. Pharmacol. Exp. Ther. 2006, 316, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, A.R.; Picano, E.; Marini, C.; Favilla, S.; Salvetti, A.; Distante, A. Activation of sympathetic tone during dipyridamole test. Chest 1992, 102, 444–447. [Google Scholar] [CrossRef]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.U.; Zamorano, J.L.; European Association of Echocardiography. Stress Echocardiography Expert Consensus Statement-Executive Summary: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur. Heart J. 2009, 30, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e8. [Google Scholar] [CrossRef] [Green Version]
- Ciampi, Q.; Zagatina, A.; Cortigiani, L.; Gaibazzi, N.; Borguezan Daros, C.; Zhuravskaya, N.; Wierzbowska-Drabik, K.; Kasprzak, J.D.; de Castro e Silva Pretto, J.L.; D’Andrea, A.; et al. Functional, Coronary Anatomic and Prognostic Correlates of Coronary Flow Velocity Reserve during Stress Echocardiography. J. Am. Coll. Cardiol. 2019, 74, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Cortigiani, L.; Carpeggiani, C.; Landi, P.; Raciti, M.; Bovenzi, F.; Picano, E. Prognostic Value of Heart Rate Reserve in Patients with Permanent Atrial Fibrillation during Dipyridamole Stress Echocardiography. Am. J. Cardiol. 2020, 125, 1661–1665. [Google Scholar] [CrossRef]
- Conradson, T.B.; Clarke, B.; Dixon, C.M.; Dalton, R.N.; Barnes, P.J. Effects of adenosine on autonomic control of heart rate in man. Acta Physiol. Scand. 1987, 131, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Bombardini, T.; Pacini, D.; Potena, L.; Maccherini, M.; Kovacevic-Preradovic, T.; Picano, E. Heart rate reserve during dipyridamole stress test applied to potential heart donors in brain death. Minerva Cardioangiol. 2020, 68, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Frøbert, O.; Mølgaard, H.; Bøtker, H.E.; Bagger, J.P. Autonomic balance in patients with angina and a normal coronary angiogram. Eur. Heart J. 1995, 16, 1356–1360. [Google Scholar] [CrossRef]
- Ponikowski, P.; Rosano, G.M.; Amadi, A.A.; Collins, P.; Coats, A.J.; Poole-Wilson, P.A.; Kaski, J.C. Transient autonomic dysfunction precedes ST-segment depression in patients with syndrome X. Am. J. Cardiol. 1996, 77, 942–947. [Google Scholar] [CrossRef]
- Rosano, G.M.; Ponikowski, P.; Adamopoulos, S.; Collins, P.; Poole-Wilson, P.A.; Coats, A.J.; Kaski, J.C. Abnormal autonomic control of the cardiovascular system in syndrome X. Am. J. Cardiol. 1994, 73, 1174–1179. [Google Scholar] [CrossRef]
- Kolasińska-Kloch, W.; Furgała, A.; Królczyk, G.; Kloch, M.; Szumańska, M.; Laskiewicz, J.; Thor, P.J. Cardiac syndrome X-autonomic system disorders. Folia Med. Cracov. 2004, 45, 19–29. [Google Scholar]
- Adamopoulos, S.; Rosano, G.M.; Ponikowski, P.; Cerquetani, E.; Piepoli, M.; Panagiota, F.; Collins, P.; Poole-Wilson, P.; Kremastinos, D.; Coats, A.J. Impaired baroreflex sensitivity and sympathovagal balance in syndrome X. Am. J. Cardiol. 1998, 82, 862–868. [Google Scholar] [CrossRef]
- Lanza, G.A.; Giordano, A.; Pristipino, C.; Calcagni, M.L.; Meduri, G.; Trani, C.; Franceschini, R.; Crea, F.; Troncone, L.; Maseri, A. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I] metaiodobenzylguanidine myocardial scintigraphy. Circulation 1997, 96, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, A.; Lanza, G.A.; Bruno, I.; Careri, G.; Pinnacchio, G.; Tarzia, P.; Battipaglia, I.; Giordano, A.; Crea, F. Usefulness of impairment of cardiac adrenergic nerve function to predict outcome in patients with cardiac syndrome X. Am. J. Cardiol. 2010, 106, 1813–1818. [Google Scholar] [CrossRef]
- Sicari, R.; Palinkas, A.; Pasanisi, E.; Venneri, L.; Picano, E. Long-term survival of patients with chest pain syndrome and angiographically normal or near-normal coronary arteries: The additional prognostic value of dipyridamole-echocardiography test. Eur. Heart J. 2005, 26, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Sicari, R.; Rigo, F.; Cortigiani, L.; Gherardi, S.; Galderisi, M.; Picano, E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am. J. Cardiol. 2009, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Cortigiani, L.; Urluescu, M.L.; Coltelli, M.; Carpeggiani, C.; Bovenzi, F.; Picano, E. Apparent Declining Prognostic Value of a Negative Stress Echocardiography Based on Regional Wall Motion Abnormalities in Patients with Normal Resting Left Ventricular Function Due to the Changing Referral Profile of the Population under Study. Circ. Cardiovasc. Imaging 2019, 12, e008564. [Google Scholar] [CrossRef]
- Marzilli, M.; Crea, F.; Morrone, D.; Bonow, R.O.; Brown, D.L.; Camici, P.G.; Chilian, W.M.; DeMaria, A.; Guarini, G.; Huqi, A.; et al. Myocardial ischemia: From disease to syndrome. Int. J. Cardiol. 2020, 314, 32–35. [Google Scholar] [CrossRef]
- Lahiri, M.K.; Kannankeril, P.J.; Goldberger, J.J. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J. Am. Coll. Cardiol. 2008, 51, 1725–1733. [Google Scholar] [CrossRef] [Green Version]
- Scheen, A.J. Effect of SGLT2 Inhibitors on the Sympathetic Nervous System and Blood Pressure. Curr. Cardiol. Rep. 2019, 21, 70. [Google Scholar] [CrossRef]
- Lauer, M.; Blackstone, E.; Young, J.; Topol, E. Cause of death in clinical research: Time for reassessment? J. Am. Coll. Cardiol. 1999, 34, 618–620. [Google Scholar] [CrossRef] [Green Version]
- Varga, A.; Picano, E.; Cortigiani, L.; Petix, N.; Margaria, F.; Magaia, O.; Heyman, J.; Bigi, R.; Mathias, W., Jr.; Gigli, G.; et al. Does stress echocardiography predict the site of future myocardial infarction? A large-scale multicenter study. EPIC (Echo Persantine International Cooperative) and EDIC (Echo Dobutamine International Cooperative) study groups. J. Am. Coll. Cardiol. 1996, 28, 45–51. [Google Scholar] [CrossRef] [Green Version]
- From, A.M.; Kane, G.; Bruce, C.; Pellikka, P.A.; Scott, C.; McCully, R.B. Characteristics and outcomes of patients with abnormal stress echocardiograms and angiographically mild coronary artery disease (<50% stenoses) or normal coronary arteries. J. Am. Soc. Echocardiogr. 2010, 23, 207–214. [Google Scholar] [CrossRef] [PubMed]
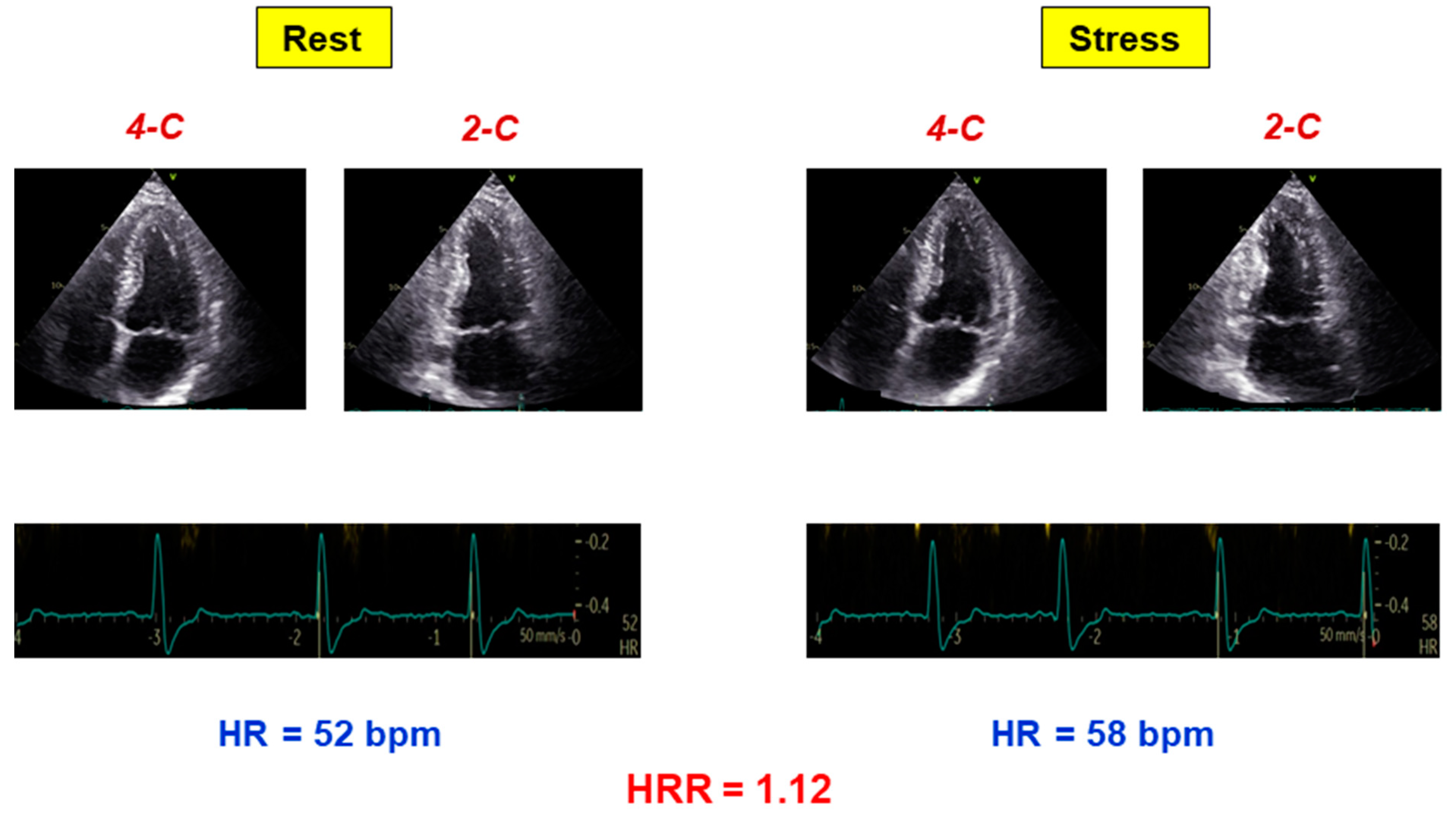
| Abnormal HRR (n = 109) | Normal HRR (n = 183) | p Value | |
|---|---|---|---|
| Age (years) | 68 ± 10 | 62 ± 11 | <0.0001 |
| Males | 63 (58%) | 98 (54%) | 0.48 |
| Clinical history | |||
| Diabetes mellitus | 32 (29%) | 39 (21%) | 0.12 |
| Arterial hypertension | 74 (68%) | 119 (65%) | 0.62 |
| Hypercholesterolemia | 57 (52%) | 96 (52%) | 0.98 |
| Cigarette smoking | 31 (28%) | 52 (28%) | 0.99 |
| Left bundle branch block | 16 (15%) | 14 (8%) | 0.06 |
| Permanent atrial fibrillation | 12 (11%) | 14 (8%) | 0.33 |
| Ongoing β-blocker therapy | 46 (42%) | 51 (28%) | 0.01 |
| Echocardiographic findings | |||
| Rest ejection fraction (%) | 56 ± 8 | 58 ± 7 | 0.03 |
| Rest WMSI | 1.18 ± 0.35 | 1.10 ± 0.26 | 0.02 |
| Stress echo-induced RWMA | 7 (6%) | 13 (7%) | 0.82 |
| Rest HR (beats/min) | 75 ± 14 | 67 ± 11 | <0.0001 |
| Peak HR (beats/min) | 83 ± 15 | 94 ± 15 | <0.0001 |
| HRR | 1.11 ± 0.10 | 1.42 ± 0.15 | <0.0001 |
| Rest SBP (mmHg) | 138 ± 24 | 135 ± 19 | 0.18 |
| Rest DBP (mmHg) | 78 ± 13 | 77 ± 13 | 0.34 |
| Peak SBP (mmHg) | 130 ± 27 | 134 ± 21 | 0.15 |
| Peak DBP (mmHg) | 71 ± 14 | 74 ± 12 | 0.03 |
| Follow-up data | |||
| Duration of follow-up (years) | 9.6 ± 5.3 | 10.8 ± 5.6 | 0.07 |
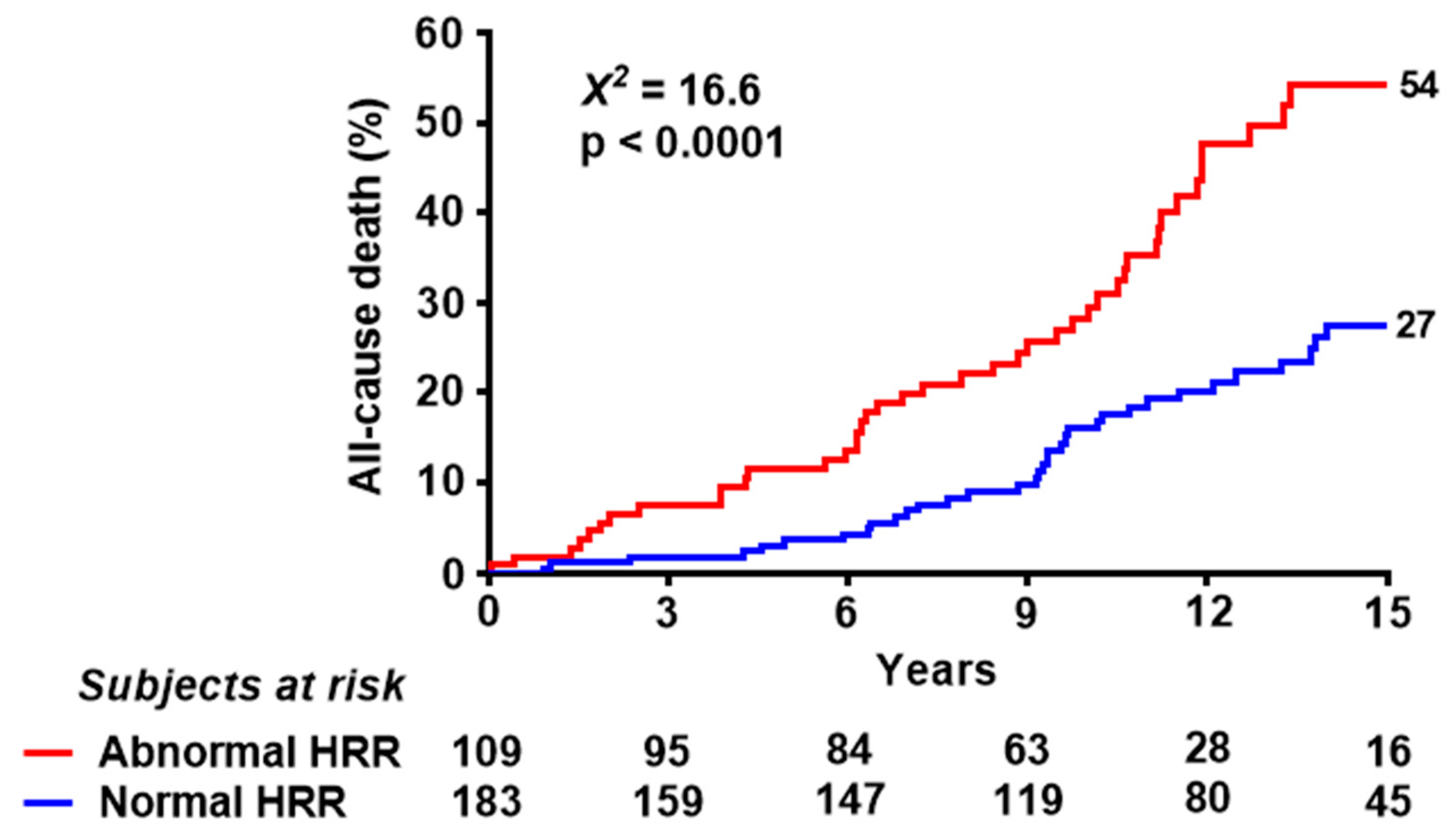
| Deaths | 47 (43%) | 42 (23%) | <0.0001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (years) | 1.09 (1.06–1.12) | <0.0001 | 1.08 (1.05–1.10) | <0.0001 |
| Gender (male) | 0.92 (0.61–1.40) | 0.70 | ||
| Diabetes mellitus | 1.40 (0.87–2.25) | 0.16 | ||
| Arterial hypertension | 0.90 (0.58–1.40) | 0.65 | ||
| Cigarette smoking | 0.81 (0.51–1.28) | 0.36 | ||
| Left bundle branch block | 1.27 (0.67–2.39) | 0.46 | ||
| Permanent atrial fibrillation | 3.36 (1.97–5.72) | <0.0001 | 2.70 (1.55–4.70) | <0.0001 |
| Ongoing β-blocker therapy | 1.25 (0.81–1.94) | 0.31 | ||
| Rest ejection fraction | 1.00 (0.97–1.03) | 0.82 | ||
| Rest WMSI | 0.96 (0.47–1.97) | 0.91 | ||
| Stress echo-induced RWMA | 1.03 (0.51–2.06) | 0.94 | ||
| Abnormal HRR | 2.34 (1.54–3.56) | <0.0001 | 1.86 (1.20–2.88) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortigiani, L.; Carpeggiani, C.; Meola, L.; Djordjevic-Dikic, A.; Bovenzi, F.; Picano, E. Reduced Sympathetic Reserve Detectable by Heart Rate Response after Dipyridamole in Anginal Patients with Normal Coronary Arteries. J. Clin. Med. 2022, 11, 52. https://doi.org/10.3390/jcm11010052
Cortigiani L, Carpeggiani C, Meola L, Djordjevic-Dikic A, Bovenzi F, Picano E. Reduced Sympathetic Reserve Detectable by Heart Rate Response after Dipyridamole in Anginal Patients with Normal Coronary Arteries. Journal of Clinical Medicine. 2022; 11(1):52. https://doi.org/10.3390/jcm11010052
Chicago/Turabian StyleCortigiani, Lauro, Clara Carpeggiani, Laura Meola, Ana Djordjevic-Dikic, Francesco Bovenzi, and Eugenio Picano. 2022. "Reduced Sympathetic Reserve Detectable by Heart Rate Response after Dipyridamole in Anginal Patients with Normal Coronary Arteries" Journal of Clinical Medicine 11, no. 1: 52. https://doi.org/10.3390/jcm11010052
APA StyleCortigiani, L., Carpeggiani, C., Meola, L., Djordjevic-Dikic, A., Bovenzi, F., & Picano, E. (2022). Reduced Sympathetic Reserve Detectable by Heart Rate Response after Dipyridamole in Anginal Patients with Normal Coronary Arteries. Journal of Clinical Medicine, 11(1), 52. https://doi.org/10.3390/jcm11010052






