Effect of Early Supraglottic Airway Device Insertion on Chest Compression Fraction during Simulated Out-of-Hospital Cardiac Arrest: Randomised Controlled Trial
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
2. Materials and Methods
2.1. Study Design
2.2. Setting
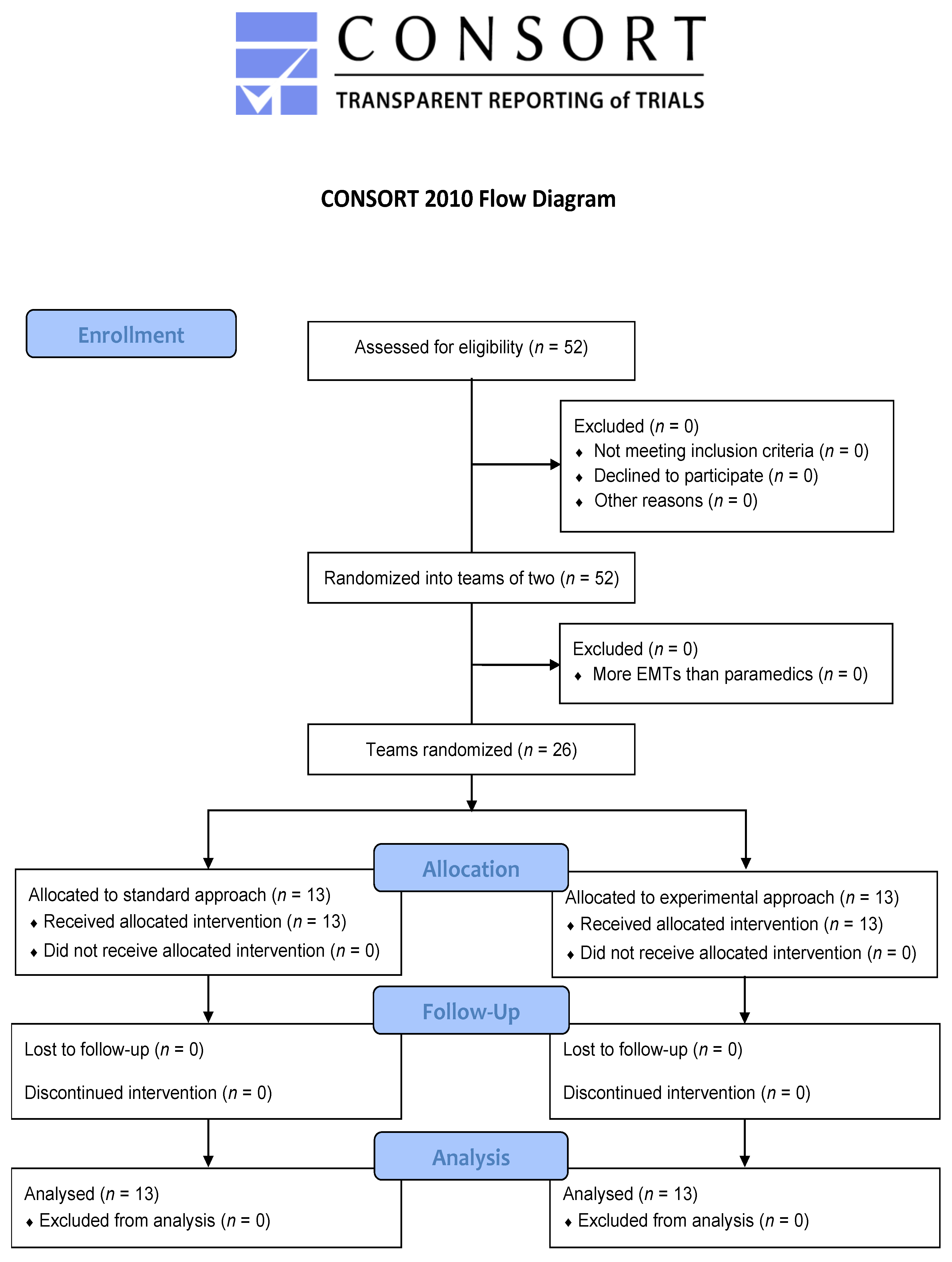
2.3. Participants and Recruitment
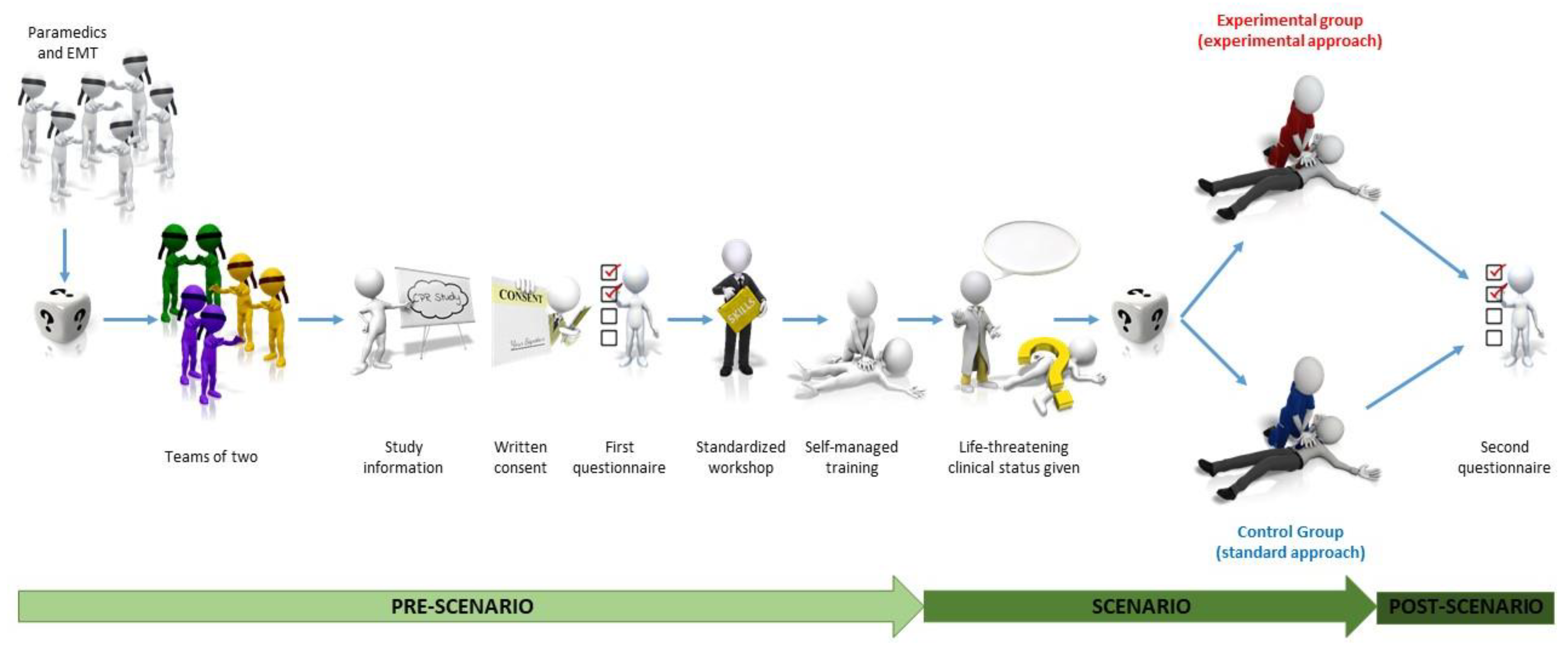
2.4. Study Sequence
2.4.1. Randomisation
2.4.2. Standardised Workshop
2.4.3. Training Session of Experimental Approach
2.4.4. Study Scenario
2.5. Equipment
2.6. Study Outcomes
2.6.1. Primary Outcome
2.6.2. Secondary Outcomes
2.7. Data Collection, Extraction and Curation
2.8. Sample Size Calculation
2.9. Statistical Analysis
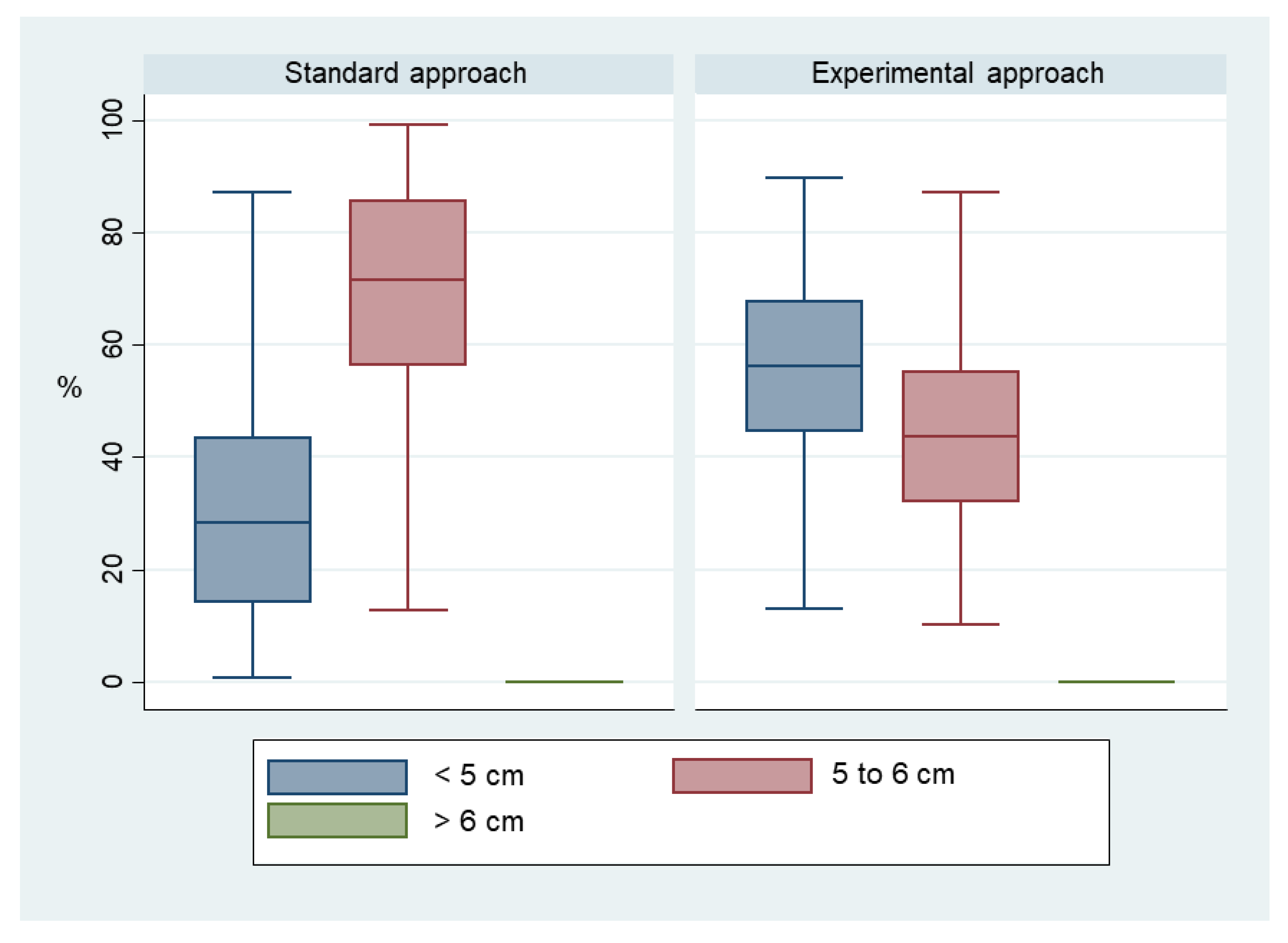
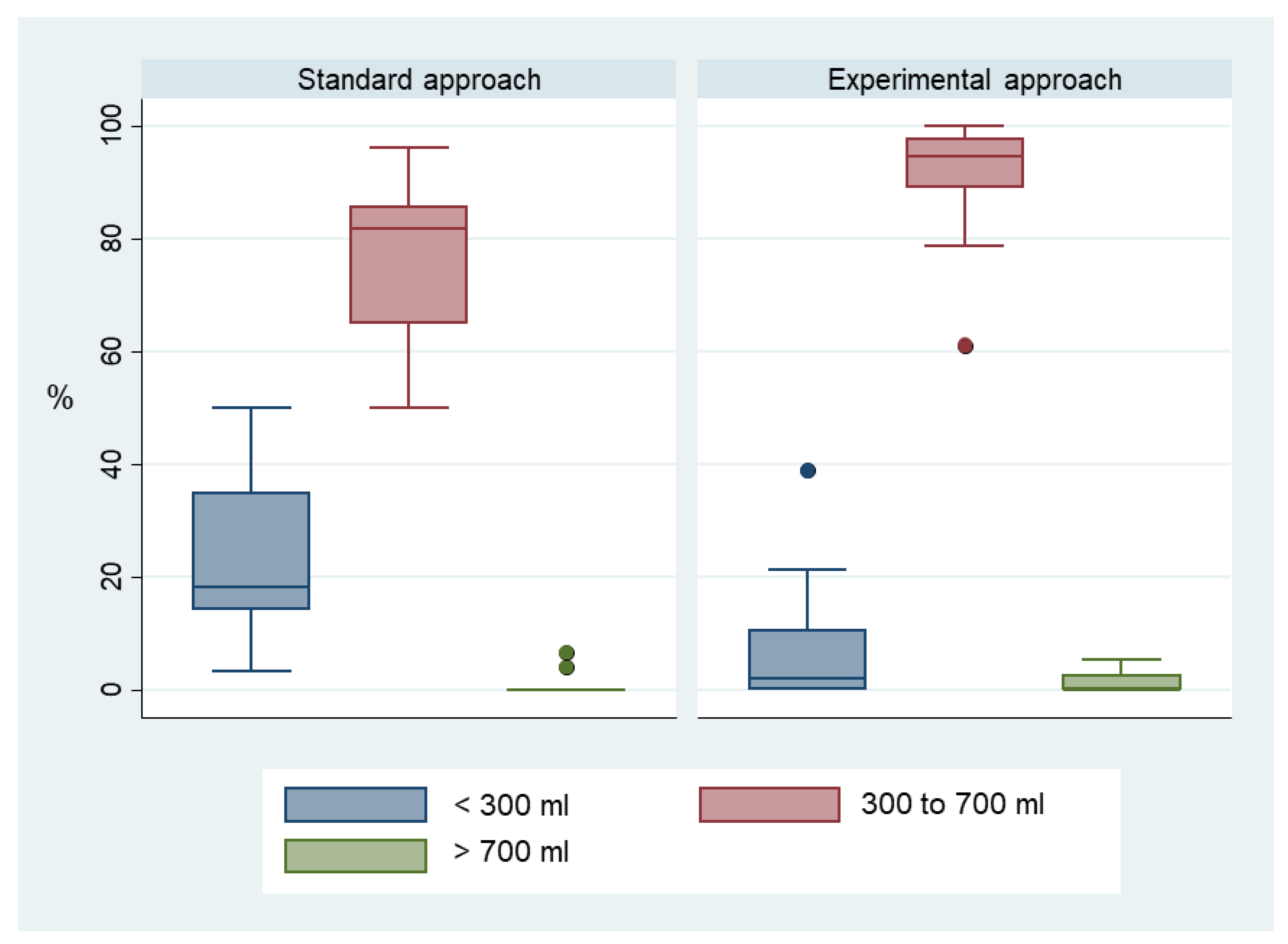
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Rea, T.; Olsufka, M.; Yin, L.; Maynard, C.; Cobb, L. The relationship between chest compression fraction and outcome from ventricular fibrillation arrests in prolonged resuscitations. Resuscitation 2014, 85, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, C.; Everson-Stewart, S.; Christenson, J.; Andrusiek, D.; Powell, J.; Nichol, G.; Cheskes, S.; Aufderheide, T.P.; Berg, R.; Stiell, I. The impact of increased chest compression fraction on return of spontaneous circulation for out-of-hospital cardiac arrest patients not in ventricular fibrillation. Resuscitation 2011, 82, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
- Uppiretla, A.K.; Gangalal, G.M.; Rao, S.; Bosco, D.D.; Shareef, S.M.; Sampath, V. Effects of Chest Compression Fraction on Return of Spontaneous Circulation in Patients with Cardiac Arrest: A Brief Report. Adv. J. Emerg. Med. 2019, 4, e8. [Google Scholar] [CrossRef] [PubMed]
- Christenson, J.; Andrusiek, D.; Everson-Stewart, S.; Kudenchuk, P.; Hostler, D.; Powell, J.; Callaway, C.; Bishop, D.; Vaillancourt, C.; Davis, D.; et al. Chest Compression Fraction Determines Survival in Patients with out-of-Hospital Ventricular Fibrillation. Circulation 2009, 120, 1241–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaillancourt, C.; Petersen, A.; Meier, E.N.; Christenson, J.; Menegazzi, J.J.; Aufderheide, T.P.; Nichol, G.; Berg, R.; Callaway, C.W.; Idris, A.H.; et al. The impact of increased chest compression fraction on survival for out-of-hospital cardiac arrest patients with a non-shockable initial rhythm. Resuscitation 2020, 154, 93–100. [Google Scholar] [CrossRef]
- Wik, L.; Olsen, J.-A.; Persse, D.; Sterz, F.; Lozano, M.; Brouwer, M.A.; Westfall, M.; Souders, C.M.; Travis, D.T.; Herken, U.R.; et al. Why do some studies find that CPR fraction is not a predictor of survival? Resuscitation 2016, 104, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Bobrow, B.J.; Clark, L.L.; Ewy, G.A.; Chikani, V.; Sanders, A.B.; Berg, R.A.; Richman, P.B.; Kern, K.B. Minimally Interrupted Cardiac Resuscitation by Emergency Medical Services for out-of-Hospital Cardiac Arrest. JAMA 2008, 299, 1158–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Wakasugi, M.; Kawagishi, T.; Hatano, T.; Fuchigami, T.; Okudera, H. Effect of Advanced Airway Management by Paramedics during out-of-Hospital Cardiac Arrest on Chest Compression Fraction and Return of Spontaneous Circulation. Open Access Emerg. Med. 2021, 13, 305–310. [Google Scholar] [CrossRef]
- Newell, C.; Grier, S.; Soar, J. Airway and ventilation management during cardiopulmonary resuscitation and after successful resuscitation. Crit. Care 2018, 22, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granfeldt, A.; Avis, S.R.; Nicholson, T.C.; Holmberg, M.J.; Moskowitz, A.; Coker, A.; Berg, K.M.; Parr, M.; Donnino, M.W.; Soar, J.; et al. Advanced airway management during adult cardiac arrest: A systematic review. Resuscitation 2019, 139, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Olasveengen, T.; Mancini, M.; Berg, R.; Brooks, S.; Castren, M.; Chung, S.; Considine, J.; Escalante, R. CPR: Chest Compression to Ventilation Ratio—EMS Delivered (BLS): Systematic Review. Available online: https://costr.ilcor.org/document/cpr-chest-compression-to-ventilation-ratio-ems-delivered (accessed on 12 February 2021).
- Carney, N.; Totten, A.M.; Cheney, T.; Jungbauer, R.; Neth, M.R.; Weeks, C.; Davis-O’Reilly, C.; Fu, R.; Yu, Y.; Chou, R.; et al. Prehospital Airway Management: A Systematic Review. Prehospital Emerg. Care 2021, 1–12. [Google Scholar] [CrossRef]
- Malinverni, S.; Bartiaux, M.; Cavallotto, F.; De Longueville, D.; Mols, P.; Gorlicki, J.; Adnet, F. Does endotracheal intubation increases chest compression fraction in out of hospital cardiac arrest: A substudy of the CAAM trial. Resuscitation 2019, 137, 35–40. [Google Scholar] [CrossRef]
- Kurz, M.C.; Prince, D.K.; Christenson, J.; Carlson, J.; Stub, D.; Cheskes, S.; Lin, S.; Aziz, M.; Austin, M.; Vaillancourt, C.; et al. Association of advanced airway device with chest compression fraction during out-of-hospital cardiopulmonary arrest. Resuscitation 2016, 98, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.L.; Cheung, K.W.; Tallon, J.M.; Travers, A.H. Comparison of tracheal intubation and alternative airway techniques performed in the prehospital setting by paramedics: A systematic review. Can. J. Emerg. Med. 2010, 12, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saracoglu, A.; Saracoglu, K. Advanced airway management in out-of-hospital cardiac arrest—to intubate or not to intubate: A narrative review of the existing literature. Anaesthesiol. Intensive Ther. 2020, 52, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Buis, M.L.; Maissan, I.M.; Hoeks, S.E.; Klimek, M.; Stolker, R.J. Defining the learning curve for endotracheal intubation using direct laryngoscopy: A systematic review. Resuscitation 2016, 99, 63–71. [Google Scholar] [CrossRef]
- Chan, M.; Fehlmann, C.A.; Pasquier, M.; Suppan, L.; Savoldelli, G.L. Endotracheal Intubation Success Rate in an Urban, Supervised, Resident-Staffed Emergency Mobile System: An 11-Year Retrospective Cohort Study. J. Clin. Med. 2020, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Duckett, J.; Fell, P.; Han, K.; Kimber, C.; Taylor, C. Introduction of the i-gel supraglottic airway device for prehospital airway management in a UK ambulance service. Emerg. Med. J. 2014, 31, 505–507. [Google Scholar] [CrossRef]
- Wharton, N.M.; Gibbison, B.; Gabbott, D.A.; Haslam, G.M.; Muchatuta, N.; Cook, T.M. I-gel insertion by novices in manikins and patients. Anaesthesia 2008, 63, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Leventis, C.; Chalkias, A.; Sampanis, M.A.; Foulidou, X.; Xanthos, T. Emergency airway management by paramedics: Comparison between standard endotracheal intubation, Laryngeal Mask Airway, and I-Gel. Eur. J. Emerg. Med. 2014, 21, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Castle, N.; Owen, R.; Hann, M.; Naidoo, R.; Reeves, D. Assessment of the speed and ease of insertion of three supraglottic airway devices by paramedics: A manikin study. Emerg. Med. J. 2010, 27, 860–863. [Google Scholar] [CrossRef]
- Goliasch, G.; Ruetzler, A.; Fischer, H.; Frass, M.; Sessler, D.I.; Ruetzler, K. Evaluation of advanced airway management in absolutely inexperienced hands: A randomized manikin trial. Eur. J. Emerg. Med. 2013, 20, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Ruetzler, K.; Roessler, B.; Potura, L.; Priemayr, A.; Robak, O.; Schuster, E.; Frass, M. Performance and skill retention of intubation by paramedics using seven different airway devices—A manikin study. Resuscitation 2011, 82, 593–597. [Google Scholar] [CrossRef]
- Chauhan, G.; Nayar, P.; Seth, A.; Gupta, K.; Panwar, M.; Agrawal, N. Comparison of clinical performance of the I-gel with LMA proseal. J. Anaesthesiol. Clin. Pharmacol. 2013, 29, 56–60. [Google Scholar] [CrossRef]
- Gabbott, D.A.; Beringer, R. The iGEL supraglottic airway: A potential role for resuscitation? Resuscitation 2007, 73, 161–162. [Google Scholar] [CrossRef]
- Middleton, P.M.; Simpson, P.; Thomas, R.; Bendall, J.C. Higher insertion success with the i-gel® supraglottic airway in out-of-hospital cardiac arrest: A randomised controlled trial. Resuscitation 2014, 85, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Häske, D.; Gaier, G.; Heinemann, N.; Schempf, B.; Renz, J.-U. Minimal training for first responders with the i-gel™ leads to successful use in prehospital cardiopulmonary resuscitation. Resuscitation 2019, 134, 167–168. [Google Scholar] [CrossRef] [Green Version]
- Theiler, L.; Gutzmann, M.; Kleine-Brueggeney, M.; Urwyler, N.; Kaempfen, B.; Greif, R. i-gel ™ supraglottic airway in clinical practice: A prospective observational multicentre study. Br. J. Anaesth. 2012, 109, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Benger, J.R.; Kirby, K.; Black, S.; Brett, S.J.; Clout, M.; Lazaroo, M.J.; Nolan, J.; Reeves, B.C.; Robinson, M.; Scott, L.J.; et al. Effect of a Strategy of a Supraglottic Airway Device vs. Tracheal Intubation during out-of-Hospital Cardiac Arrest on Functional Outcome. JAMA 2018, 320, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.; Chantler, P.; Baskett, P. The incidence of regurgitation during cardiopulmonary resuscitation: A comparison between the bag valve mask and laryngeal mask airway: The AIRWAYS-2 randomized clinical trial. Resuscitation 1998, 38, 3–6. [Google Scholar] [CrossRef]
- Piegeler, T.; Roessler, B.; Goliasch, G.; Fischer, H.; Schlaepfer, M.; Lang, S.; Ruetzler, K. Evaluation of six different airway devices regarding regurgitation and pulmonary aspiration during cardio-pulmonary resuscitation (CPR)—A human cadaver pilot study. Resuscitation 2016, 102, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Häske, D.; Schempf, B.; Gaier, G.; Niederberger, C. Performance of the i-gel™ during pre-hospital cardiopulmonary resuscitation. Resuscitation 2013, 84, 1229–1232. [Google Scholar] [CrossRef]
- Soar, J.; Berg, K.M.; Andersen, L.W.; Böttiger, B.W.; Cacciola, S.; Callaway, C.W.; Couper, K.; Cronberg, T.; D’Arrigo, S.; Deakin, C.D.; et al. Adult Advanced Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2020, 156, A80–A119. [Google Scholar] [CrossRef]
- Nolan, J.P.; Maconochie, I.; Soar, J.; Olasveengen, T.M.; Greif, R.; Wyckoff, M.H.; Singletary, E.M.; Aickin, R.; Berg, K.M.; Mancini, M.E.; et al. Executive Summary: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020, 142, S2–S27. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Stuby, L.; Jampen, L.; Sierro, J.; Paus, E.; Spichiger, T.; Suppan, L.; Thurre, D. Effect on Chest Compression Fraction of Continuous Manual Compressions with Asynchronous Ventilations Using an i-gel® versus 30:2 Approach during Simulated out-of-Hospital Cardiac Arrest: Protocol for a Manikin Multicenter Randomized Controlled Trial. Healthcare 2021, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Keamk-Create Random and Balanced Teams. Available online: https://www.keamk.com/ (accessed on 5 January 2021).
- Create a Blocked Randomisation List. Sealed Envelope. Available online: https://www.sealedenvelope.com/simple-randomiser/v1/lists (accessed on 6 December 2020).
- Peyton, J.W.R. Teaching & Learning in Medical Practice; Manticore Europe Ltd.: Rickmansworth, Herts, UK, 1998; ISBN 978-1-900887-00-7. [Google Scholar]
- Stuby, L.; Currat, L.; Gartner, B.; Mayoraz, M.; Harbarth, S.; Suppan, L.; Suppan, M. Impact of Face-to-Face Teaching in Addition to Electronic Learning on Personal Protective Equipment Doffing Proficiency in Student Paramedics: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2021, 10, e26927. [Google Scholar] [CrossRef]
- Giacomino, K.; Caliesch, R.; Sattelmayer, K.M. The effectiveness of the Peyton’s 4-step teaching approach on skill acquisition of procedures in health professions education: A systematic review and meta-analysis with integrated meta-regression. PeerJ 2020, 8, e10129. [Google Scholar] [CrossRef]
- Dörges, V.; Ocker, H.; Hagelberg, S.; Wenzel, V.; Schmucker, P. Optimisation of tidal volumes given with self-inflatable bags without additional oxygen. Resuscitation 2000, 43, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Dörges, V.; Ocker, H.; Hagelberg, S.; Wenzel, V.; Idris, A.H.; Schmucker, P. Smaller tidal volumes with room-air are not sufficient to ensure adequate oxygenation during bag–valve–mask ventilation. Resuscitation 2000, 44, 37–41. [Google Scholar] [CrossRef]
- Baskett, P.; Nolan, J.; Parr, M. Tidal volumes which are perceived to be adequate for resuscitation. Resuscitation 1996, 31, 231–234. [Google Scholar] [CrossRef]
- Aramendi, E.; Lu, Y.; Chang, M.P.; Elola, A.; Irusta, U.; Owens, P.; Idris, A.H. A novel technique to assess the quality of ventilation during pre-hospital cardiopulmonary resuscitation. Resuscitation 2018, 132, 41–46. [Google Scholar] [CrossRef]
- Neto, A.S.; Cardoso, S.O.; Manetta, J.A.; Pereira, V.G.M.; Espósito, D.C.; Pasqualucci, M.D.O.P.; Damasceno, M.C.T.; Schultz, M.J. Association between Use of Lung-Protective Ventilation with Lower Tidal Volumes and Clinical Outcomes among Patients without Acute Respiratory Distress Syndrome: A Meta-Analysis. JAMA 2012, 308, 1651–1659. [Google Scholar] [CrossRef] [Green Version]
- Stuby, L. CPR—Early Insertion Effect of a Supraglottic Airway Device on CCF in Simulated OHCA—PHP Code for Data Extraction from SimMan® 3G. Mendeley Data, V1. 2021. Available online: https://data.mendeley.com/datasets/s8d2gpfhyw/1 (accessed on 30 November 2021).
- Christiansen, T.; Lauritsen, J. EpiData Software. Available online: https://www.epidata.dk/ (accessed on 18 December 2020).
- Stuby, L.; Thurre, D.; Jampen, L.; Spichiger, T.; Sierro, J.; Bergeron, M.; Paus, E. CPR-Early Insertion Effect of a Supraglottic Airway Device on CCF in simulated OHCA-Dataset. Mendeley Data, V1. 2021. Available online: https://data.mendeley.com/datasets/98rf9psgvb/1 (accessed on 30 November 2021).
- Sealed Envelope. Power Calculator for Continuous Outcome Superiority Trial. Available online: https://www.sealedenvelope.com/power/continuous-superiority/ (accessed on 8 June 2021).
- Vogt, L.; Sellmann, T.; Wetzchewald, D.; Schwager, H.; Russo, S.; Marsch, S. Effects of Bag Mask Ventilation and Advanced Airway Management on Adherence to Ventilation Recommendations and Chest Compression Fraction: A Prospective Randomized Simulator-Based Trial. J. Clin. Med. 2020, 9, 2045. [Google Scholar] [CrossRef]
- Cereceda-Sánchez, F.; Molina-Mula, J.; Clar-Terradas, J.; Mascaró-Galmes, A.; Montero-París, P.; Martinez-Cuellar, N. 7 Bag-valve-mask vs. laryngeal mask (I-Gel®) during basic instrumental CPR with capnography monitoring: Preliminary results of a randomized cluster trial. BMJ Open 2019, 9, A3. [Google Scholar] [CrossRef]
- Lavonas, E.J.; Ohshimo, S.; Nation, K.; Van de Voorde, P.; Nuthall, G.; Maconochie, I.; Torabi, N.; Morrison, L.J.; DeCaen, A.; Atkins, D.; et al. International Liaison Committee on Resuscitation (ILCOR) Pediatric Life Support Task Force Advanced Airway Interventions for Paediatric Cardiac Arrest: A Systematic Review and Meta-Analysis. Resuscitation 2019, 138, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.L.; Walker, M.; Leroux, Y.; Carter, A. Chest Compression Fraction in Simulated Cardiac Arrest Management by Primary Care Paramedics: King Laryngeal Tube Airway Versus Basic Airway Management. Prehospital Emerg. Care 2013, 17, 285–290. [Google Scholar] [CrossRef]
- Koka, A.; Suppan, L.; Cottet, P.; Carrera, E.; Stuby, L.; Suppan, M. Teaching the National Institutes of Health Stroke Scale to Paramedics (E-Learning vs. Video): Randomized Controlled Trial. J. Med Internet Res. 2020, 22, e18358. [Google Scholar] [CrossRef]
- Suppan, L.; Abbas, M.; Stuby, L.; Cottet, P.; Larribau, R.; Golay, E.; Iten, A.; Harbarth, S.; Gartner, B.; Suppan, M. Effect of an E-Learning Module on Personal Protective Equipment Proficiency among Prehospital Personnel: Web-Based Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e21265. [Google Scholar] [CrossRef]
- Suppan, L.; Stuby, L.; Gartner, B.; Larribau, R.; Iten, A.; Abbas, M.; Harbarth, S.; Suppan, M. Impact of an e-learning module on personal protective equipment knowledge in student paramedics: A randomized controlled trial. Antimicrob. Resist. Infect. Control. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Perkins, G.D.; Stephenson, B.T.; Smith, C.M.; Gao, F. A comparison between over-the-head and standard cardiopulmonary resuscitation. Resuscitation 2004, 61, 155–161. [Google Scholar] [CrossRef]
- Chi, C.-H.; Tsou, J.-Y.; Su, F.-C. Comparison of chest compression kinematics associated with over-the-head and standard cardiopulmonary resuscitation. Am. J. Emerg. Med. 2009, 27, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Ćwiertnia, M.; Kawecki, M.; Ilczak, T.; Mikulska, M.; Dutka, M.; Bobiński, R. Comparison of standard and over-the-head method of chest compressions during cardiopulmonary resuscitation—A simulation study. BMC Emerg. Med. 2019, 19, 73. [Google Scholar] [CrossRef] [Green Version]
- Maisch, S.; Issleib, M.; Kuhls, B.; Mueller, J.; Horlacher, T.; Goetz, A.E.; Schmidt, G.N. A Comparison between over-The-Head and Standard Cardiopulmonary Resuscitation Performed by Two Rescuers: A Simulation Study. J. Emerg. Med. 2010, 39, 369–376. [Google Scholar] [CrossRef]
- Davis, D.P.; Graham, P.G.; Husa, R.D.; Lawrence, B.; Minokadeh, A.; Altieri, K.; Sell, R.E. A performance improvement-based resuscitation programme reduces arrest incidence and increases survival from in-hospital cardiac arrest. Resuscitation 2015, 92, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bobrow, B.J.; Vadeboncoeur, T.F.; Stolz, U.; Silver, A.E.; Tobin, J.M.; Crawford, S.A.; Mason, T.K.; Schirmer, J.; Smith, G.A.; Spaite, D. The Influence of Scenario-Based Training and Real-Time Audiovisual Feedback on out-of-Hospital Cardiopulmonary Resuscitation Quality and Survival from out-of-Hospital Cardiac Arrest. Ann. Emerg. Med. 2013, 62, 47–56.e1. [Google Scholar] [CrossRef]
- Smith, K.; Dyson, K.; Stub, D.; Magnuson, N.; Anastasopoulos, K.; Bernard, S. 29 Feasibility of using a defibrillator to provide real-time and post-event feedback to paramedics on the quality of their CPR. BMJ Open 2019, 9, A11. [Google Scholar] [CrossRef]
- Lakomek, F.; Lukas, R.-P.; Brinkrolf, P.; Mennewisch, A.; Steinsiek, N.; Gutendorf, P.; Sudowe, H.; Heller, M.; Kwiecien, R.; Zarbock, A.; et al. Real-time feedback improves chest compression quality in out-of-hospital cardiac arrest: A prospective cohort study. PLoS ONE 2020, 15, e0229431. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.P.; Maxey, V.A.; Watson, W.A. Hawthorne Effect: Implications for Prehospital Research. Ann. Emerg. Med. 1995, 26, 590–594. [Google Scholar] [CrossRef]
| Standard Approach (n= 26 Participants) | Experimental Approach (n = 26 Participants) | |
|---|---|---|
| Age, mean (SD), years | 36.6 (9.0) | 35.9 (8.0) |
| Gender, n (%) | ||
| - female | 11 (42.3) | 8 (30.8) |
| - male | 15 (57.7) | 18 (69.2) |
| - other | 0 (0.0) | 0 (0.0) |
| Experience, mean (SD), years | 10.3 (8.5) | 10.6 (8.3) |
| Function—Paramedic, n (%) | 23 (88.5) | 21 (80.8) |
| Number of i-gel® insertions on manikin in the past year, median (Q1; Q3) | 0 (0; 1) | 0 (0; 2) |
| Number of insertions on human in the past year, median (Q1; Q3) | 0 (0; 0) | 0 (0; 0) |
| Minutes | Standard Approach (n = 13 Teams) | Experimental Approach (n = 13 Teams) | p-Value |
|---|---|---|---|
| 0–2 | 83.6% (81.2–86.1) | 89.0% (87.0–91.1) | 0.001 |
| 2–4 | 67.4% (64.6–70.2) | 79.0% (74.0–84.0) | <0.001 |
| 4–6 | 63.7% (59.9–67.6) | 72.0% (67.4–76.6) | <0.001 |
| 6–8 | 60.1% (57.2–63.0) | 70.9% (66.2–75.6) | <0.001 |
| 8–10 | 64.4% (59.5–69.3) | 71.2% (67.1–75.4) | 0.03 |
| Overall | 67.8% (65.7–70.0) | 76.4% (73.1–79.7) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuby, L.; Jampen, L.; Sierro, J.; Bergeron, M.; Paus, E.; Spichiger, T.; Suppan, L.; Thurre, D. Effect of Early Supraglottic Airway Device Insertion on Chest Compression Fraction during Simulated Out-of-Hospital Cardiac Arrest: Randomised Controlled Trial. J. Clin. Med. 2022, 11, 217. https://doi.org/10.3390/jcm11010217
Stuby L, Jampen L, Sierro J, Bergeron M, Paus E, Spichiger T, Suppan L, Thurre D. Effect of Early Supraglottic Airway Device Insertion on Chest Compression Fraction during Simulated Out-of-Hospital Cardiac Arrest: Randomised Controlled Trial. Journal of Clinical Medicine. 2022; 11(1):217. https://doi.org/10.3390/jcm11010217
Chicago/Turabian StyleStuby, Loric, Laurent Jampen, Julien Sierro, Maxime Bergeron, Erik Paus, Thierry Spichiger, Laurent Suppan, and David Thurre. 2022. "Effect of Early Supraglottic Airway Device Insertion on Chest Compression Fraction during Simulated Out-of-Hospital Cardiac Arrest: Randomised Controlled Trial" Journal of Clinical Medicine 11, no. 1: 217. https://doi.org/10.3390/jcm11010217
APA StyleStuby, L., Jampen, L., Sierro, J., Bergeron, M., Paus, E., Spichiger, T., Suppan, L., & Thurre, D. (2022). Effect of Early Supraglottic Airway Device Insertion on Chest Compression Fraction during Simulated Out-of-Hospital Cardiac Arrest: Randomised Controlled Trial. Journal of Clinical Medicine, 11(1), 217. https://doi.org/10.3390/jcm11010217








