Atrioventricular Nodal Reentrant Tachycardia Ablation Using Mini-Electrode Recordings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
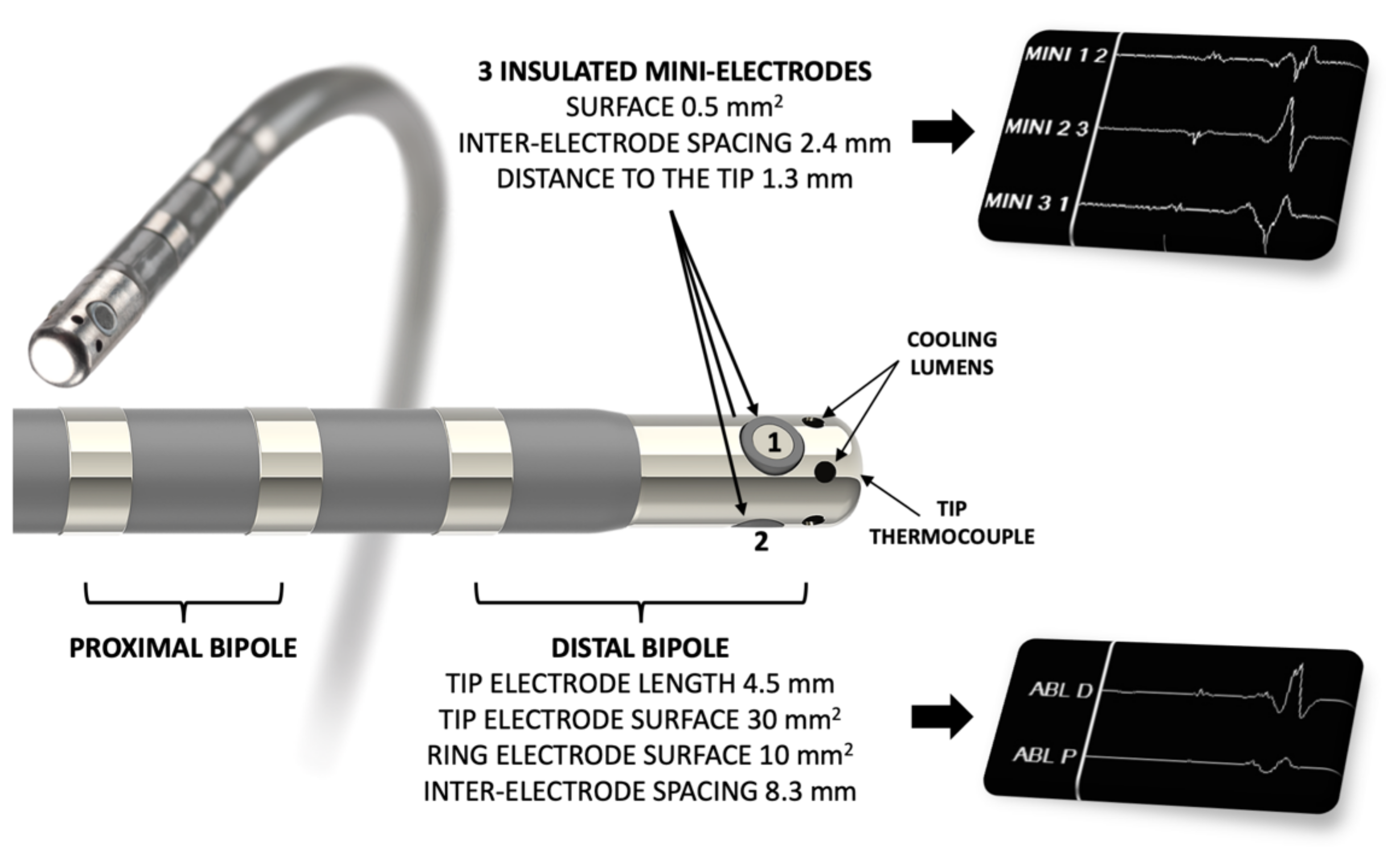
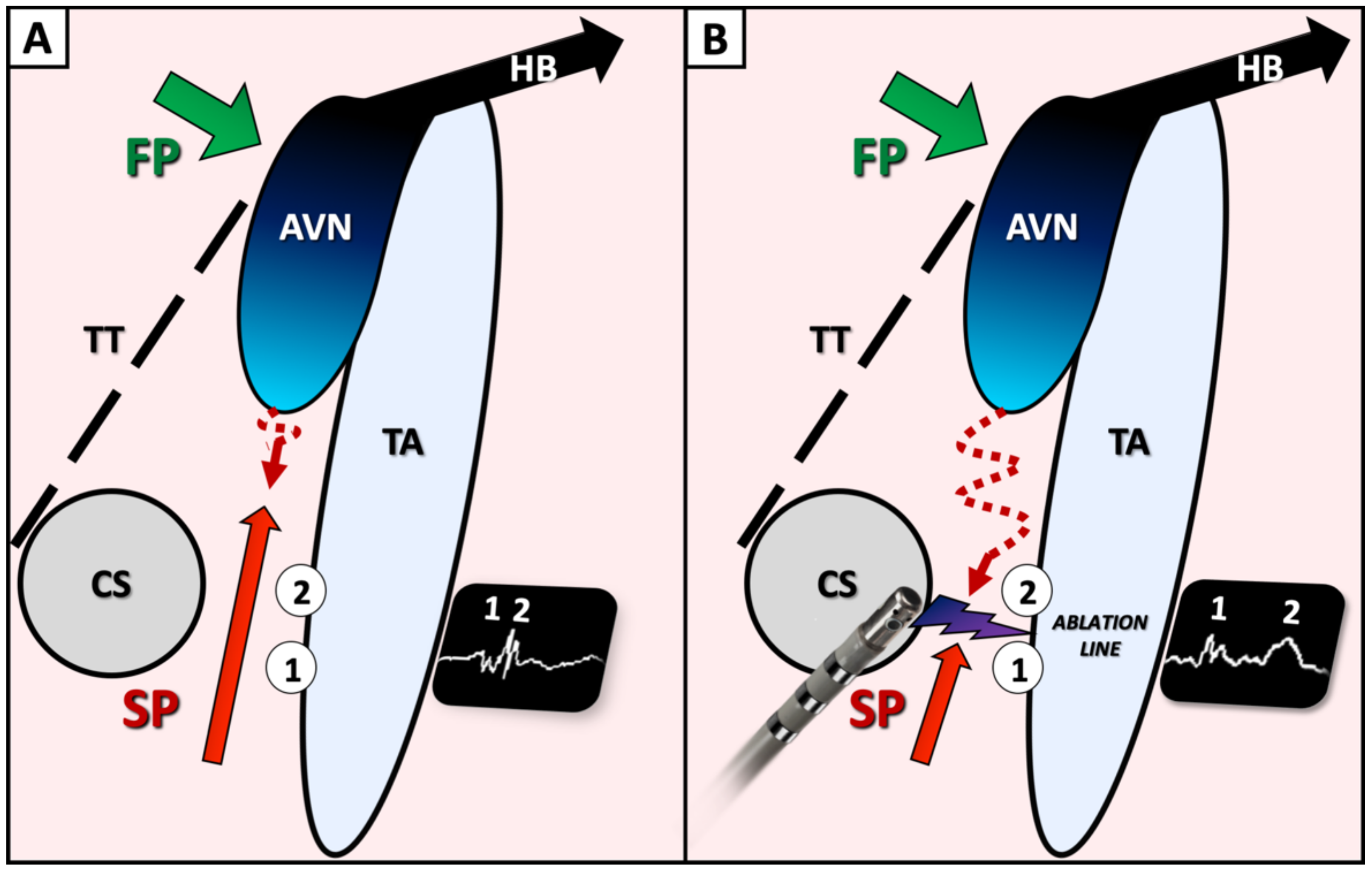
2.2. Ablation Procedure
2.3. Follow-Up
2.4. Statistical Analyses
3. Results
3.1. Population
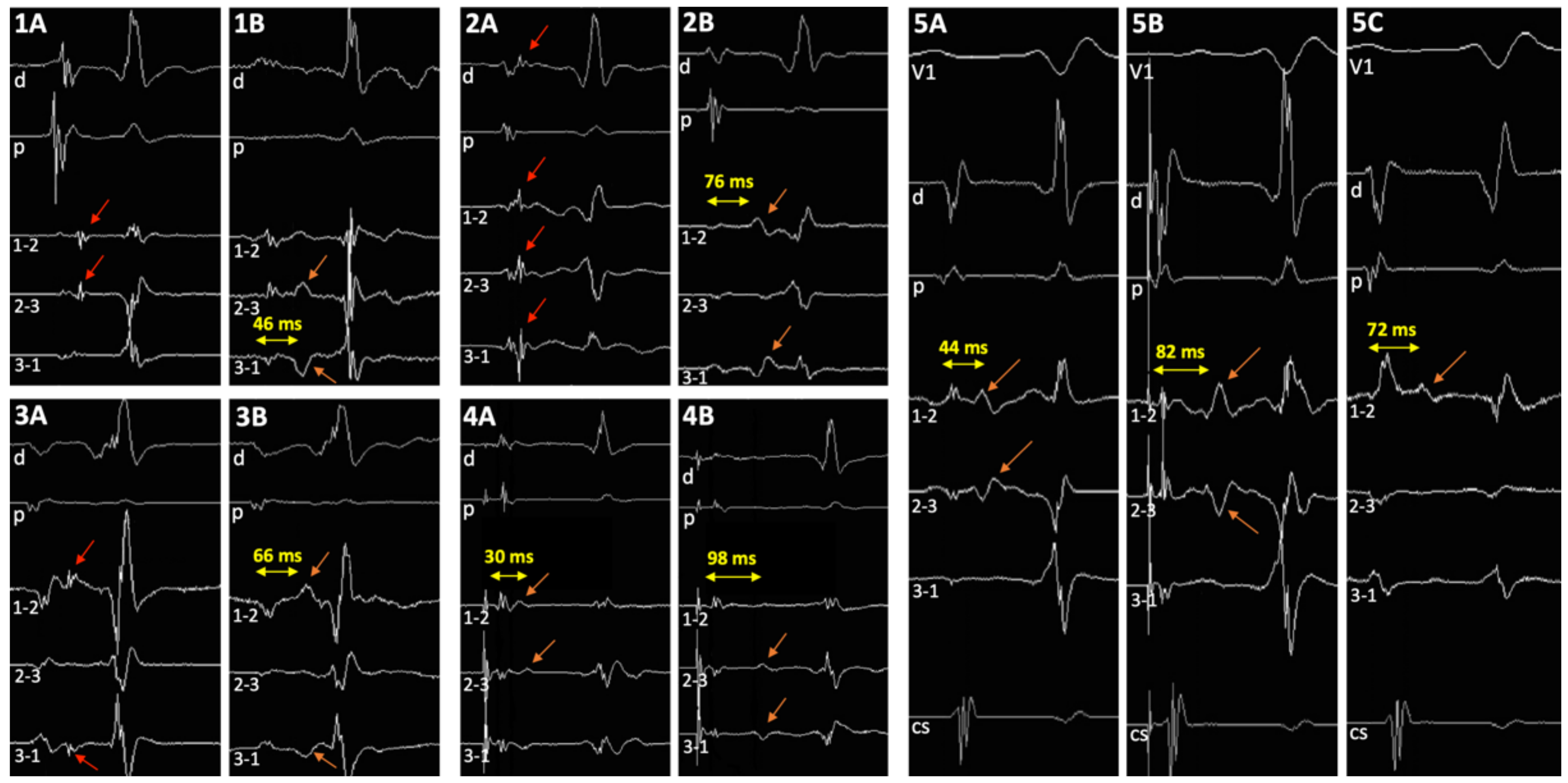
3.2. EP Study
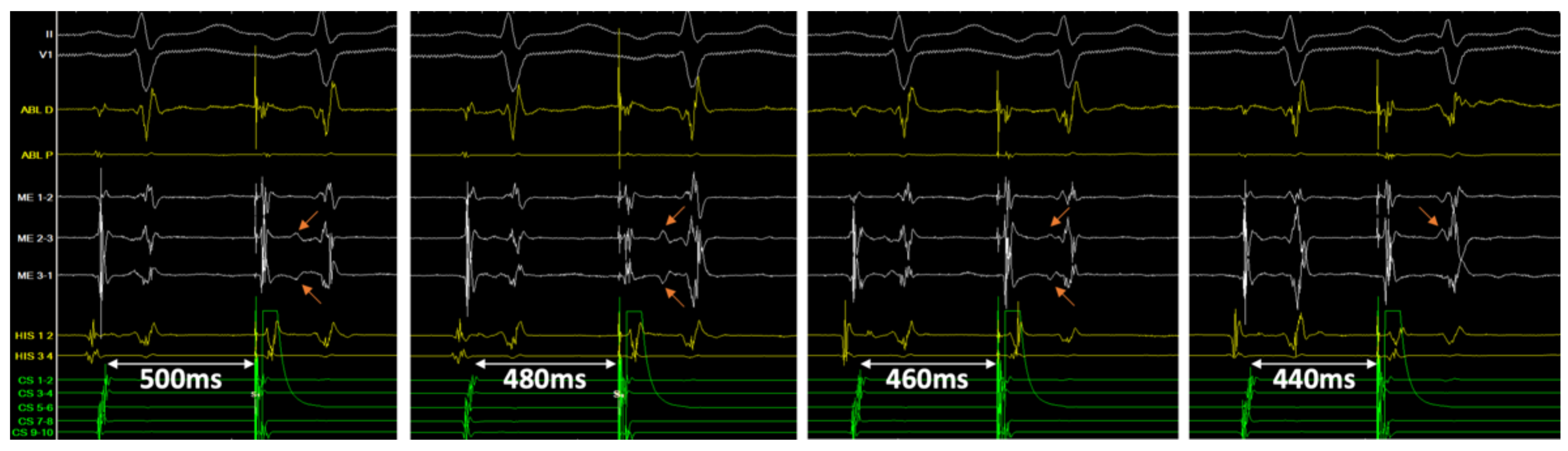
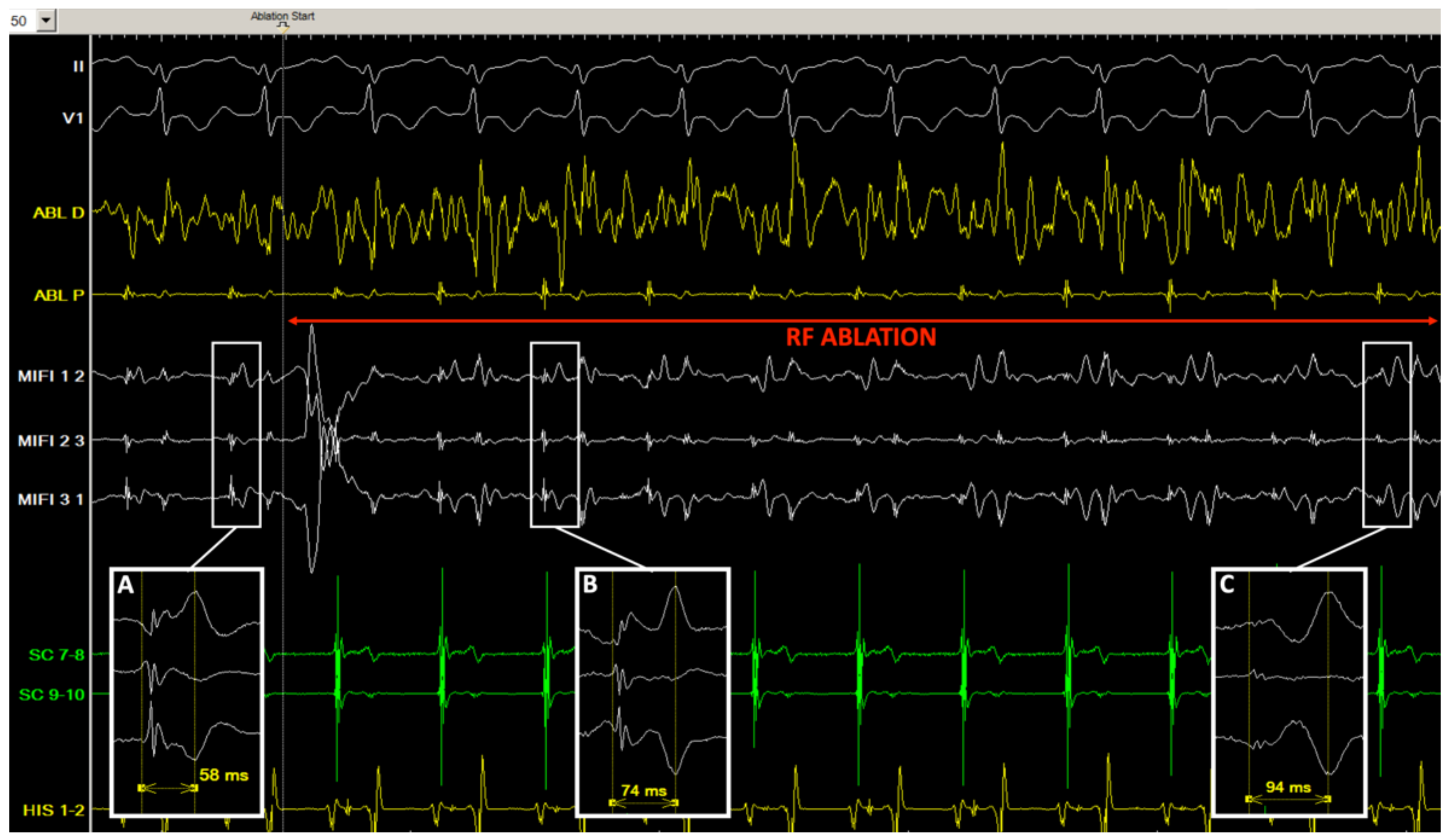
3.3. Ablation
3.4. Follow-Up
3.5. Double Potentials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia; The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haissaguerre, M.; Gaita, F.; Fischer, B.; Commenges, D.; Montserrat, P.; d’Ivernois, C.; Lemetayer, P.; Warin, J.F. Elimination of atrioventricular nodal reentrant tachycardia using discrete slow potentials to guide application of radiofrequency energy. Circulation 1992, 85, 2162–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, W.M.; Beckman, K.J.; McClelland, J.H.; Wang, X.; Friday, K.J.; Roman, C.A.; Moulton, K.P.; Twidale, N.; Hazlitt, H.A.; Prior, M.I.; et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow-pathway conduction. N. Engl. J. Med. 1992, 327, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.C.; Takahashi, A.; Jaïs, P.; Hocini, M.; Clémenty, J.; Haïssaguerre, M. Local electrogram-based criteria of cavotricuspid isthmus block. J. Cardiovasc. Electrophysiol. 1999, 10, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Oral, H.; Sticherling, C.; Chough, S.P.; Baker, R.L.; Wasmer, K.; Pelosi, F., Jr.; Knight, B.P.; Strickberger, S.A.; Morady, F. Double potentials along the ablation line as a guide to radiofrequency ablation of typical atrial flutter. J. Am. Coll. Cardiol. 2001, 38, 750–755. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.A.; de Bakker, J.M.; Vermeulen, J.T.; Opthof, T.; Becker, A.E.; Janse, M.J. Origin and significance of double potentials near the atrioventricular node. Correlation of extracellular potentials, intracellular potentials, and histology. Circulation 1994, 89, 2351–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuire, M.A.; Bourke, J.P.; Robotin, M.C.; Johnson, D.C.; Meldrum-Hanna, W.; Nunn, G.R.; Uther, J.B.; Ross, D.L. High resolution mapping of Koch’s triangle using sixty electrodes in humans with atrioventricular junctional (AV nodal) reentrant tachycardia. Circulation 1993, 88, 2315–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Hocini, M.; Takahashi, A.; Gaïta, F.; Barold, S.S.; Clémenty, J. Analysis of electrophysiological activity in Koch’s triangle relevant to ablation of the slow AV nodal pathway. Pacing Clin. Electrophysiol. 1997, 20, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- De Bakker, J.M.; Loh, P.; Hocini, M.; Thibault, B.; Janse, M.J. Double component action potentials in the posterior approach to the atrioventricular node: Do they reflect activation delay in the slow pathway? J. Am. Coll. Cardiol. 1999, 34, 570–577. [Google Scholar] [CrossRef] [Green Version]
| # | Age (Years) | Sex | Symptoms | Cardiac Disease | Treatment | Documentation |
|---|---|---|---|---|---|---|
| 1 | 22 | F | Palpitations | 0 | 0 | 0 |
| 2 | 67 | M | Palpitations | 0 | 0 | HOLTER |
| 3 | 43 | F | Palpitations | 0 | 0 | 0 |
| 4 | 61 | M | Palpitations | 0 | 0 | ECG |
| 5 | 42 | F | Palpitations | 0 | 0 | HOLTER |
| 6 | 71 | F | Palpitations | 0 | Betablocker | ECG |
| 7 | 60 | F | Palpitations | DCM | Betablocker | ECG |
| 8 | 35 | F | Presyncope | 0 | 0 | 0 |
| 9 | 49 | F | Palpitations | 0 | Verapamil | ECG |
| 10 | 69 | F | Palpitations | 0 | 0 | ECG |
| 11 | 44 | F | Palpitations | 0 | Betablocker | 0 |
| 12 | 46 | F | Palpitations | 0 | 0 | ECG |
| 13 | 36 | F | Presyncope | 0 | 0 | 0 |
| PRE-ABLATION | POST-ABLATION | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | AH (ms) | HV (ms) | AV (ms) | VA (ms) | J | H | DP SR | DP AP | TCL (ms) | AH (ms) | HV (ms) | AV (ms) | DP SR | DP AP |
| 1 | 146 | 34 | 353 | - | + | - | 30 | - | 300 | 95 | 38 | - | 60 | - |
| 2 | 112 | 48 | 400 | 429 | - | - | - | - | 270 | 100 | 42 | 340 | 52 | 64 |
| 3 | 108 | 40 | 286 | 324 | + | - | 52 | - | 350 | 92 | 40 | 333 | 66 | - |
| 4 | 95 | 72 | 375 | 261 | - | + | 44 | - | 310 | - | - | - | 72 | 90 |
| 5 | 115 | 55 | 261 | 286 | - | + | 35 | 52 | 380 | 100 | 52 | 273 | 58 | 74 |
| 6 | 106 | 50 | 300 | 316 | + | + | 28 | - | 410 | - | - | - | 76 | - |
| 7 | 110 | 62 | 333 | 375 | - | + | - | 39 | 415 | - | - | 333 | - | 100 |
| 8 | 88 | 50 | 600 | 600 | - | - | - | - | 300 | 95 | 46 | 333 | - | 80 |
| 9 | 94 | 50 | 316 | 353 | - | + | - | 61 | 230* | 85 | 40 | 240 | 30 | - |
| 10 | 72 | 52 | 353 | - | - | + | 45 | - | 350 | 90 | 54 | 353 | 63 | - |
| 11 | 118 | 32 | 274 | 316 | - | + | - | 30 | 350 | 110 | 36 | 414 | 62 | 98 |
| 12 | 86 | 40 | 316 | 353 | - | + | - | 25 | 375 | 90 | 38 | 316 | 76 | - |
| 13 | 164 | 46 | 343 | 350 | - | - | - | 25 | 280 | - | - | 353 | - | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clementy, N.; Pineaud, G.; Bisson, A.; Babuty, D. Atrioventricular Nodal Reentrant Tachycardia Ablation Using Mini-Electrode Recordings. J. Clin. Med. 2022, 11, 282. https://doi.org/10.3390/jcm11010282
Clementy N, Pineaud G, Bisson A, Babuty D. Atrioventricular Nodal Reentrant Tachycardia Ablation Using Mini-Electrode Recordings. Journal of Clinical Medicine. 2022; 11(1):282. https://doi.org/10.3390/jcm11010282
Chicago/Turabian StyleClementy, Nicolas, Gérôme Pineaud, Arnaud Bisson, and Dominique Babuty. 2022. "Atrioventricular Nodal Reentrant Tachycardia Ablation Using Mini-Electrode Recordings" Journal of Clinical Medicine 11, no. 1: 282. https://doi.org/10.3390/jcm11010282
APA StyleClementy, N., Pineaud, G., Bisson, A., & Babuty, D. (2022). Atrioventricular Nodal Reentrant Tachycardia Ablation Using Mini-Electrode Recordings. Journal of Clinical Medicine, 11(1), 282. https://doi.org/10.3390/jcm11010282






