Temporal Artery Vascular Diseases
Abstract
1. Introduction
2. Temporal Arteritis
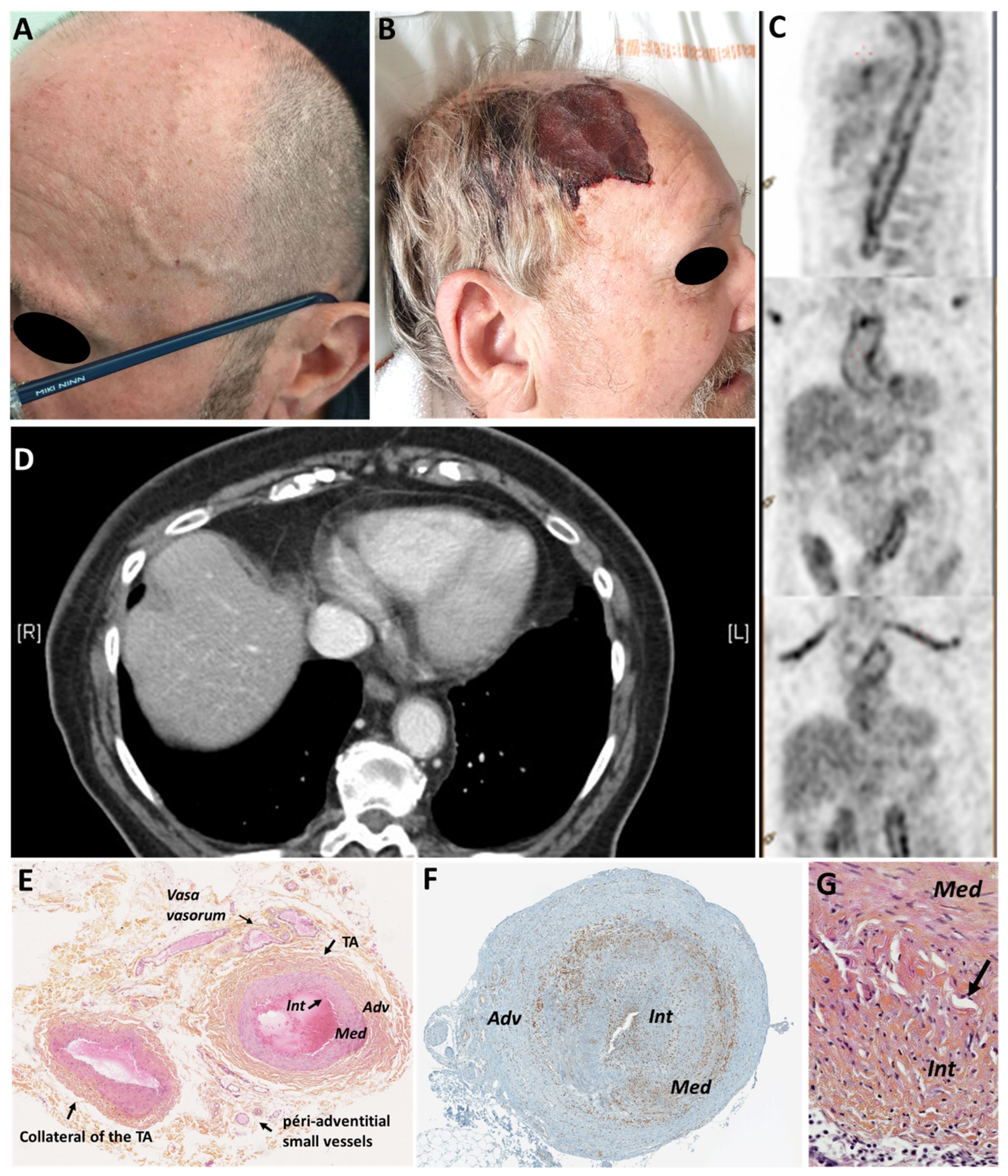
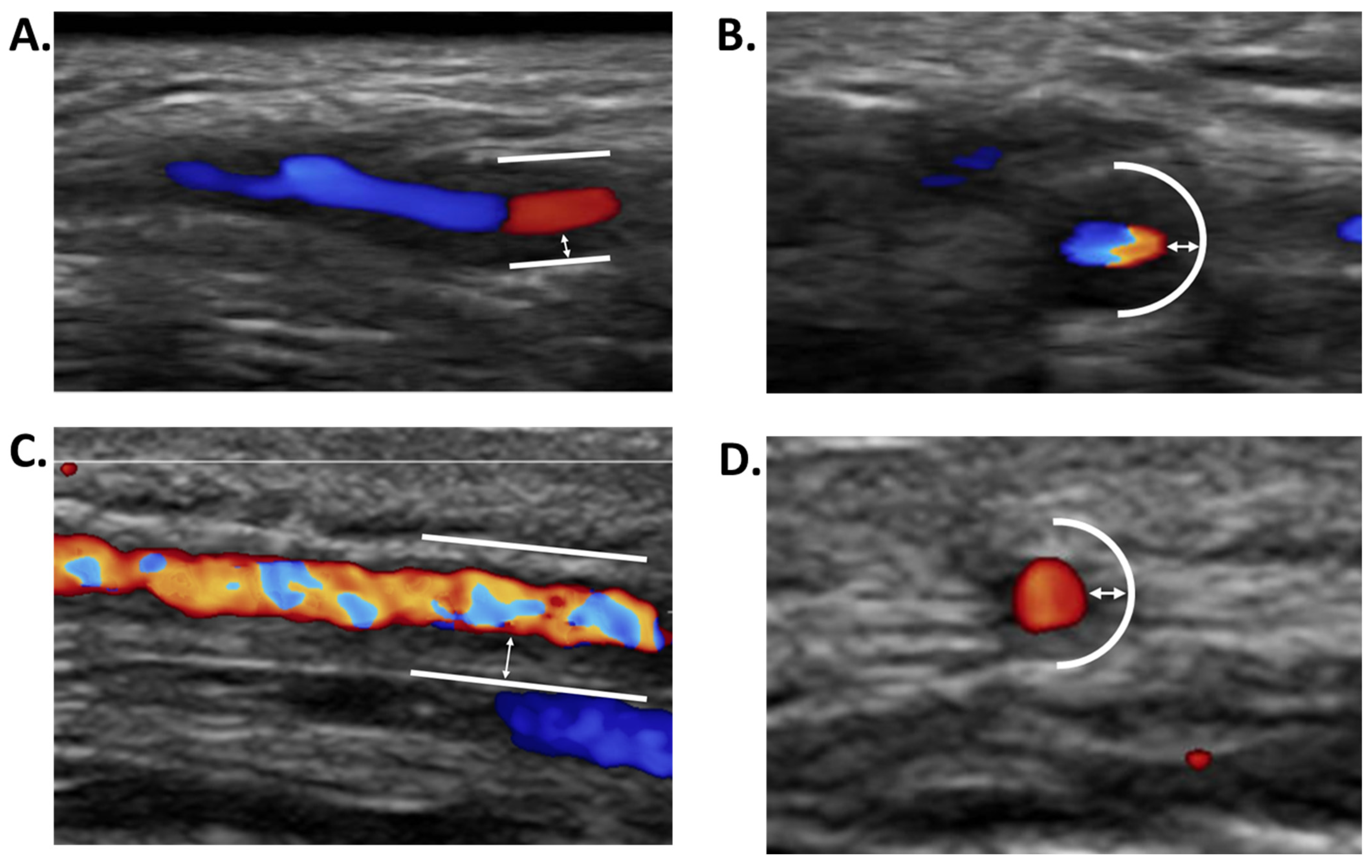
2.1. Giant Cell Arteritis (GCA)
2.2. Differential Diagnoses of GCA
2.2.1. Necrotizing Vasculitis
2.2.2. Inflammatory Diseases Limited to the Adventitia
2.2.3. IgG4-Related Disease
2.2.4. Sarcoidosis
2.2.5. VZV Vasculitis
3. Others Temporal Artery Vascular Diseases
3.1. Post-Traumatic Complications of the Temporal Arteries
3.2. Atheromatous Disease
3.3. Calcifying Uremic Arteriolopathy (Calciphylaxis)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salvarani, C.; Cantini, F.; Boiardi, L.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. N. Engl. J. Med. 2002, 347, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Hunder, G.G.; Bloch, D.A.; Michel, B.A.; Stevens, M.B.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990, 33, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Medium- and large-vessel vasculitis. N. Engl. J. Med. 2003, 349, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C. Widespread headache as the first clinical manifestation of giant cell arteritis in patients affected by polymyalgia rheumatica. Reumatologia 2016, 54, 236–238. [Google Scholar] [CrossRef]
- Salvarani, C.; Cantini, F.; Hunder, G.G. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008, 372, 234–245. [Google Scholar] [CrossRef]
- Mournet, S.; Sene, T.; Charbonneau, F.; Poillon, G.; Vignal, C.; Clavel, G.; Zuber, K.; Savatovsky, J.; Lecler, A. High-resolution MRI demonstrates signal abnormalities of the 3rd cranial nerve in giant cell arteritis patients with 3rd cranial nerve impairment. Eur. Radiol. 2021, 31, 4472–4480. [Google Scholar] [CrossRef]
- de Boysson, H.; Liozon, E.; Larivière, D.; Samson, M.; Parienti, J.J.; Boutemy, J.; Maigné, G.; Martin Silva, N.; Ly, K.; Touzé, E.; et al. Giant Cell Arteritis-related Stroke: A Retrospective Multicenter Case-control Study. J. Rheumatol. 2017, 44, 297–303. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Vazquez-Rodriguez, T.R.; Gomez-Acebo, I.; Pego-Reigosa, R.; Lopez-Diaz, M.J.; Vazquez-Trinanes, M.C.; Miranda-Filloy, J.A.; Blanco, R.; Dierssen, T.; Gonzalez-Juanatey, C.; et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine 2009, 88, 227–235. [Google Scholar] [CrossRef]
- Samson, M.; Jacquin, A.; Audia, S.; Daubail, B.; Devilliers, H.; Petrella, T.; Martin, L.; Durier, J.; Besancenot, J.F.; Lorcerie, B.; et al. Stroke associated with giant cell arteritis: A population-based study. J. Neurol. Neurosurg. Psychiatry 2015, 86, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Horton, B.T.; Magath, T.B.; Brown, G.E. An undescribed form of arteritis of the temporal vessels. Proc. Staff Meet Mayo Clin. 1932, 7, 700–701. [Google Scholar]
- Dejaco, C.; Duftner, C.; Buttgereit, F.; Matteson, E.L.; Dasgupta, B. The spectrum of giant cell arteritis and polymyalgia rheumatica: Revisiting the concept of the disease. Rheumatology 2017, 56, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Kermani, T.A.; Warrington, K.J.; Crowson, C.S.; Ytterberg, S.R.; Hunder, G.G.; Gabriel, S.E.; Matteson, E.L. Large-vessel involvement in giant cell arteritis: A population-based cohort study of the incidence-trends and prognosis. Ann. Rheum. Dis. 2013, 72, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Schönau, V.; Vogel, K.; Englbrecht, M.; Wacker, J.; Schmidt, D.; Manger, B.; Kuwert, T.; Schett, G. The value of 18F-FDG-PET/CT in identifying the cause of fever of unknown origin (FUO) and inflammation of unknown origin (IUO): Data from a prospective study. Ann. Rheum. Dis. 2018, 77, 70–77. [Google Scholar] [CrossRef]
- Prieto-Gonzalez, S.; Depetris, M.; Garcia-Martinez, A.; Espigol-Frigole, G.; Tavera-Bahillo, I.; Corbera-Bellata, M.; Planas-Rigol, E.; Alba, M.A.; Hernandez-Rodriguez, J.; Grau, J.M.; et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: A prospective, case-control study. Ann. Rheum. Dis. 2014, 73, 1388–1392. [Google Scholar] [CrossRef]
- Ghinoi, A.; Pipitone, N.; Nicolini, A.; Boiardi, L.; Silingardi, M.; Germano, G.; Salvarani, C. Large-vessel involvement in recent-onset giant cell arteritis: A case-control colour-Doppler sonography study. Rheumatology 2012, 51, 730–734. [Google Scholar] [CrossRef]
- Kermani, T.A.; Warrington, K.J. Prognosis and monitoring of giant cell arteritis and associated complications. Expert Rev. Clin. Immunol. 2018, 14, 379–388. [Google Scholar] [CrossRef]
- Blockmans, D.; de Ceuninck, L.; Vanderschueren, S.; Knockaert, D.; Mortelmans, L.; Bobbaers, H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A prospective study of 35 patients. Arthritis Rheum. 2006, 55, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef]
- Rinagel, M.; Chatelus, E.; Jousse-Joulin, S.; Sibilia, J.; Gottenberg, J.E.; Chasset, F.; Arnaud, L. Diagnostic performance of temporal artery ultrasound for the diagnosis of giant cell arteritis: A systematic review and meta-analysis of the literature. Autoimmun. Rev. 2019, 18, 56–61. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, E.; Monjo-Henry, I.; Bonilla, G.; Plasencia, C.; Miranda-Carus, M.E.; Balsa, A.; De Miguel, E. False positives in the ultrasound diagnosis of giant cell arteritis: Some diseases can also show the halo sign. Rheumatology 2020, 59, 2443–2447. [Google Scholar] [CrossRef]
- Molina Collada, J.; Ruiz Bravo-Burguillos, E.; Monjo, I.; Bonilla, G.; Fernandez, E.; Balsa, A.; De Miguel, E. Positive ultrasound halo sign of temporal arteries due to amyloidosis. Rheumatology 2019, 58, 2067–2069. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, A.; Muratore, F.; Boiardi, L.; Restuccia, G.; Pipitone, N.; Pazzola, G.; Tagliavini, E.; Ragazzi, M.; Rossi, G.; Salvarani, C. Inflamed temporal artery: Histologic findings in 354 biopsies, with clinical correlations. Am. J. Surg. Pathol. 2014, 38, 1360–1370. [Google Scholar] [CrossRef]
- Kanaan, M.Z.; Lorenzi, A.R.; Thampy, N.; Pandit, R.; Dayan, M. Bilateral Non-arteritic Anterior Ischaemic Optic Neuropathy as the Presentation of Systemic Amyloidosis. Neuroophthalmology 2017, 41, 330–334. [Google Scholar] [CrossRef]
- Luqmani, R.; Lee, E.; Singh, S.; Gillett, M.; Schmidt, W.A.; Bradburn, M.; Dasgupta, B.; Diamantopoulos, A.P.; Forrester-Barker, W.; Hamilton, W.; et al. The Role of Ultrasound Compared to Biopsy of Temporal Arteries in the Diagnosis and Treatment of Giant Cell Arteritis (TABUL): A diagnostic accuracy and cost-effectiveness study. Health Technol. Assess. 2016, 20, 1–238. [Google Scholar] [CrossRef]
- De Boysson, H.; Daumas, A.; Vautier, M.; Parienti, J.J.; Liozon, E.; Lambert, M.; Samson, M.; Ebbo, M.; Dumont, A.; Sultan, A.; et al. Large-vessel involvement and aortic dilation in giant-cell arteritis. A multicenter study of 549 patients. Autoimmun. Rev. 2018, 17, 391–398. [Google Scholar] [CrossRef]
- Espitia, O.; Agard, C. Aortitis in giant cell arteritis and its complications. Rev. Med. Interne 2013, 34, 412–420. [Google Scholar] [CrossRef]
- Espitia, O.; Neel, A.; Leux, C.; Connault, J.; Espitia-Thibault, A.; Ponge, T.; Dupas, B.; Barrier, J.H.; Hamidou, M.A.; Agard, C. Giant cell arteritis with or without aortitis at diagnosis. A retrospective study of 22 patients with longterm followup. J. Rheumatol. 2012, 39, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.C.; Kiran, A.; Maskell, J.; Hutchings, A.; Arden, N.; Dasgupta, B.; Hamilton, W.; Emin, A.; Culliford, D.; Luqmani, R.A. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann. Rheum. Dis. 2015, 74, 129–135. [Google Scholar] [CrossRef]
- Prieto-Gonzalez, S.; Arguis, P.; Garcia-Martinez, A.; Espigol-Frigole, G.; Tavera-Bahillo, I.; Butjosa, M.; Sanchez, M.; Hernandez-Rodriguez, J.; Grau, J.M.; Cid, M.C. Large vessel involvement in biopsy-proven giant cell arteritis: Prospective study in 40 newly diagnosed patients using CT angiography. Ann. Rheum. Dis. 2012, 71, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Tomasson, G.; Peloquin, C.; Mohammad, A.; Love, T.J.; Zhang, Y.; Choi, H.K.; Merkel, P.A. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: A cohort study. Ann. Intern. Med. 2014, 160, 73–80. [Google Scholar] [CrossRef]
- Greigert, H.; Zeller, M.; Putot, A.; Steinmetz, E.; Terriat, B.; Maza, M.; Falvo, N.; Muller, G.; Arnould, L.; Creuzot-Garcher, C.; et al. Myocardial infarction during giant cell arteritis: A cohort study. Eur. J. Intern. Med. 2021, 89, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Lopez-Diaz, M.J.; Barros, S.; Garcia-Porrua, C.; Sanchez-Andrade, A.; Paz-Carreira, J.; Martin, J.; Llorca, J. Giant cell arteritis: Laboratory tests at the time of diagnosis in a series of 240 patients. Medicine 2005, 84, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Hunder, G.G. Giant cell arteritis with low erythrocyte sedimentation rate: Frequency of occurence in a population-based study. Arthritis Rheum. 2001, 45, 140–145. [Google Scholar] [CrossRef]
- Cid, M.C.; Hernandez-Rodriguez, J.; Esteban, M.J.; Cebrian, M.; Gho, Y.S.; Font, C.; Urbano-Marquez, A.; Grau, J.M.; Kleinman, H.K. Tissue and serum angiogenic activity is associated with low prevalence of ischemic complications in patients with giant-cell arteritis. Circulation 2002, 106, 1664–1671. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Immune mechanisms in medium and large-vessel vasculitis. Nat. Rev. Rheumatol. 2013, 9, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Zhang, H.; Berry, G.; Goronzy, J.J.; Weyand, C.M. Immune checkpoint dysfunction in large and medium vessel vasculitis. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1052–H1059. [Google Scholar] [CrossRef]
- Borchers, A.T.; Gershwin, M.E. Giant cell arteritis: A review of classification, pathophysiology, geoepidemiology and treatment. Autoimmun. Rev. 2012, 11, A544–A554. [Google Scholar] [CrossRef]
- Muratore, F.; Kermani, T.A.; Crowson, C.S.; Green, A.B.; Salvarani, C.; Matteson, E.L.; Warrington, K.J. Large-vessel giant cell arteritis: A cohort study. Rheumatology 2015, 54, 463–470. [Google Scholar] [CrossRef]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; de Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef]
- Maz, M.; Chung, S.A.; Abril, A.; Langford, C.A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021, 73, 1349–1365. [Google Scholar] [CrossRef]
- Bienvenu, B.; Ly, K.H.; Lambert, M.; Agard, C.; Andre, M.; Benhamou, Y.; Bonnotte, B.; de Boysson, H.; Espitia, O.; Fau, G.; et al. Management of giant cell arteritis: Recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev. Med. Interne 2016, 37, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Aussedat, M.; Lobbes, H.; Samson, M.; Euvrard, R.; Lega, J.C.; Mainbourg, S. Epidemiology of major relapse in giant cell arteritis: A study-level meta-analysis. Autoimmun. Rev. 2022, 21, 102930. [Google Scholar] [CrossRef]
- Mainbourg, S.; Addario, A.; Samson, M.; Puechal, X.; Francois, M.; Durupt, S.; Gueyffier, F.; Cucherat, M.; Durieu, I.; Reynaud, Q.; et al. Prevalence of Giant Cell Arteritis Relapse in Patients Treated With Glucocorticoids: A Meta-Analysis. Arthritis Care Res. 2020, 72, 838–849. [Google Scholar] [CrossRef]
- Proven, A.; Gabriel, S.E.; Orces, C.; O’Fallon, W.M.; Hunder, G.G. Glucocorticoid therapy in giant cell arteritis: Duration and adverse outcomes. Arthritis Rheum. 2003, 49, 703–708. [Google Scholar] [CrossRef]
- Mahr, A.D.; Jover, J.A.; Spiera, R.F.; Hernandez-Garcia, C.; Fernandez-Gutierrez, B.; Lavalley, M.P.; Merkel, P.A. Adjunctive methotrexate for treatment of giant cell arteritis: An individual patient data meta-analysis. Arthritis Rheum. 2007, 56, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of Tocilizumab in Giant-Cell Arteritis. N. Engl. J. Med. 2017, 377, 317–328. [Google Scholar] [CrossRef]
- Villiger, P.M.; Adler, S.; Kuchen, S.; Wermelinger, F.; Dan, D.; Fiege, V.; Butikofer, L.; Seitz, M.; Reichenbach, S. Tocilizumab for induction and maintenance of remission in giant cell arteritis: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016, 387, 1921–1927. [Google Scholar] [CrossRef]
- Hoffman, G.S.; Cid, M.C.; Hellmann, D.B.; Guillevin, L.; Stone, J.H.; Schousboe, J.; Cohen, P.; Calabrese, L.H.; Dickler, H.; Merkel, P.A.; et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002, 46, 1309–1318. [Google Scholar] [CrossRef]
- Jover, J.A.; Hernandez-Garcia, C.; Morado, I.C.; Vargas, E.; Banares, A.; Fernandez-Gutierrez, B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2001, 134, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Spiera, R.F.; Mitnick, H.J.; Kupersmith, M.; Richmond, M.; Spiera, H.; Peterson, M.G.; Paget, S.A. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin. Exp. Rheumatol. 2001, 19, 495–501. [Google Scholar]
- Hoffman, G.S.; Cid, M.C.; Rendt-Zagar, K.E.; Merkel, P.A.; Weyand, C.M.; Stone, J.H.; Salvarani, C.; Xu, W.; Visvanathan, S.; Rahman, M.U. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: A randomized trial. Ann. Intern. Med. 2007, 146, 621–630. [Google Scholar] [CrossRef]
- Martinez-Taboada, V.M.; Rodriguez-Valverde, V.; Carreno, L.; Lopez-Longo, J.; Figueroa, M.; Belzunegui, J.; Mola, E.M.; Bonilla, G. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Ann. Rheum. Dis. 2008, 67, 625–630. [Google Scholar] [CrossRef]
- Seror, R.; Baron, G.; Hachulla, E.; Debandt, M.; Larroche, C.; Puechal, X.; Maurier, F.; de Wazieres, B.; Quemeneur, T.; Ravaud, P.; et al. Adalimumab for steroid sparing in patients with giant-cell arteritis: Results of a multicentre randomised controlled trial. Ann. Rheum. Dis. 2014, 73, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.; Hazleman, B.L. Azathioprine in giant cell arteritis/polymyalgia rheumatica: A double-blind study. Ann. Rheum. Dis. 1986, 45, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Langford, C.A.; Cuthbertson, D.; Ytterberg, S.R.; Khalidi, N.; Monach, P.A.; Carette, S.; Seo, P.; Moreland, L.W.; Weisman, M.; Koening, C.L.; et al. A randomized, double-blind trial of abatacept (CTLA4-IG) for the treatment of giant cell arteritis. Arthritis Rheumatol. 2017, 69, 837–845. [Google Scholar] [CrossRef]
- Conway, R.; O’Neill, L.; Gallagher, P.; McCarthy, G.M.; Murphy, C.C.; Veale, D.J.; Fearon, U.; Molloy, E.S. Ustekinumab for refractory giant cell arteritis: A prospective 52-week trial. Semin. Arthritis Rheum. 2018, 48. [Google Scholar] [CrossRef]
- Conway, R.; O’Neill, L.; O’Flynn, E.; Gallagher, P.; McCarthy, G.M.; Murphy, C.C.; Veale, D.J.; Fearon, U.; Molloy, E.S. Ustekinumab for the treatment of refractory giant cell arteritis. Ann. Rheum. Dis. 2016, 75, 1578–1579. [Google Scholar] [CrossRef] [PubMed]
- Matza, M.A.; Fernandes, A.D.; Stone, J.H.; Unizony, S.H. Ustekinumab for the Treatment of Giant Cell Arteritis. Arthritis Care Res. 2020, 73, 893–897. [Google Scholar] [CrossRef]
- Cid, M.C.; Unizony, S.; Pupim, L.; Fang, F.; Pirrello, J.; Ren, A.; Samant, M.; Zhou, T.; Paolini, J.F. Mavrilimumab (anti GM-CSF receptor a monoclonal antibody) reduces the risk of flare and increases sustained remission in a phase 2 trial of patients with giant cell arteritis. Arthritis Rheum. 2020, 72, 4147–4148. [Google Scholar] [CrossRef]
- Venhoff, N.; Schmidt, W.A.; Bergner, R.; Rech, J.; Unger, L.; Tony, H.P.; Mendelson, M.; Sieder, C.; Maricos, M.; Thiel, J. Secukinumab in giant cell arteritis: A randomized, parallel-group, double-blind, placebo-controlled, multicenter phase 2 trial. Arthritis Rheum. 2021, 73. Available online: https://acrabstracts.org/abstract/secukinumab-in-giant-cell-arteritis-a-randomized-parallel-group-double-blind-placebo-controlled-multicenter-phase-2-trial/ (accessed on 6 December 2021).
- Samson, M.; Corbera-Bellalta, M.; Audia, S.; Planas-Rigol, E.; Martin, L.; Cid, M.C.; Bonnotte, B. Recent advances in our understanding of giant cell arteritis pathogenesis. Autoimmun. Rev. 2017, 16, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.H.; Regent, A.; Tamby, M.C.; Mouthon, L. Pathogenesis of giant cell arteritis: More than just an inflammatory condition? Autoimmun. Rev. 2010, 9, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Shirai, T.; Namkoong, H.; Zhang, H.; Berry, G.J.; Wallis, B.B.; Schaefgen, B.; Harrison, D.G.; Tremmel, J.A.; Giacomini, J.C.; et al. Pyruvate controls the checkpoint inhibitor PD-L1 and suppresses T cell immunity. J. Clin. Investig. 2017, 127, 2725–2738. [Google Scholar] [CrossRef]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Vaglio, A.; Liao, Y.J.; Warrington, K.J.; Goronzy, J.J.; Weyand, C.M. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc. Natl. Acad. Sci. USA 2017, 114, E970–E979. [Google Scholar] [CrossRef] [PubMed]
- Narala, R.; Reddy, S.A.; Mruthyunjaya, P. Giant cell arteritis manifesting as retinal arterial occlusion and paracentral acute middle maculopathy in a patient on pembrolizumab for metastatic uveal melanoma. Am. J. Ophthalmol. Case Rep. 2020, 20, 100891. [Google Scholar] [CrossRef]
- Goldstein, B.L.; Gedmintas, L.; Todd, D.J. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol. 2014, 66, 768–769. [Google Scholar] [CrossRef]
- Samson, M.; Espígol-Frigolé, G.; Terrades-García, N.; Prieto-González, S.; Corbera-Bellalta, M.; Alba-Rovira, R.; Hernández-Rodríguez, J.; Audia, S.; Bonnotte, B.; Cid, M.C. Biological treatments in giant cell arteritis & Takayasu arteritis. Eur. J. Intern. Med. 2018, 50, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Miyata, T.; Tanemoto, K. Current Clinical Features of New Patients With Takayasu Arteritis Observed From Cross-Country Research in Japan: Age and Sex Specificity. Circulation 2015, 132, 1701–1709. [Google Scholar] [CrossRef]
- Seyahi, E. Takayasu arteritis: An update. Curr. Opin. Rheumatol. 2017, 29, 51–56. [Google Scholar] [CrossRef]
- Bloch, D.A.; Michel, B.A.; Hunder, G.G.; McShane, D.J.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Fries, J.F.; Leavitt, R.Y.; et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Patients and methods. Arthritis Rheum. 1990, 33, 1068–1073. [Google Scholar] [CrossRef]
- Genereau, T.; Lortholary, O.; Pottier, M.A.; Michon-Pasturel, U.; Ponge, T.; de Wazieres, B.; Liozon, E.; Pinede, L.; Hachulla, E.; Roblot, P.; et al. Temporal artery biopsy: A diagnostic tool for systemic necrotizing vasculitis. French Vasculitis Study Group. Arthritis Rheum. 1999, 42, 2674–2681. [Google Scholar] [CrossRef]
- Delaval, L.; Samson, M.; Schein, F.; Agard, C.; Trefond, L.; Deroux, A.; Dupuy, H.; Garrouste, C.; Godmer, P.; Landron, C.; et al. Temporal Arteritis Revealing Antineutrophil Cytoplasmic Antibody-Associated Vasculitides: A Case-Control Study. Arthritis Rheumatol. 2020, 73, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.A.; Hatemi, G.; Burns, J.C.; Mohammad, A.J. Global epidemiology of vasculitis. Nat. Rev. Rheumatol. 2022, 18, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, P.; Turkiewicz, A.; Englund, M.; Turesson, C.; Mohammad, A.J. Epidemiology of biopsy-confirmed giant cell arteritis in southern Sweden—An update on incidence and first prevalence estimate. Rheumatology 2021, keab269. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Masamune, A.; Shimosegawa, T.; Okazaki, K. Prevalence of IgG4-Related Disease in Japan Based on Nationwide Survey in 2009. Int. J. Rheumatol. 2012, 2012, 358371. [Google Scholar] [CrossRef]
- Specks, U.; Merkel, P.A.; Seo, P.; Spiera, R.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.; St Clair, E.W.; Fessler, B.J.; Ding, L.; et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N. Engl. J. Med. 2013, 369, 417–427. [Google Scholar] [CrossRef]
- Restuccia, G.; Cavazza, A.; Boiardi, L.; Pipitone, N.; Macchioni, P.; Bajocchi, G.; Catanoso, M.G.; Muratore, F.; Ghinoi, A.; Magnani, L.; et al. Small-vessel vasculitis surrounding an uninflamed temporal artery and isolated vasa vasorum vasculitis of the temporal artery: Two subsets of giant cell arteritis. Arthritis Rheum. 2012, 64, 549–556. [Google Scholar] [CrossRef]
- Galli, E.; Muratore, F.; Boiardi, L.; Restuccia, G.; Cavazza, A.; Catanoso, M.; Macchioni, P.; Spaggiari, L.; Casali, M.; Pipitone, N.; et al. Significance of inflammation restricted to adventitial/periadventitial tissue on temporal artery biopsy. Semin. Arthritis Rheum. 2020, 50, 1064–1072. [Google Scholar] [CrossRef]
- Le Pendu, C.; Meignin, V.; Gonzalez-Chiappe, S.; Hij, A.; Galateau-Sallé, F.; Mahr, A. Poor Predictive Value of Isolated Adventitial and Periadventitial Infiltrates in Temporal Artery Biopsies for Diagnosis of Giant Cell Arteritis. J. Rheumatol. 2017, 44. [Google Scholar] [CrossRef]
- Jia, L.; Couce, M.; Barnholtz-Sloan, J.S.; Cohen, M.L. Is all inflammation within temporal artery biopsies temporal arteritis? Hum. Pathol. 2016, 57, 17–21. [Google Scholar] [CrossRef]
- Kasashima, F.; Kawakami, K.; Matsumoto, Y.; Endo, M.; Kasashima, S.; Kawashima, A. IgG4-Related Arterial Disease. Ann. Vasc. Dis. 2018, 11, 72–77. [Google Scholar] [CrossRef]
- Kuma, S.; Takeshima, T.; Ohga, T.; Nozoe, T.; Sueishi, K. Superficial temporal artery aneurysm associated with immunoglobulin G4-related disease. J. Vasc. Surg. Cases Innov. Tech. 2017, 3, 243–246. [Google Scholar] [CrossRef][Green Version]
- Ferfar, Y.; Charlotte, F.; Cacoub, P.; Saadoun, D. Temporal arteritis in IgG4 related disease. Jt. Bone Spine 2021, 88, 105087. [Google Scholar] [CrossRef]
- Fernandes, S.R.; Singsen, B.H.; Hoffman, G.S. Sarcoidosis and systemic vasculitis. Semin. Arthritis Rheum. 2000, 30, 33–46. [Google Scholar] [CrossRef]
- Wang, L.W.; Omari, A.; Emmett, L.; Jansz, P.C.; Huilgol, R.; Rainer, S.; Subbiah, R.N. Granulomatous sarcoid aortitis: A serious complication of a well-known multisystem disease. Lancet 2015, 385, 2014. [Google Scholar] [CrossRef]
- Muller, R.; Benyamine, A.; Masson, E.; Escoda, T.; Bagneres, D.; Demoux, A.L.; Bernard-Guervilly, F.; Laas, O.; Granel, B.; Rossi, P. Artérite granulomateuse au cours d’une sarcoïdose systémique: Horton ou manifestation vasculaire de la sarcoïdose? Rev. Méd. Interne 2017, 38, A122. [Google Scholar] [CrossRef]
- Nagel, M.A.; Cohrs, R.J.; Mahalingam, R.; Wellish, M.C.; Forghani, B.; Schiller, A.; Safdieh, J.E.; Kamenkovich, E.; Ostrow, L.W.; Levy, M.; et al. The varicella zoster virus vasculopathies: Clinical, CSF, imaging, and virologic features. Neurology 2008, 70, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.H.; Kleinschmidt-DeMasters, B.K.; Wellish, M.; Hedley-Whyte, E.T.; Rentier, B.; Mahalingam, R. Varicella zoster virus, a cause of waxing and waning vasculitis: The New England Journal of Medicine case 5-1995 revisited. Neurology 1996, 47, 1441–1446. [Google Scholar] [CrossRef]
- Gilden, D.; Nagel, M.A. Varicella zoster virus triggers the immunopathology of giant cell arteritis. Curr. Opin. Rheumatol. 2016, 28, 376–382. [Google Scholar] [CrossRef]
- Asai, K.; Tani, S.; Imai, Y.; Mineharu, Y.; Sakai, N. Traumatic arteriovenous fistula of the superficial temporal artery. J. Surg. Case Rep. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Balligand, A.; Mulquin, N. Pseudoaneurysm of the Superficial Temporal Artery After Blunt Trauma. Mayo Clin. Proc. 2020, 95, 226–227. [Google Scholar] [CrossRef]
- Stapleton, C.J.; Fusco, M.R.; Thomas, A.J.; Levy, E.I.; Ogilvy, C.S. Traumatic pseudoaneurysms of the superficial temporal artery: Case series, anatomy, and multidisciplinary treatment considerations. J. Clin. Neurosci. 2014, 21, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.H.; Haque, M.R.; Kawsar, K.A.; Sarker, M.H.; Momtazul Haque, A.F. Surgical management of scalp arterio-venous malformation and scalp venous malformation: An experience of eleven cases. Indian J. Plast. Surg. 2013, 46, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Tisetso Morare, N.M.; Baloyi, E.R.J. Post-traumatic arteriovenous malformation of the superficial temporal artery. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 50–54. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, E.; Beltran, L.M.; Monjo, I.; Deodati, F.; Schmidt, W.A.; Garcia-Puig, J. Atherosclerosis as a potential pitfall in the diagnosis of giant cell arteritis. Rheumatology 2018, 57, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Kroshinsky, D.; Nazarian, R.M.; Goverman, J.; Malhotra, R.; Jackson, V.A.; Kamdar, M.M.; Steele, D.J.; Thadhani, R.I. Calciphylaxis: Risk factors, diagnosis, and treatment. Am. J. Kidney Dis. 2015, 66, 133–146. [Google Scholar] [CrossRef]
- Roverano, S.; Ortiz, A.; Henares, E.; Eletti, M.; Paira, S. Calciphylaxis of the temporal artery masquerading as temporal arteritis: A case presentation and review of the literature. Clin. Rheumatol. 2015, 34, 1985–1988. [Google Scholar] [CrossRef]

| Epidemiology | Cephalic Clinical Signs | Extra-Cephalic Clinical Signs | Biology | Histological Signs (TAB) | Treatment | |
|---|---|---|---|---|---|---|
| GCA | Incidence: 14.6 (range: 6.0–43.6) per 100,000 persons aged ≥50 years [73] Prevalence: 75.5 per 100,000 inhabitants (107.8 in women, 40.1 in men) [74] | - Headache, scalp tenderness, or jaw claudication.Abnormal TA exam: induration, tenderness to palpation, edema, decrease/abolition of the temporal pulses - No involvement of the intracerebral vessels. | - PMR - Claudication of a limb - Abolition/decrease of a peripheral pulse - Aortic complication (aneurysm, dissection) - Ischemic complications: Ophthalmological: AAION, CRAO, PION, diplopia Neurological: Stroke (posterior vertebral territory) Myocardial infarction, limb ischemia | - Inflammatory syndrome (95%) - Inflammatory anemia, thrombocytosis (40–50%) | Granulomatous, non-necrotizing panarteritis, inflammatory cellular infiltrate of the media and/or intima made of mononuclear cells, multinuclear giant cells, fragmentation of the internal elastic lamina, destruction of the media, stenosing hyperplasia of the intima | - Glucocorticoids (constant effectiveness except for ischemic sequelae) - 2nd line (relapse(s), corticodependence): tocilizumab, methotrexate |
| Necrotizing vasculitis (AAV and PAN) | PAN: annual incidence = 0.9–8.0 per million in Europe prevalence = 31 per million [73] AAV: Combined incidence annual rate for GPA, MPA and EGPA = 24.7 to 33.0 per million [73] | - Cephalic symptoms in 88% of cases in case of temporal involvement (headache, jaw claudication, scalp tenderness, abolition of the temporal pulse). - Other systemic signs (renal, skin, peripheral neuropathy, ENT...) | - Arthralgia, myalgia - Skin signs (purpura, necrosis, livedo) - ENT signs (rhinitis, sinusitis) - Ophthalmologic signs (vasculitis, exophthalmia, and scleritis in GPA) - peripheral neuropathy (mononeuritis > polyneuritis) - Glomerular nephropathy (AAV) or vascular nephropathy (PAN) - Pachymeningitis (GPA) - Pulmonary signs (asthma (EGPA), nodules (GPA), alveolar hemorrhage (GPA and MPA)) - Heart disease (EGPA) - Digestive signs (perforation, pancreatitis, appendicitis, peritonitis), mainly in PAN | - Inflammatory syndrome - Biological signs related to organ damage - ANCA Anti-PR3: GPA Anti-MPO: EGPA, MPA No ANCA: PAN | Necrotizing vasculitis (fibrinoid necrosis) with cellular infiltrate (T lymphocytes, macrophages, neutrophils, and/or eosinophils): - AAV: small vessels (vasa vasorum or peri-adventitial vessels) without the involvement of the media and intima - PAN: temporal artery and/or its collaterals | - Corticosteroids - Immunosuppressants (rituximab, cyclophosphamide, methotrexate) |
| IgG4-RD | Prevalence = 6/100,000 inhabitants [75] | Aneurysm of the TA, isolated or associated with other vascular localizations (large vessel vasculitis, aortitis). | - retroperitoneal fibrosis - adenopathies - pseudo-tumors - pancreatitis - cholangitis - dacryoadenitis, orbital pseudotumor - involvement of the salivary glands (Mikulicz, Küttner) - pachymeningitis - interstitial lung disease - glomerular or tubulointerstitial nephropathy | - Biological signs related to organ damage - Mild inflammatory syndrome (30–40%) - Hypereosinophilia (30–40%) - Hypocomplementemia (30%) - Polyclonal hypergammaglobulinemia (70%) - Elevation of serum IgG4 (>80%) - Elevation of total IgE (>80%) | Parietal thickening of the adventitia, lymphoplasmacytic and eosinophilic infiltrate, +/- storiform fibrosis, numerous lymphoid follicles, IgG4-positive plasma cells disseminated throughout the vessel wall, arterial thrombosis | - Corticosteroid therapy (constant effectiveness) - 2nd line: rituximab - 3rd line: azathioprine, mycophenolate mofetil |
| VZV | - No specific data, very rare | - Headaches - Possible involvement of intracerebral arteries (ischemic or hemorrhagic stroke, intracerebral aneurysm, cerebral thrombophlebitis, spinal cord infarction, cranial nerve involvement) | - Zoster +/− recent or ophthalmic shingles - VZV necrotizing retinitis - Ischemic optic neuropathy - Other arterial attacks of VZV vasculitis (stenoses, occlusions, thromboses, dissections of large and medium caliber arteries) | - Positive VZV PCR (CSF) - Measurement of anti-VZV IgG and IgM in serum and CSF (intrathecal synthesis of anti-VZV IgG) | Granulomatous arteritis, often transmural inflammation, media necrosis, multinucleated giant cells, epithelioid macrophages combined with the presence of VZV antigens | - Acyclovir 10–15 mg/Kg/8 h IV for 14 days - No effectiveness of corticosteroids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greigert, H.; Ramon, A.; Tarris, G.; Martin, L.; Bonnotte, B.; Samson, M. Temporal Artery Vascular Diseases. J. Clin. Med. 2022, 11, 275. https://doi.org/10.3390/jcm11010275
Greigert H, Ramon A, Tarris G, Martin L, Bonnotte B, Samson M. Temporal Artery Vascular Diseases. Journal of Clinical Medicine. 2022; 11(1):275. https://doi.org/10.3390/jcm11010275
Chicago/Turabian StyleGreigert, Hélène, André Ramon, Georges Tarris, Laurent Martin, Bernard Bonnotte, and Maxime Samson. 2022. "Temporal Artery Vascular Diseases" Journal of Clinical Medicine 11, no. 1: 275. https://doi.org/10.3390/jcm11010275
APA StyleGreigert, H., Ramon, A., Tarris, G., Martin, L., Bonnotte, B., & Samson, M. (2022). Temporal Artery Vascular Diseases. Journal of Clinical Medicine, 11(1), 275. https://doi.org/10.3390/jcm11010275






