The 2020 “Padua Criteria” for Diagnosis and Phenotype Characterization of Arrhythmogenic Cardiomyopathy in Clinical Practice
Abstract
:1. Background
2. The Padua Criteria for ACM Diagnosis
2.1. STEP 1: The Multiparametric Diagnostic Approach
- 1. Morpho-functional abnormalities
- 2. Structural myocardial abnormalities
- 3. ECG repolarization abnormalities
- 4. ECG depolarization abnormalities
- 5. Ventricular arrhythmias
- 6. Family history and molecular genetics.
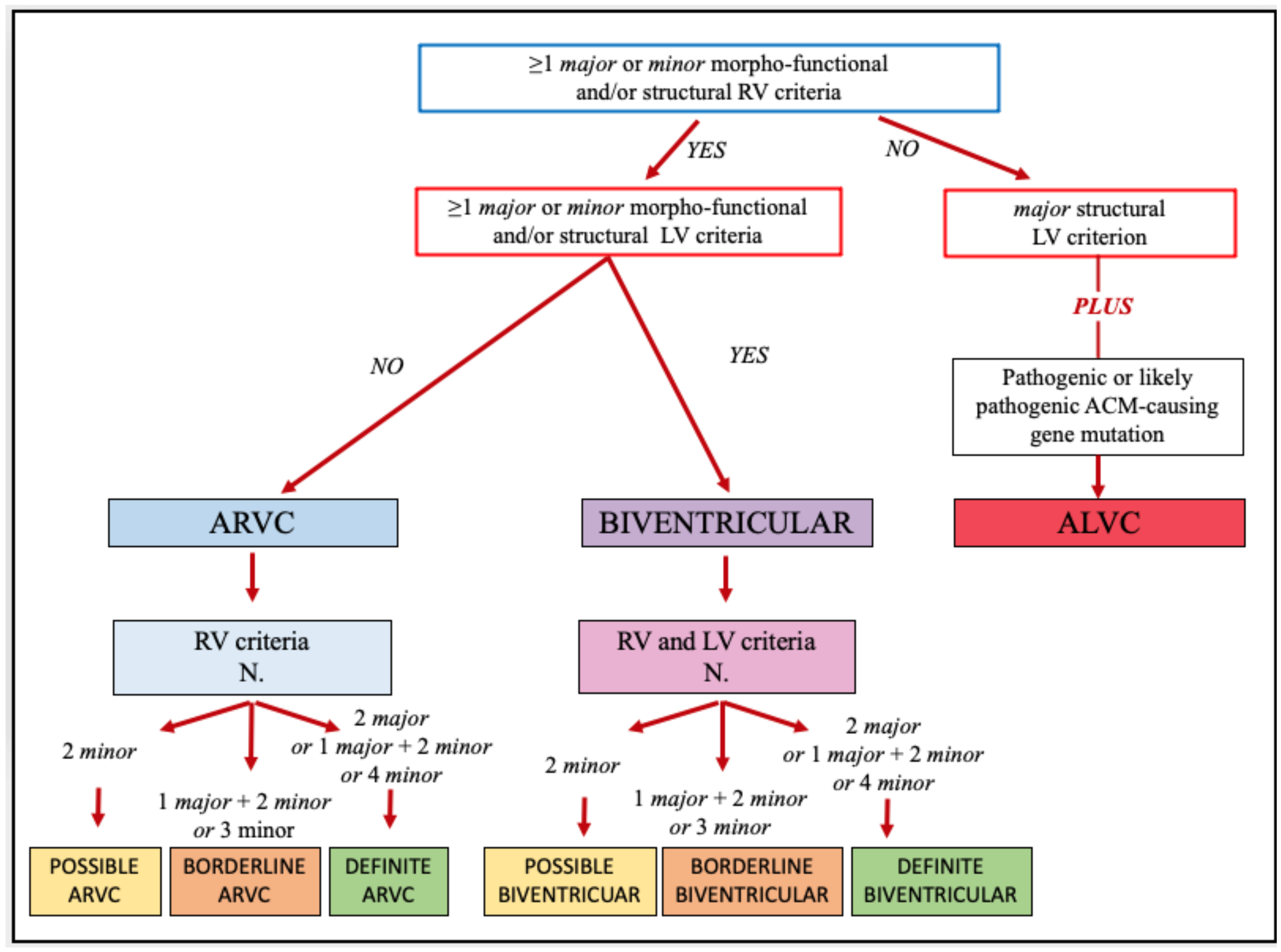
2.2. STEP 2: The Phenotype Characterization
3. Practical Application of the Padua Criteria
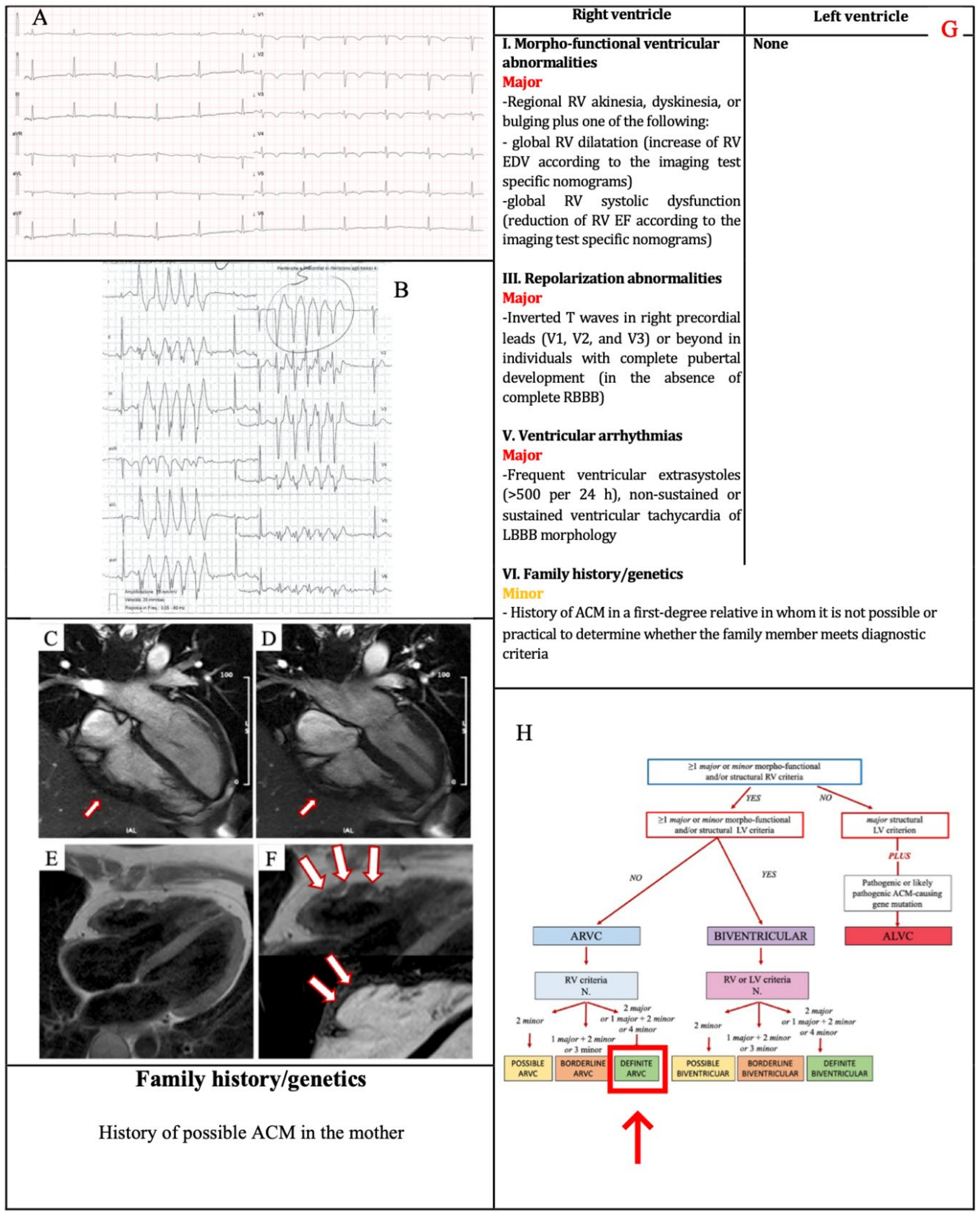
3.1. Example 1
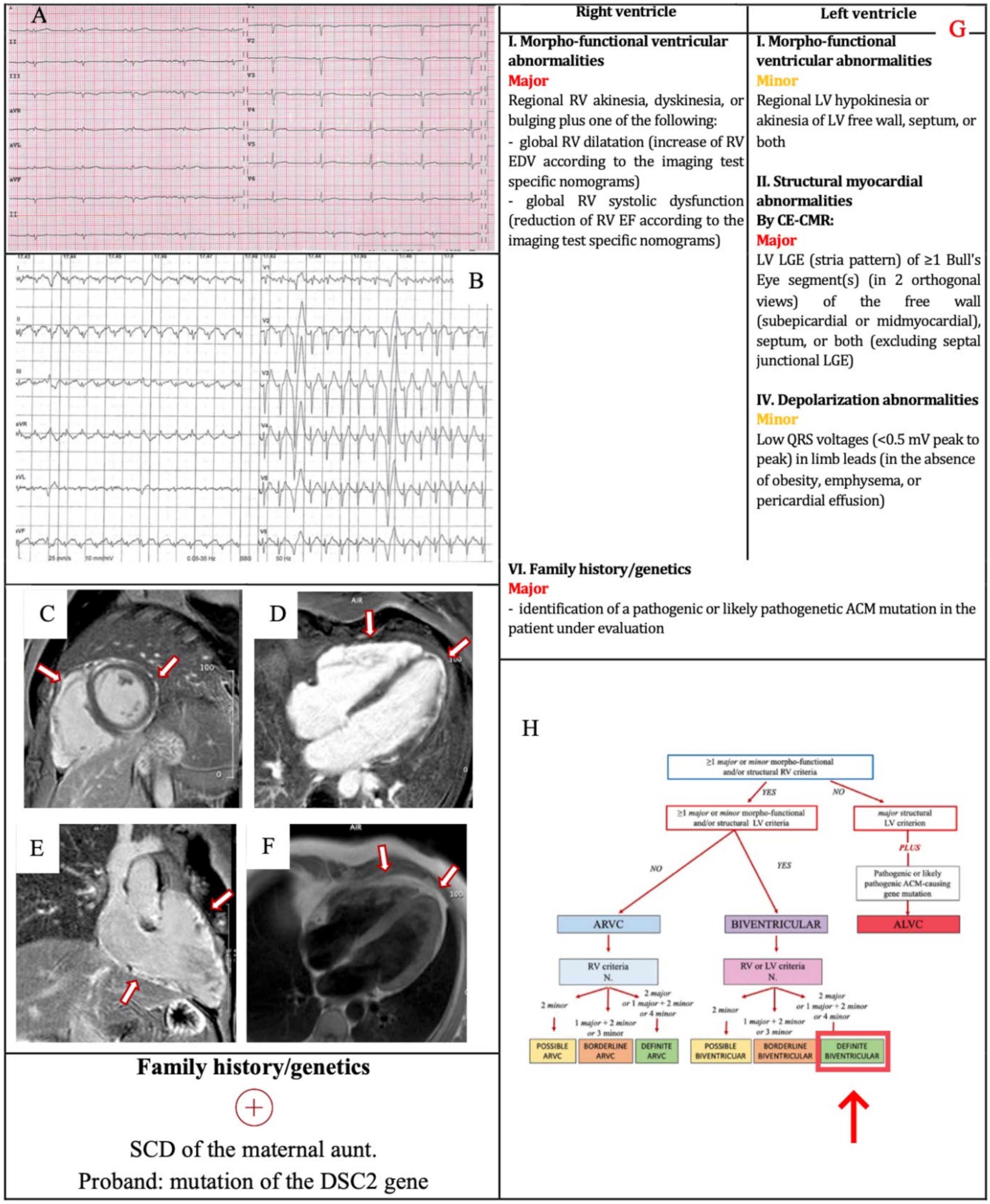
3.2. Example 2
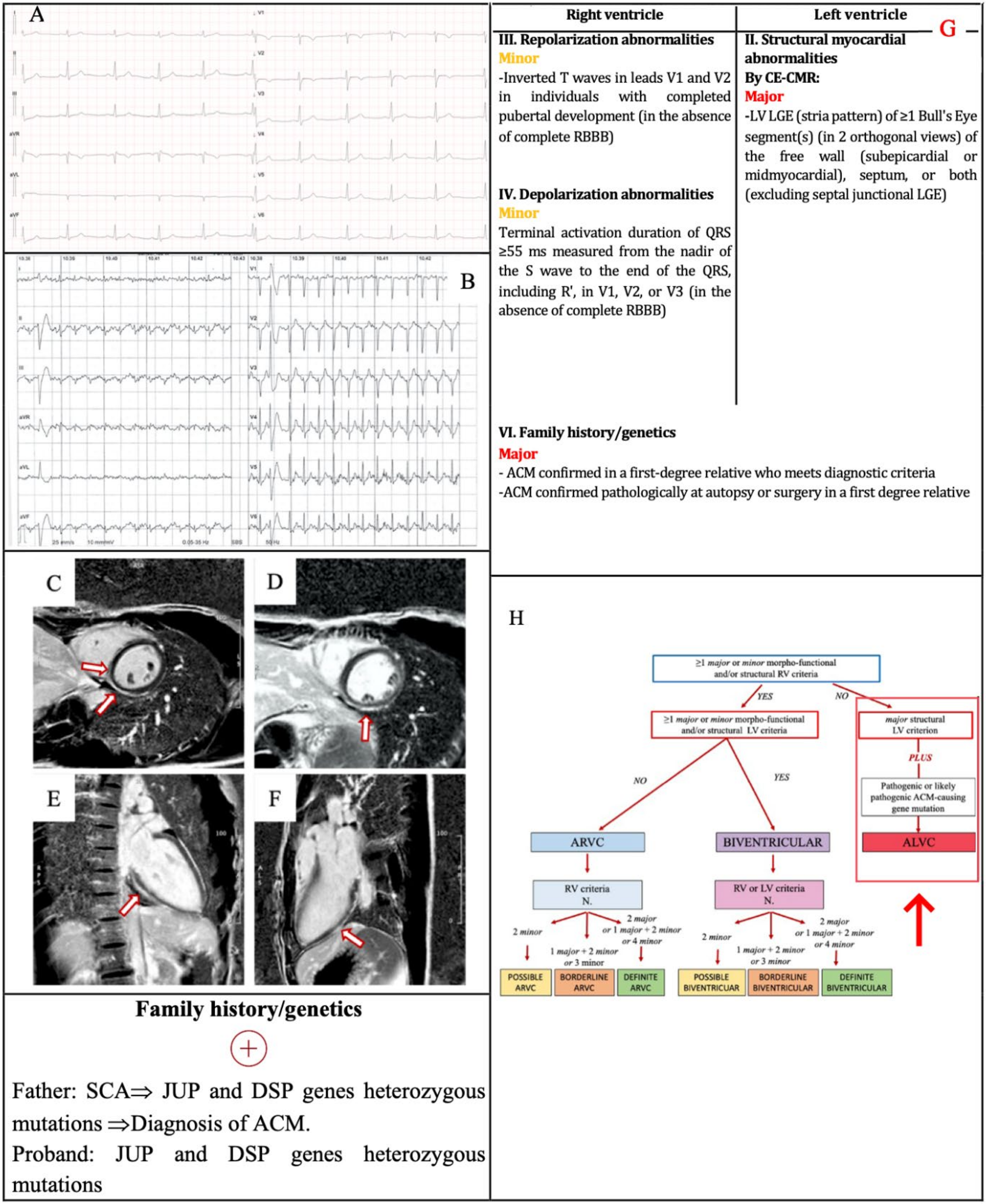
3.3. Example 3
4. Conclusions
Funding
Conflicts of Interest
References
- Thiene, G.; Nava, A.; Corrado, D.; Rossi, L.; Pennelli, N. Right Ventricular Cardiomyopathy and Sudden Death in Young People. N. Engl. J. Med. 1988, 318, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Right Ventricular Cardiomyopathy: Another Cause of Sudden Death in the Young. N. Engl. J. Med. 1988, 318, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; Fontaine, G.H.; Guiraudon, G.; Frank, R.; Laurenceau, J.L.; Malergue, C.; Grosgogeat, Y. Right Ventricular Dysplasia: A Report of 24 Adult Cases. Circulation 1982, 65, 384–398. [Google Scholar] [CrossRef] [Green Version]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef]
- Norman, M.; Simpson, M.; Mogensen, J.; Shaw, A.; Hughes, S.; Syrris, P.; Sen-Chowdhry, S.; Rowland, E.; Crosby, A.; McKenna, W.J. Novel Mutation in Desmoplakin Causes Arrhythmogenic Left Ventricular Cardiomyopathy. Circulation 2005, 112, 636–642. [Google Scholar] [CrossRef]
- McKenna, W.J.; Thiene, G.; Nava, A.; Fontaliran, F.; Blomstrom-Lundqvist, C.; Fontaine, G.; Camerini, F. Diagnosis of Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart J. 1994, 71, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Hamid, M.S.; Norman, M.; Quraishi, A.; Firoozi, S.; Thaman, R.; Gimeno, J.R.; Sachdev, B.; Rowland, E.; Elliott, P.M.; McKenna, W.J. Prospective Evaluation of Relatives for Familial Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia Reveals a Need to Broaden Diagnostic Criteria. J. Am. Coll. Cardiol. 2002, 40, 1445–1450. [Google Scholar] [CrossRef] [Green Version]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.P.J.; Daubert, J.P.; et al. Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: Proposed Modification of the Task Force Criteria. Eur. Heart J. 2010, 31, 806–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrado, D.; van Tintelen, P.J.; McKenna, W.J.; Hauer, R.N.W.; Anastastakis, A.; Asimaki, A.; Basso, C.; Bauce, B.; Brunckhorst, C.; Bucciarelli-Ducci, C.; et al. Arrhythmogenic Right Ventricular Cardiomyopathy: Evaluation of the Current Diagnostic Criteria and Differential Diagnosis. Eur. Heart J. 2020, 41, 1414–1429. [Google Scholar] [CrossRef] [Green Version]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; Lazzari, M.D.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of Arrhythmogenic Cardiomyopathy: The Padua Criteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef]
- Corrado, D.; Zorzi, A.; Cipriani, A.; Bauce, B.; Bariani, R.; Beffagna, G.; De Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; et al. Evolving Diagnostic Criteria for Arrhythmogenic Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e021987. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Cipriani, A.; Rizzo, S.; De Lazzari, M.; De Gaspari, M.; Akrami, N.; Bariani, R.; Zorzi, A.; Migliore, F.; Rigato, I.; et al. Myocardial Tissue Characterization in Arrhythmogenic Cardiomyopathy: Comparison between Endomyocardial Biopsy and Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2021, 14, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, N.; Burt, J.R.; Corona-Villalobos, C.P.; Te Riele, A.S.; James, C.A.; Murray, B.; Calkins, H.; Tandri, H.; Bluemke, D.A.; Zimmerman, S.L.; et al. Cardiac MR Findings and Potential Diagnostic Pitfalls in Patients Evaluated for Arrhythmogenic Right Ventricular Cardiomyopathy. Radiographics 2014, 34, 1553–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Khanji, M.Y.; Plein, S.; Lancellotti, P.; Bucciarelli-Ducci, C. European Association of Cardiovascular Imaging Expert Consensus Paper: A Comprehensive Review of Cardiovascular Magnetic Resonance Normal Values of Cardiac Chamber Size and Aortic Root in Adults and Recommendations for Grading Severity. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1321–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascenzi, F.; Anselmi, F.; Piu, P.; Fiorentini, C.; Carbone, S.F.; Volterrani, L.; Focardi, M.; Bonifazi, M.; Mondillo, S. Cardiac Magnetic Resonance Normal Reference Values of Biventricular Size and Function in Male Athlete’s Heart. JACC Cardiovasc. Imaging 2019, 12, 1755–1765. [Google Scholar] [CrossRef]
- Augusto, J.B.; Eiros, R.; Nakou, E.; Moura-Ferreira, S.; Treibel, T.A.; Captur, G.; Akhtar, M.M.; Protonotarios, A.; Gossios, T.D.; Savvatis, K.; et al. Dilated Cardiomyopathy and Arrhythmogenic Left Ventricular Cardiomyopathy: A Comprehensive Genotype-Imaging Phenotype Study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 326–336. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Barison, A.; Todiere, G.; Grigoratos, C.; Ait Ali, L.; Di Bella, G.; Emdin, M.; Festa, P. Usefulness of Combined Functional Assessment by Cardiac Magnetic Resonance and Tissue Characterization Versus Task Force Criteria for Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy. Am. J. Cardiol. 2016, 118, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, M.; Zorzi, A.; Cipriani, A.; Susana, A.; Mastella, G.; Rizzo, A.; Rigato, I.; Bauce, B.; Giorgi, B.; Lacognata, C.; et al. Relationship between Electrocardiographic Findings and Cardiac Magnetic Resonance Phenotypes in Arrhythmogenic Cardiomyopathy. J. Am. Heart Assoc. 2018, 7, e009855. [Google Scholar] [CrossRef] [Green Version]
- Platonov, P.G.; Calkins, H.; Hauer, R.N.; Corrado, D.; Svendsen, J.H.; Wichter, T.; Biernacka, E.K.; Saguner, A.M.; Te Riele, A.S.J.M.; Zareba, W. High Interobserver Variability in the Assessment of Epsilon Waves: Implications for Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia. Heart Rhythm. 2016, 13, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriani, A.; Zorzi, A.; Sarto, P.; Donini, M.; Rigato, I.; Bariani, R.; De Lazzari, M.; Pilichou, K.; Thiene, G.; Iliceto, S.; et al. Predictive Value of Exercise Testing in Athletes with Ventricular Ectopy Evaluated by Cardiac Magnetic Resonance. Heart Rhythm. 2019, 16, 239–248. [Google Scholar] [CrossRef] [PubMed]
| Criteria for RV Involvement | Criteria for LV Involvement | |
|---|---|---|
| I. Morpho-functional ventricular abnormalities | By 2D echocardiogram,CMR or angiography: Major • Regional RV akinesia, dyskinesia or bulging plus one of the following: - global RV dilatation (increase of RV EDV according to the imaging test specific monograms for age, sex and BSA) or - global RV systolic dysfunction (reduction of RV EF according to the imaging test specific monograms for age and sex) | By 2D echocardiogram,CMR or angiography: Minor • Global LV systolic dysfunction (depression of LV EF or reduction of echocardiographic global longitudinal strain), with or without LV dilatation (increase in LV EDV according to the imaging test specific nomograms for age, sex, and BSA) |
| Minor • Regional RV akinesia, dyskinesia or aneurysm of RV free wall | Minor • Regional LV hypokinesia or akinesia of LV free wall, septum or both | |
| II. Structural myocardial abnormalities | By CECMR: Major • Transmural LGE (stria pattern) of ≥1 RV region(s) (inlet, outlet, and apex in 2 orthogonal views) | By CECMR: Major • LV LGE (stria pattern) of ≥1 Bull’s Eye segment(s) (in 2 orthogonal views) of the free wall (subepicardial or midmyocardial), septum or both (excluding septal junctional LGE) |
| By EMB (limited indications): Major • Fibrous replacement of the myocardium in ≥1 sample, with or without fatty tissue | ||
| III. ECG repolarization abnormalities | Major • Inverted T waves in right precordial leads (V1, V2 and V3) or beyond in individuals with complete pubertal development (in the absence of complete RBBB) Minor
| Minor • Inverted T waves in left precordial leads (V4–V6) without complete LBBB |
| IV. ECG depolarization abnormalities | Minor
| Minor • Low QRS voltages (<0.5 mV peak to peak) in limb leads (in the absence of obesity, emphysema or pericardial effusion) |
| V. Ventricular arrhythmias | Major • Frequent ventricular extrasystoles (>500 per 24 h) or non-sustained or sustained ventricular tachycardia of LBBB morphology Minor • Frequent ventricular extrasystoles (>500 per 24 h) or non-sustained or sustained ventricular tachycardia of LBBB morphology with inferior axis (“RVOT pattern”) | Minor • Frequent ventricular extrasystoles (>500 per 24 h) or non-sustained or sustained ventricular tachycardia with an RBBB morphology (excluding the “fascicular pattern”) |
| VI. Family history/genetics | Major
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziano, F.; Zorzi, A.; Cipriani, A.; De Lazzari, M.; Bauce, B.; Rigato, I.; Brunetti, G.; Pilichou, K.; Basso, C.; Perazzolo Marra, M.; et al. The 2020 “Padua Criteria” for Diagnosis and Phenotype Characterization of Arrhythmogenic Cardiomyopathy in Clinical Practice. J. Clin. Med. 2022, 11, 279. https://doi.org/10.3390/jcm11010279
Graziano F, Zorzi A, Cipriani A, De Lazzari M, Bauce B, Rigato I, Brunetti G, Pilichou K, Basso C, Perazzolo Marra M, et al. The 2020 “Padua Criteria” for Diagnosis and Phenotype Characterization of Arrhythmogenic Cardiomyopathy in Clinical Practice. Journal of Clinical Medicine. 2022; 11(1):279. https://doi.org/10.3390/jcm11010279
Chicago/Turabian StyleGraziano, Francesca, Alessandro Zorzi, Alberto Cipriani, Manuel De Lazzari, Barbara Bauce, Ilaria Rigato, Giulia Brunetti, Kalliopi Pilichou, Cristina Basso, Martina Perazzolo Marra, and et al. 2022. "The 2020 “Padua Criteria” for Diagnosis and Phenotype Characterization of Arrhythmogenic Cardiomyopathy in Clinical Practice" Journal of Clinical Medicine 11, no. 1: 279. https://doi.org/10.3390/jcm11010279
APA StyleGraziano, F., Zorzi, A., Cipriani, A., De Lazzari, M., Bauce, B., Rigato, I., Brunetti, G., Pilichou, K., Basso, C., Perazzolo Marra, M., & Corrado, D. (2022). The 2020 “Padua Criteria” for Diagnosis and Phenotype Characterization of Arrhythmogenic Cardiomyopathy in Clinical Practice. Journal of Clinical Medicine, 11(1), 279. https://doi.org/10.3390/jcm11010279







