Metabolites of L-ARG in Exhaled Breath Condensate and Serum Are Not Biomarkers of Bronchial Asthma in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Medical History and Physical Examination
2.3. Exhaled Breath Condensate Collection
2.4. Baseline Functional Respiratory Test
2.5. Atopy Status
2.6. Blood Sample Collection, Storage, and Preparation
2.7. Interleukin-4 Concentrations
2.8. L-ARG, ADMA, SDMA, CIT, ORN, and DMA Concentrations
- 100 µL aliquots of calibration standards or 100 µL of serum, 10 µL of internal standard solution (50 µM D6-DMA, 20 µM D7-ADMA, 100 µM D6-ornithine, and 100 µM D7-arginine, respectively), and 50 µL of borate buffer (0.025 M Na2B4O7 × 10H2O, 1.77 mM NaOH, pH = 9.2) were added into 2.0 mL polypropylene tubes and vortexed (1 min, 25 °C).
- Derivatization was performed using 400 µL of acetonitrile (ACN) and 10 µL of 10% BCl in ACN. The samples were incubated and vortexed (5 min, 25 °C), and centrifuged (7 min, 17,000 RCF, 4 °C). After this, 100 µL of the clear supernatant was transferred into glass tubes containing 400 µL of water.
- Preparation of standard calibration curves was performed using the following concentration ranges: 5; 12.5; 25; 50; 100; 150; 200; 250 µM for L-ARG, 0.05; 0.13; 0.25; 0.5; 1.0; 1.5; 2; 2.5 µM for ADMA and SDMA, 1; 2.5; 5; 10; 20; 30; to 50 µM for CIT, 2.5; 7.5; 15; 30; 60; 90; 120; 150 µM for ORN, and 0.14; 0.35; 0.7; 1.4; 2.8; 4.2; 5.6; 7.0 µM for DMA.
- Analytical chromatography was performed using an Acquity UPLC system containing a cooled autosampler (Waters, Milford, MA, USA) and Acquity HSS T3 column (50 × 1.0 mm, 1.75 µm) from Waters. Elution was conducted with 0.1% formic acid (FA) in water as mobile phase A and 0.1% FA in methanol as mobile phase B. The volume was 2 µL. Total run time was 7 min, with a total flow rate of 250 µL/min. The following gradient was used: 5% B for 0–0.5 min, 5–14% B for 0.5–3 min, 14–60% B for 3–4 min, 60–90% B for 4–4.5 min, 90% B for 4.5–5 min and 90–5% B for 5–5.10 min.
- Mass spectrometric analysis was conducted using Xevo G2 QTOF MS (Waters, Milford, MA, USA) with ESI in positive ion mode. Parameters such as the spray voltage, source temperature, and the desolvation temperature were set at 0.5 kV, 120 °C, and 450 °C, respectively. Nitrogen was used as the nebulizing and drying gas. Data were acquired by using MassLynx software (Waters, Milford, MA, USA) for the following ions (m/z): 279.1457 (for L-ARG), 286.1749 (for D7-arginine), 307.1717 (for ADMA and SDMA), 314.2076 (for D7-ADMA), 280.1297 (for CIT), 341.1501 (for ORN), 347.1878 (for D6-ornithine), 150.0919 (for DMA), and 156.1113 (for D6-DMA).
2.9. Statistical Analysis
3. Results
3.1. Characteristics of Participants
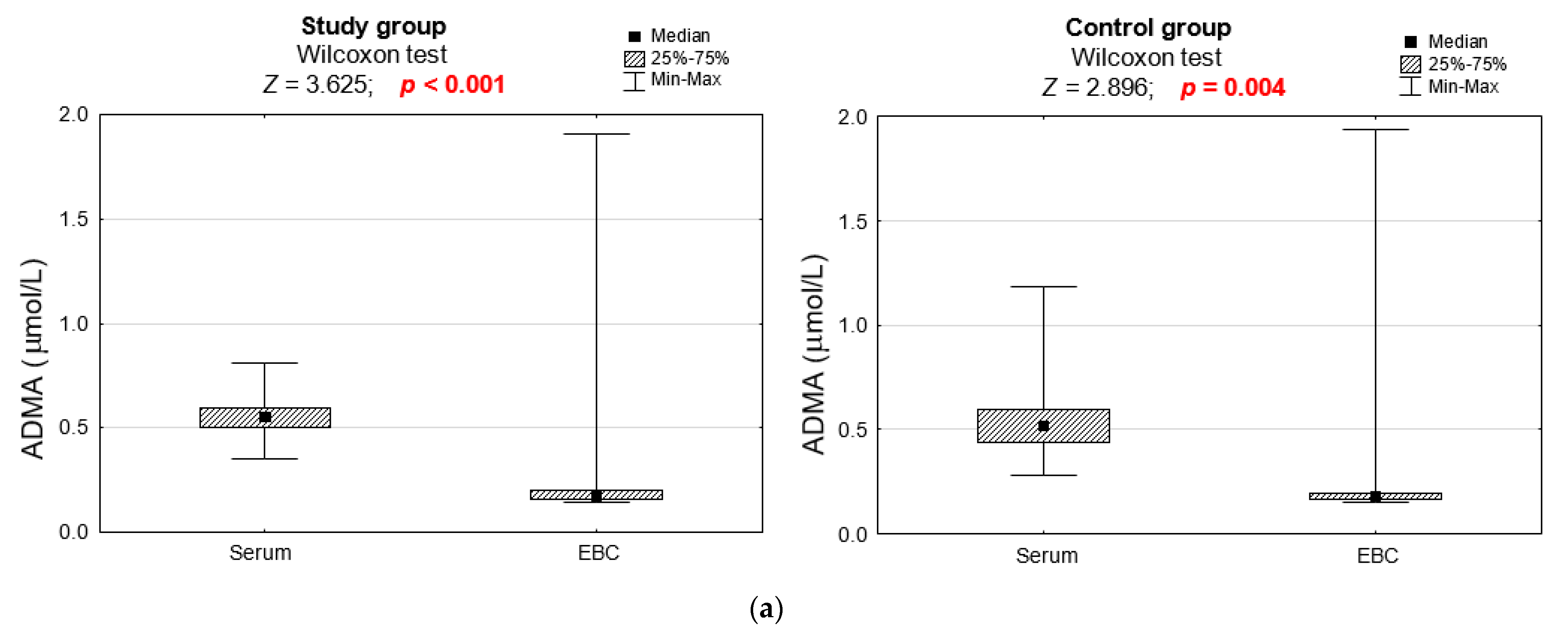
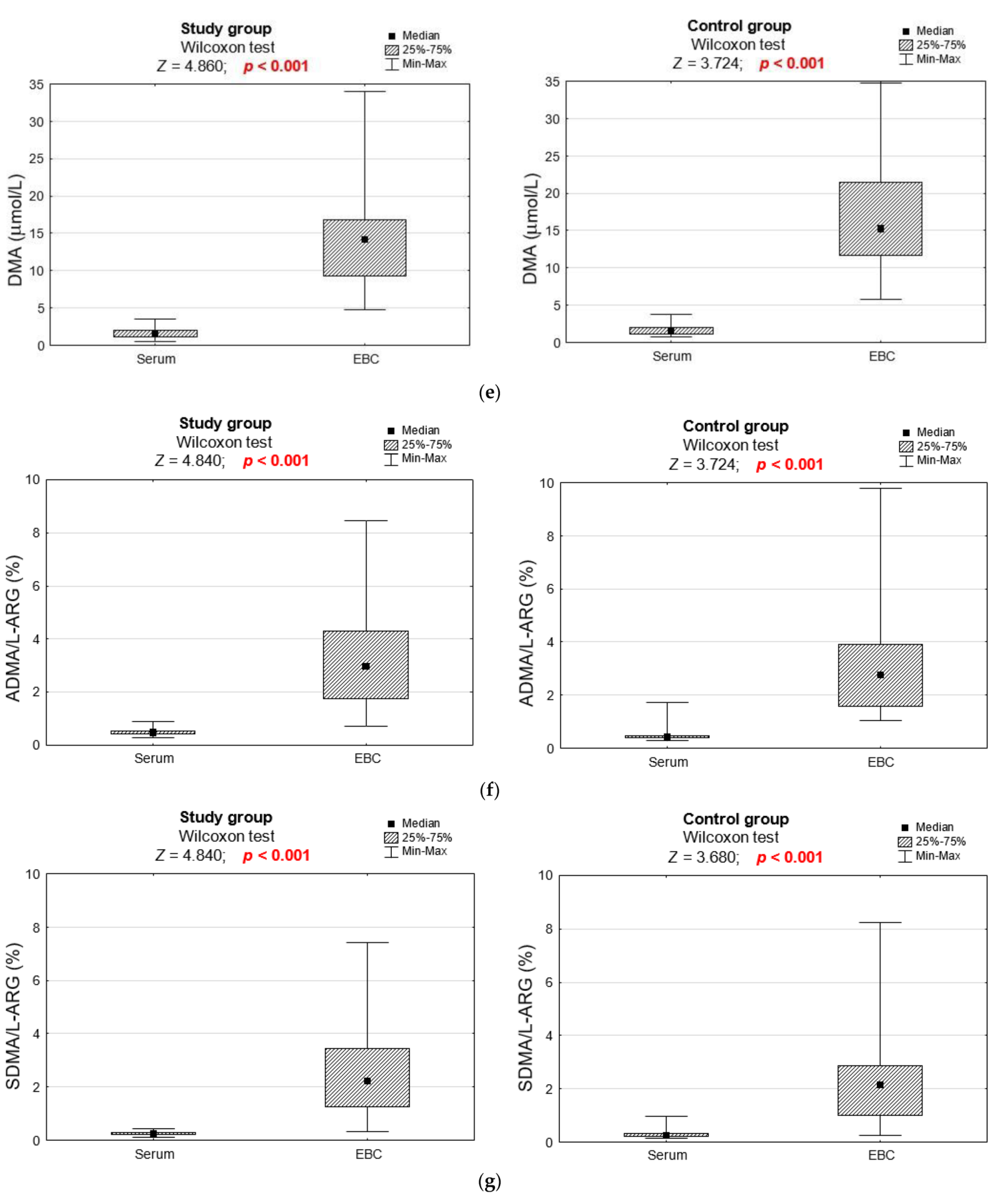
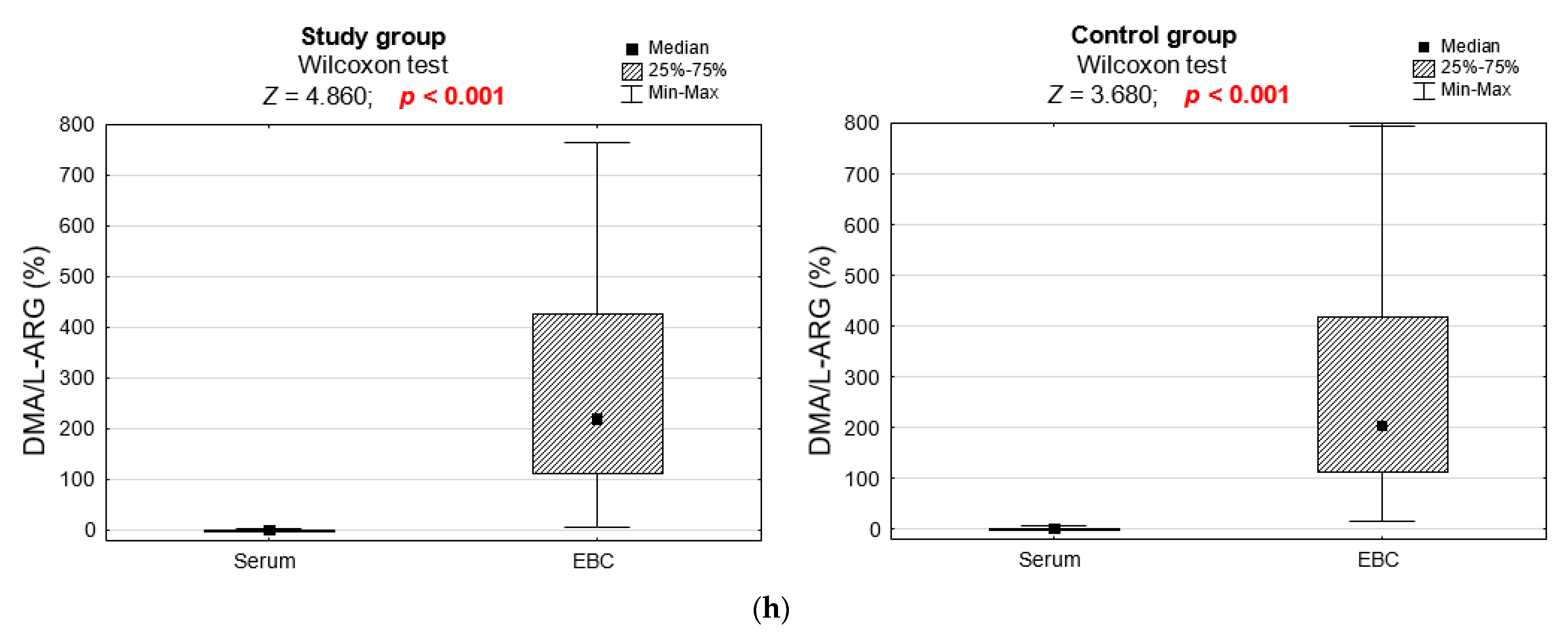
3.2. Biochemical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef]
- Faro, A.; Wood, R.E.; Schechter, M.S.; Leong, A.B.; Wittkugel, E.; Abode, K.; Chmiel, J.F.; Daines, C.; Davis, S.; Eber, E.; et al. Official American Thoracic Society technical standards: Flexible airway endoscopy in children. Am. J. Respir. Crit. Care Med. 2015, 191, 1066–1080. [Google Scholar] [CrossRef]
- Scott, J.A.; North, M.L.; Rafii, M.; Huang, H.; Pencharz, P.; Subbarao, P.; Belik, J.; Grasemann, H. Asymmetric dimethylarginine is increased in asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Bucciarelli, V.; Verini, M.; Consilvio, N.P.; Gallina, S.; Martini, F.; Aceto, A.; Scotti, L.; Bucciarelli, T. ADMA, SDMA, L-Arginine and nitric oxide in allergic pediatric bronchial asthma. J. Biol. Regul. Homeost. Agents 2012, 26, 561–566. [Google Scholar] [PubMed]
- Carraro, S.; Giordano, G.; Piacentini, G.; Kantar, A.; Moser, S.; Cesca, L.; Berardi, M.; Di Gangi, I.M.; Baraldi, E. Asymmetric dimethylarginine in exhaled breath condensate and serum of children with asthma. Chest 2013, 144, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Comhair, S.A.A.; Hazen, S.L.; Powers, R.W.; Khatri, S.S.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Fitzpatrick, A.M.; et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am. J. Respir. Crit. Care Med. 2013, 187, 153–159. [Google Scholar] [CrossRef]
- Quinn, K.D.; Schedel, M.; Nkrumah-Elie, Y.; Joetham, A.; Armstrong, M.; Cruickshank-Quinn, C.; Reisdorph, N.; Gelfand, E.W. Dysregulation of metabolic pathways in a mouse model of allergic asthma. Allergy 2017, 72, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Martins, M.A.; Tibério, I.F. Nitric oxide in asthma physiopathology. ISRN Allergy 2011, 2011, 832560. [Google Scholar] [CrossRef]
- Bulau, P.; Zakrzewicz, D.; Kitowska, K.; Leiper, J.; Gunther, A.; Grimminger, F.; Eickelberg, O. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L18–L24. [Google Scholar] [CrossRef]
- Barnes, P.J. NO or no NO in asthma? Thorax 1996, 51, 218–220. [Google Scholar] [CrossRef][Green Version]
- Kharitonov, S.A.; Yates, D.; Robbins, R.A.; Logan-Sinclair, R.; Shinebourne, E.A.; Barnes, P.J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994, 343, 133–135. [Google Scholar] [CrossRef]
- Lane, C.; Knight, D.; Burgess, S.; Franklin, P.; Horak, F.; Legg, J.; Moeller, A.; Stick, S. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax 2004, 59, 757–760. [Google Scholar] [CrossRef]
- Wells, S.M.; Buford, M.C.; Migliaccio, C.T.; Holian, A. Elevated asymmetric dimethylarginine alters lung function and induces collagen deposition in mice. Am. J. Respir. Cell Mol. Biol. 2009, 40, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.M.; Holian, A. Asymmetric dimethylarginine induces oxidative and nitrosative stress in murine lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 2007, 36, 520–528. [Google Scholar] [CrossRef]
- Dweik, R.A. The lung in the balance: Arginine, methylated arginines, and nitric oxide. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L15–L17. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, M.P.; Meurs, H.; Gosens, R. Targeting arginase and nitric oxide metabolism in chronic airway diseases and their co-morbidities. Curr. Opin. Pharmacol. 2018, 40, 126–133. [Google Scholar] [CrossRef]
- Maarsingh, H.; Zaagsma, J.; Meurs, H. Arginase: A key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br. J. Pharmacol. 2009, 158, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Horvath, I.; Hunt, J.; Barnes, P.J.; Alving, K.; Antczak, A.; Baraldi, E.; Becher, G.; van Beurden, W.J.; Corradi, M.; Dekhuijzen, R.; et al. Exhaled breath condensate: Methodological recommendations and unresolved questions. Eur. Respir. J. 2005, 26, 523–548. [Google Scholar] [CrossRef]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.-C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Bernstein, I.L.; Storms, W.W. Practice parameters for allergy diagnostic testing. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann. Allergy Asthma Immunol. 1995, 75, 543–625. [Google Scholar] [PubMed]
- Konstantinou, G.N.; Bousquet, P.J.; Zuberbier, T.; Papadopoulos, N.G. The longest wheal diameter is the optimal measurement for the evaluation of skin prick tests. Int. Arch. Allergy Immunol. 2010, 151, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Fleszar, M.G.; Wiśniewski, J.; Zboch, M.; Diakowska, D.; Gamian, A.; Krzystek-Korpacka, M. Targeted metabolomic analysis of nitric oxide/L-arginine pathway metabolites in dementia: Association with pathology, severity, and structural brain changes. Sci. Rep. 2019, 9, 13764. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Mabalirajan, U.; Ghosh, B.; Agrawal, A. Altered asymmetric dimethyl arginine metabolism in allergically inflamed mouse lungs. Am. J. Respir. Cell Mol. Biol. 2010, 42, 3–8. [Google Scholar] [CrossRef]
- Lara, A.; Khatri, S.B.; Wang, Z.; Comhair, S.A.A.; Xu, W.; Dweik, R.A.; Bodine, M.; Levison, B.S.; Hammel, J.; Bleecker, E.; et al. Alterations of the arginine metabolome in asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Kraj, L.; Krawiec, M.; Koter, M.; Graboń, W.; Kraj, G.; Chołojczyk, M.; Kulus, M.; Barańczyk-Kuźma, A. Altered L-arginine metabolism in children with controlled asthma. Allergy Asthma Proc. 2014, 35, 80–83. [Google Scholar] [CrossRef]
- Lau, E.M.; Morgan, P.E.; Belousova, E.G.; Toelle, B.G.; Ayer, J.G.; Celermajer, D.S.; Marks, G.B. Asymmetric dimethylarginine and asthma: Results from the Childhood Asthma Prevention Study. Eur. Respir. J. 2013, 41, 1234–1237. [Google Scholar] [CrossRef]
- MEDIVAC S.r.l. Available online: www.medivac.it/en/ (accessed on 10 May 2021).
- Respiratory Research, Inc. Available online: https://respiratoryresearch.com/rtube/ (accessed on 10 May 2021).

| Characteristic | Study Group n = 37 | Control Group n = 28 | p |
|---|---|---|---|
| Gender, n (%): | 0.547 | ||
| Girls | 12 (32.4%) | 12 (42.9%) | |
| Boys | 25 (67.6%) | 16 (57.1%) | |
| Age (years): | 0.010 | ||
| M ± SD | 10.9 ± 2.7 | 12.6 ± 2.5 | |
| Me [Q1; Q3] | 10 [9; 13] | 13 [12; 14] | |
| Min–Max | 6–17 | 7–17 | |
| Age groups, n (%): | 0.002 | ||
| 6–11 years | 25 (67.6%) | 7 (25.0%) | |
| 12–17 years | 12 (32.4%) | 21 (75.0%) | |
| Residential area: | 0.291 | ||
| Rural | 10 (27.0%) | 12 (42.9%) | |
| <15.000 residents | 4 (10.8%) | 1 (3.6%) | |
| >15.000 residents | 23 (62.2%) | 15 (53.6%) | |
| BMI (kg/m2): | 0.012 | ||
| M ± SD | 18.6 ± 3.9 | 21.0 ± 3.4 | |
| Me [Q1; Q3] | 17 [16; 21] | 21 [19; 23] | |
| Min–Max | 12–29 | 15–30 | |
| Number of siblings, n (%) | 0.429 | ||
| M ± SD | 1.4 ± 1.1 | 1.3 ± 1.6 | |
| Me [Q1; Q3] | 1 [1; 2] | 1 [1; 1] | |
| Min–Max | 0–5 | 0–9 | |
| Contact with an animal in the home environment: | |||
| Dog | 12 (32.4%) | 10 (35.7%) | 0.990 |
| Cat | 11 (29.7%) | 10 (35.7%) | 0.808 |
| Exposure to tobacco smoke in the home | 13 (35.1%) | 12 (42.9%) | 0.707 |
| Participation in physical education classes | 36 (97.3%) | 28 (100.0%) | 1.000 |
| Pneumococcal vaccines | 16 (43.2%) | 7 (25.0%) | 0.207 |
| FEV1 (% predicted) | 96.5 ± 15.0 | 104.8 ± 16.9 | 0.041 |
| FVC (% predicted) | 107.8 ± 15.0 | 110.6 ± 14.3 | 0.441 |
| FEV1/FVC | 89.3 ± 11.0 | 94.1 ± 10.2 | 0.075 |
| Characteristic | Results |
|---|---|
| The age of diagnosis of asthma | |
| M ± SD | 6.4 ± 3.6 |
| Me [Q1; Q3] | 6 [4; 9] |
| Min–Max | 1–13 |
| Asthma duration | |
| M ± SD | 4.6 ± 3.7 |
| Me [Q1; Q3] | 4 [1; 7] |
| Min–Max | 0.5–12 |
| Diagnosis of asthma before 6 years of age | 17/37 (45.9%) |
| Other atopic diseases in the child | 23/37 (62.2%) |
| Positive family history of atopic diseases | 23/37 (62.2%) |
| Atopy | 27/37 (73.0%) |
| Treatment with inhaled corticosteroids in the previous 4 weeks | 30/37 (81.1%) |
| FEV 1%FVC (% predicted) | |
| M ± SD | 89.3 ± 11.0 |
| Me [Q1; Q3] | 92 [83; 95] |
| Min–Max | 64–116 |
| FEV 1 (% predicted) | |
| M ± SD | 96.5 ± 15.0 |
| Me [Q1; Q3] | 97 [91; 105] |
| Min–Max | 57–130 |
| FVC (% predicted) | |
| M ± SD | 107.8 ± 15.0 |
| Me [Q1; Q3] | 108 [96; 113] |
| Min–Max | 85–152 |
| Serum L-ARG and Its Metabolite Concentration | Study Group n = 36 | Control Group n = 26 | p |
|---|---|---|---|
| L-ARG (μmol/L) | 0.881 | ||
| M ± SD | 118.16 ± 28.32 | 121.81 ± 48.52 | |
| Me [Q1; Q3] | 115.0 [96.4; 132.7] | 122.7 [90.1; 139.1] | |
| Min–Max | 76.7–220.9 | 45.3–290.7 | |
| ADMA (μmol/L) | 0.304 | ||
| M ± SD | 0.56 ± 0.11 | 0.54 ± 0.18 | |
| Me [Q1; Q3] | 0.55 [0.50; 0.60] | 0.52 [0.44; 0.60] | |
| Min–Max | 0.35–0.81 | 0.28–1.18 | |
| SDMA (μmol/L) | 0.416 | ||
| M ± SD | 0.30 ± 0.07 | 0.32 ± 0.10 | |
| Me [Q1; Q3] | 0.29 [0.25; 0.36] | 0.31 [0.27; 0.35] | |
| Min–Max | 0.17–0.47 | 0.17–0.70 | |
| CIT (μmol/L) | 0.653 | ||
| M ± SD | 29.59 ± 8.69 | 28.45 ± 7.83 | |
| Me [Q1; Q3] | 28.1 [24.7; 33.5] | 27.5 [24.3; 33.1] | |
| Min–Max | 12.3–54.3 | 16.0–48.9 | |
| ORN (μmol/L) | 0.972 | ||
| M ± SD | 52.87 ± 20.38 | 63.40 ± 52.35 | |
| Me [Q1; Q3] | 45.5 [37.0; 70.0] | 50.8 [38.0; 58.7] | |
| Min–Max | 27.6–111.9 | 16.7–242.7 | |
| DMA (μmol/L) | 0.858 | ||
| M ± SD | 1.62 ± 0.67 | 1.67 ± 0.73 | |
| Me [Q1; Q3] | 1.6 [1.1; 2.1] | 1.6 [1.0; 2.1] | |
| Min–Max | 0.5–3.5 | 0.7–3.8 | |
| ADMA/L-ARG ratio (%) | 0.112 | ||
| M ± SD | 0.48 ± 0.12 | 0.49 ± 0.29 | |
| Me [Q1; Q3] | 0.5 [0.4; 0.5] | 0.4 [0.4; 0.5] | |
| Min–Max | 0.3–0.9 | 0.3–1.7 | |
| SDMA/L-ARG ratio (%) | 0.633 | ||
| M ± SD | 0.26 ± 0.07 | 0.29 ± 0.15 | |
| Me [Q1; Q3] | 0.3 [0.2; 0.3] | 0.3 [0.2; 0.3] | |
| Min–Max | 0.1–0.4 | 0.2–1.0 | |
| DMA/L-ARG ratio (%) | 0.960 | ||
| M ± SD | 1.40 ± 0.57 | 1.54 ± 1.11 | |
| Me [Q1; Q3] | 1.3 [1.0; 1.7] | 1.3 [1.1; 1.6] | |
| Min–Max | 0.5–2.7 | 0.6–6.5 |
| Serum IL-4 Concentration | Study Group n = 37 | Control Group n = 27 | p |
|---|---|---|---|
| IL-4 (pg/mL) | 0.716 | ||
| M ± SD | 0.16 ± 0.05 | 0.15 ± 0.04 | |
| Me [Q1; Q3] | 0.14 [0.13; 0.17] | 0.14 [0.12; 0.16] | |
| Min–Max | 0.10–0.34 | 0.11–0.28 |
| L-ARG and Its Metabolite Concentrations in EBC | Study Group n = 32 | Control Group n = 20 | p |
|---|---|---|---|
| L-ARG (μmol/L) | 0.672 | ||
| M ± SD | 17.01 ± 29.38 | 16.48 ± 29.23 | |
| Me [Q1; Q3] | 5.9 [3,7; 10.6] | 5.9 [4.5; 13.3] | |
| Min–Max | 1.7–121.5 | 1.5–127.9 | |
| ADMA (μmol/L) | 0.323 | ||
| M ± SD | 0.31 ± 0.42 | 0.29 ± 0.40 | |
| Me [Q1; Q3] | 0.2 [0.2; 0.2] | 0.2 [0.2; 0.2] | |
| Min–Max | 0.1–1.9 | 0.1–1.9 | |
| SDMA (μmol/L) | 0.352 | ||
| M ± SD | 0.16 ± 0.08 | 0.14 ± 0.05 | |
| Me [Q1; Q3] | 0.1 [0.1; 0.1] | 0.1 [0.1; 0.1] | |
| Min–Max | 0.1–0.4 | 0.1–0.3 | |
| CIT (μmol/L) | 0.125 | ||
| M ± SD | 27.39 ± 34.99 | 37.77 ± 70.70 | |
| Me [Q1; Q3] | 15.9 [13.4; 20.3] | 18.0 [16.0; 25.7] | |
| Min–Max | 10.8–159.3 | 10.9–332.7 | |
| ORN (μmol/L) | 0.108 | ||
| M ± SD | 40.23 ± 65.02 | 80.73 ± 212.05 | |
| Me [Q1; Q3] | 15.7 [11.1; 29.5] | 28.4 [15.3; 38.3] | |
| Min–Max | 3.8–295.2 | 7.8–967.6 | |
| DMA (μmol/L) | 0.211 | ||
| M ± SD | 14.46 ± 6.48 | 17.13 ± 7.28 | |
| Me [Q1; Q3] | 14.2 [9.2; 16.8] | 15.2 [11.6; 21.5] | |
| Min–Max | 4.8–33.9 | 5.8–34.7 | |
| ADMA/L-ARG ratio (%) | 0.579 | ||
| M ± SD | 3.31 ± 1.80 | 3.17 ± 2.23 | |
| Me [Q1; Q3] | 3.0 [1.7; 4.3] | 2.8 [1.6; 3.9] | |
| Min–Max | 0.7–8.5 | 1.1–9.8 | |
| SDMA/L-ARG ratio (%) | 0.592 | ||
| M ± SD | 2.49 ± 1.68 | 2.32 ± 1.92 | |
| Me [Q1; Q3] | 2.2 [1.2; 3.5] | 2.2 [1.0; 2.9] | |
| Min–Max | 0.3–7.4 | 0.3–8.3 | |
| DMA/L-ARG ratio (%) | 0.843 | ||
| M ± SD | 274.29 ± 219.47 | 290.41 ± 231.87 | |
| Me [Q1; Q3] | 219.3 [112.3; 428.9] | 203.2 [110.5; 420.4] | |
| Min–Max | 8.1–764.7 | 15.6–795.0 |
| Parameter | L-ARG | ADMA | SDMA | CIT | ORN | DMA | ADMA/ L-ARG | SDMA/ L-ARG | DMA/ L-ARG |
|---|---|---|---|---|---|---|---|---|---|
| FEV1 and serum (n = 62) measurements | 0.076 | 0.142 | 0.175 | 0.110 | −0.022 | 0.109 | −0.064 | 0.048 | 0.045 |
| FEV1 and EBC (n = 52) measurements | −0.090 | −0.151 | −0.040 | −0.202 | −0.161 | 0.012 | 0.011 | 0.077 | 0.071 |
| IL-4 and serum (n = 62) measurements | −0.014 | 0.039 | 0.109 | 0.213 | 0.160 | 0.080 | 0.065 | 0.082 | 0.136 |
| IL-4 and EBC (n = 51) measurements | −0.050 | −0.014 | 0.018 | −0.053 | 0.080 | 0.102 | 0.064 | 0.038 | 0.088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Połomska, J.; Sozańska, B. Metabolites of L-ARG in Exhaled Breath Condensate and Serum Are Not Biomarkers of Bronchial Asthma in Children. J. Clin. Med. 2022, 11, 252. https://doi.org/10.3390/jcm11010252
Połomska J, Sozańska B. Metabolites of L-ARG in Exhaled Breath Condensate and Serum Are Not Biomarkers of Bronchial Asthma in Children. Journal of Clinical Medicine. 2022; 11(1):252. https://doi.org/10.3390/jcm11010252
Chicago/Turabian StylePołomska, Joanna, and Barbara Sozańska. 2022. "Metabolites of L-ARG in Exhaled Breath Condensate and Serum Are Not Biomarkers of Bronchial Asthma in Children" Journal of Clinical Medicine 11, no. 1: 252. https://doi.org/10.3390/jcm11010252
APA StylePołomska, J., & Sozańska, B. (2022). Metabolites of L-ARG in Exhaled Breath Condensate and Serum Are Not Biomarkers of Bronchial Asthma in Children. Journal of Clinical Medicine, 11(1), 252. https://doi.org/10.3390/jcm11010252






