Sex Hormone Receptor Expression in Craniopharyngiomas and Association with Tumor Aggressiveness Characteristics
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients and Samples
2.2. Histopathology and Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. Patient and Sample Characteristics
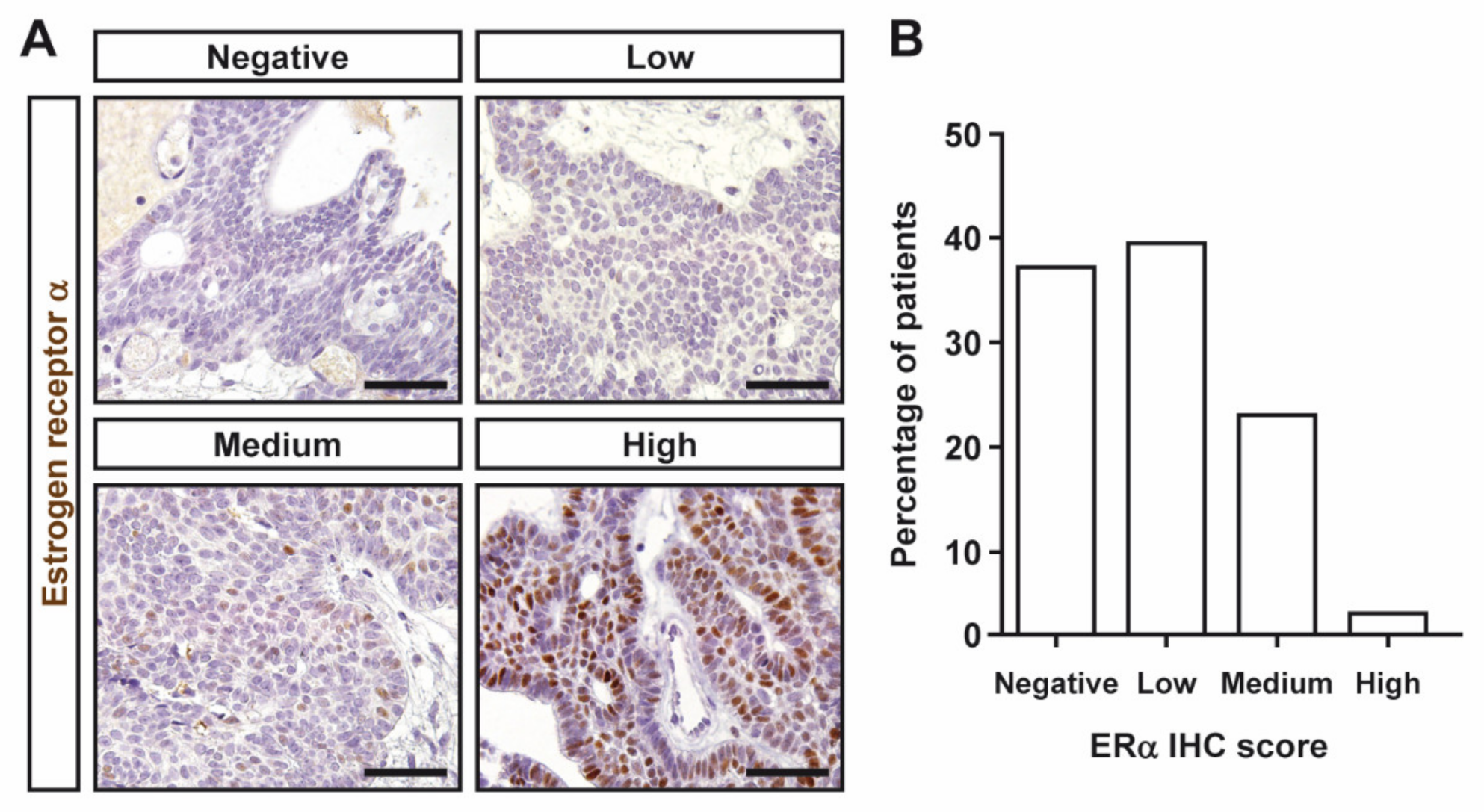
3.2. Estrogen and Progesterone Receptor Expression in Craniopharyngiomas
3.3. Association between Estrogen and Progesterone Receptor Levels and Clinical Features of Craniopharyngiomas
3.4. β-Catenin Expression and CP Recurrence
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller, H.L. Craniopharyngioma. Endocr. Rev. 2014, 35, 513–543. [Google Scholar] [CrossRef]
- Muller, H.L.; Merchant, T.E.; Warmuth-Metz, M.; Martinez-Barbera, J.P.; Puget, S. Craniopharyngioma. Nat. Rev. Dis. Primers 2019, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Bunin, G.R.; Surawicz, T.S.; Witman, P.A.; Preston-Martin, S.; Davis, F.; Bruner, J.M. The descriptive epidemiology of craniopharyngioma. J. Neurosurg. 1998, 89, 547–551. [Google Scholar] [CrossRef]
- Sekine, S.; Shibata, T.; Kokubu, A.; Morishita, Y.; Noguchi, M.; Nakanishi, Y.; Sakamoto, M.; Hirohashi, S. Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am. J. Pathol. 2002, 161, 1997–2001. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Taylor-Weiner, A.; Manley, P.E.; Jones, R.T.; Dias-Santagata, D.; Thorner, A.R.; Lawrence, M.S.; Rodriguez, F.J.; Bernardo, L.A.; Schubert, L.; et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat. Genet. 2014, 46, 161–165. [Google Scholar] [CrossRef]
- Cossu, G.; Jouanneau, E.; Cavallo, L.M.; Elbabaa, S.K.; Giammattei, L.; Starnoni, D.; Barges-Coll, J.; Cappabianca, P.; Benes, V.; Baskaya, M.K.; et al. Surgical management of craniopharyngiomas in adult patients: A systematic review and consensus statement on behalf of the EANS skull base section. Acta Neurochir. 2020, 162, 1159–1177. [Google Scholar] [CrossRef] [Green Version]
- Ottenhausen, M.; Rumalla, K.; La Corte, E.; Alalade, A.; Nair, P.; Forbes, J.; Ben Nsir, A.; Schwartz, T.H. Treatment strategies for craniopharyngiomas. J. Neurosurg. Sci. 2019, 63, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Buchfelder, M.; Schlaffer, S.M.; Lin, F.; Kleindienst, A. Surgery for craniopharyngioma. Pituitary 2013, 16, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Apra, C.; Enachescu, C.; Lapras, V.; Raverot, G.; Jouanneau, E. Is Gross Total Resection Reasonable in Adults with Craniopharyngiomas with Hypothalamic Involvement? World Neurosurg. 2019, 129, e803–e811. [Google Scholar] [CrossRef] [PubMed]
- Bulow, B.; Attewell, R.; Hagmar, L.; Malmstrom, P.; Nordstrom, C.H.; Erfurth, E.M. Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J. Clin. Endocrinol. Metab. 1998, 83, 3897–3904. [Google Scholar] [CrossRef] [PubMed]
- Dandurand, C.; Sepehry, A.A.; Asadi Lari, M.H.; Akagami, R.; Gooderham, P. Adult Craniopharyngioma: Case Series, Systematic Review, and Meta-Analysis. Neurosurgery 2018, 83, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Prieto, R.; Pascual, J.M.; Subhi-Issa, I.; Jorquera, M.; Yus, M.; Martinez, R. Predictive factors for craniopharyngioma recurrence: A systematic review and illustrative case report of a rapid recurrence. World Neurosurg. 2013, 79, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Coury, J.R.; Davis, B.N.; Koumas, C.P.; Manzano, G.S.; Dehdashti, A.R. Histopathological and molecular predictors of growth patterns and recurrence in craniopharyngiomas: A systematic review. Neurosurg. Rev. 2020, 43, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Prieto, R.; Pascual, J.M. Can tissue biomarkers reliably predict the biological behavior of craniopharyngiomas? A comprehensive overview. Pituitary 2018, 21, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, E.; De Divitiis, O.; Solari, D.; Borrelli, G.; Bracale, U.M.; Di Somma, A.; Cappabianca, P.; Del Basso De Caro, M. Can recurrences be predicted in craniopharyngiomas? Beta-catenin coexisting with stem cells markers and p-ATM in a clinicopathologic study of 45cases. J. Exp. Clin. Cancer Res. 2017, 36, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, J.; Huang, S.; You, C. Aberrant membranous expression of beta-catenin predicts poor prognosis in patients with craniopharyngioma. Ann. Diagn. Pathol. 2015, 19, 403–408. [Google Scholar] [CrossRef]
- Honegger, J.; Renner, C.; Fahlbusch, R.; Adams, E.F. Progesterone receptor gene expression in craniopharyngiomas and evidence for biological activity. Neurosurgery 1997, 41, 1359–1363, discussion 1363–1354. [Google Scholar] [CrossRef] [PubMed]
- Thapar, K.; Stefaneanu, L.; Kovacs, K.; Scheithauer, B.W.; Lloyd, R.V.; Muller, P.J.; Laws, E.R., Jr. Estrogen receptor gene expression in craniopharyngiomas: An in situ hybridization study. Neurosurgery 1994, 35, 1012–1017. [Google Scholar] [CrossRef]
- Pravdenkova, S.; Al-Mefty, O.; Sawyer, J.; Husain, M. Progesterone and estrogen receptors: Opposing prognostic indicators in meningiomas. J. Neurosurg. 2006, 105, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Delgrange, E.; Vasiljevic, A.; Wierinckx, A.; Francois, P.; Jouanneau, E.; Raverot, G.; Trouillas, J. Expression of estrogen receptor alpha is associated with prolactin pituitary tumor prognosis and supports the sex-related difference in tumor growth. Eur. J. Endocrinol. 2015, 172, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Oystese, K.A.; Casar-Borota, O.; Normann, K.R.; Zucknick, M.; Berg, J.P.; Bollerslev, J. Estrogen Receptor alpha, a Sex-Dependent Predictor of Aggressiveness in Nonfunctioning Pituitary Adenomas: SSTR and Sex Hormone Receptor Distribution in NFPA. J. Clin. Endocrinol. Metab. 2017, 102, 3581–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumoto, S.; Suzuki, T.; Kinoshita, M.; Hashiba, T.; Kagawa, N.; Wada, K.; Fujimoto, Y.; Hashimoto, N.; Saitoh, Y.; Maruno, M.; et al. Immunohistochemical detection of female sex hormone receptors in craniopharyngiomas: Correlation with clinical and histologic features. Surg. Neurol. 2005, 63, 520–525, discussion 525. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B.M.; Hoelsken, A.; Fahlbusch, R.; Blumcke, I.; Buslei, R. Hormone receptor expression in craniopharyngiomas: A clinicopathological correlation. Neurosurgery 2010, 67, 617–625, discussion 625. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Moreno, E.; Vazquez-Borrego, M.C.; Dios, E.; Gros-Herguido, N.; Flores-Martinez, A.; Rivero-Cortes, E.; Madrazo-Atutxa, A.; Japon, M.A.; Luque, R.M.; Castano, J.P.; et al. Association between dopamine and somatostatin receptor expression and pharmacological response to somatostatin analogues in acromegaly. J. Cell Mol. Med. 2018, 22, 1640–1649. [Google Scholar] [CrossRef] [Green Version]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Investig. 2018, 98, 844–855. [Google Scholar] [CrossRef]
- Magge, S.N.; Brunt, M.; Scott, R.M. Craniopharyngioma presenting during pregnancy 4 years after a normal magnetic resonance imaging scan: Case report. Neurosurgery 2001, 49, 1014–1016, conclusion 1016–1017. [Google Scholar] [CrossRef]
- Aydin, Y.; Can, S.M.; Gulkilik, A.; Turkmenoglu, O.; Alatli, C.; Ziyal, I. Rapid enlargement and recurrence of a preexisting intrasellar craniopharyngioma during the course of two pregnancies. Case report. J. Neurosurg. 1999, 91, 322–324. [Google Scholar] [CrossRef]
- Lund-Iversen, M.; Scott, H.; Strom, E.H.; Theiss, N.; Brustugun, O.T.; Gronberg, B.H. Expression of Estrogen Receptor-alpha and Survival in Advanced-stage Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 2261–2269. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Song, Y.; Xu, H.; Zhou, K.; Zhang, W.; Chen, J.; Qin, M.; Yi, H.; Gustafsson, J.A.; Yang, H.; et al. In nonfunctional pituitary adenomas, estrogen receptors and slug contribute to development of invasiveness. J. Clin. Endocrinol. Metab. 2011, 96, E1237–E1245. [Google Scholar] [CrossRef] [Green Version]


| Characteristics | |
|---|---|
| Sex (% female) | 46.3% |
| Age at diagnosis (years, median, IQR) | 30 (9.5–58) |
| Pediatric patients [younger than 16] years (n, %) | 15 (36.6%) |
| Maximum tumor diameter at diagnosis (mm, median, IQR) * | 34 (25–40) |
| Histopathologic type (n, %) | |
| Adamantinomatous | 36 (87.8%) |
| Papillary | 5 (12.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ortega, A.; Flores-Martinez, Á.; Venegas-Moreno, E.; Dios, E.; Del Can, D.; Rivas, E.; Kaen, A.; Cárdenas Ruiz-Valdepeñas, E.; Fajardo, E.; Roldán, F.; et al. Sex Hormone Receptor Expression in Craniopharyngiomas and Association with Tumor Aggressiveness Characteristics. J. Clin. Med. 2022, 11, 281. https://doi.org/10.3390/jcm11010281
Martínez-Ortega A, Flores-Martinez Á, Venegas-Moreno E, Dios E, Del Can D, Rivas E, Kaen A, Cárdenas Ruiz-Valdepeñas E, Fajardo E, Roldán F, et al. Sex Hormone Receptor Expression in Craniopharyngiomas and Association with Tumor Aggressiveness Characteristics. Journal of Clinical Medicine. 2022; 11(1):281. https://doi.org/10.3390/jcm11010281
Chicago/Turabian StyleMartínez-Ortega, Antonio, Álvaro Flores-Martinez, Eva Venegas-Moreno, Elena Dios, Diego Del Can, Eloy Rivas, Ariel Kaen, Eugenio Cárdenas Ruiz-Valdepeñas, Elena Fajardo, Florinda Roldán, and et al. 2022. "Sex Hormone Receptor Expression in Craniopharyngiomas and Association with Tumor Aggressiveness Characteristics" Journal of Clinical Medicine 11, no. 1: 281. https://doi.org/10.3390/jcm11010281
APA StyleMartínez-Ortega, A., Flores-Martinez, Á., Venegas-Moreno, E., Dios, E., Del Can, D., Rivas, E., Kaen, A., Cárdenas Ruiz-Valdepeñas, E., Fajardo, E., Roldán, F., González-Rivera, N., Oliva, R., Fernández-Peña, J. I., Soto-Moreno, A., & Cano, D. A. (2022). Sex Hormone Receptor Expression in Craniopharyngiomas and Association with Tumor Aggressiveness Characteristics. Journal of Clinical Medicine, 11(1), 281. https://doi.org/10.3390/jcm11010281







