Triage Strategies Based on C-Reactive Protein Levels and SARS-CoV-2 Tests among Individuals Referred with Suspected COVID-19: A Prospective Cohort Study
Abstract: Background
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Participants
2.3. Outcomes
2.4. Data Extraction
2.5. Quantitative Variables
2.6. SARS-CoV-2 Detection and CRP Measurements
2.7. Statistical Methods
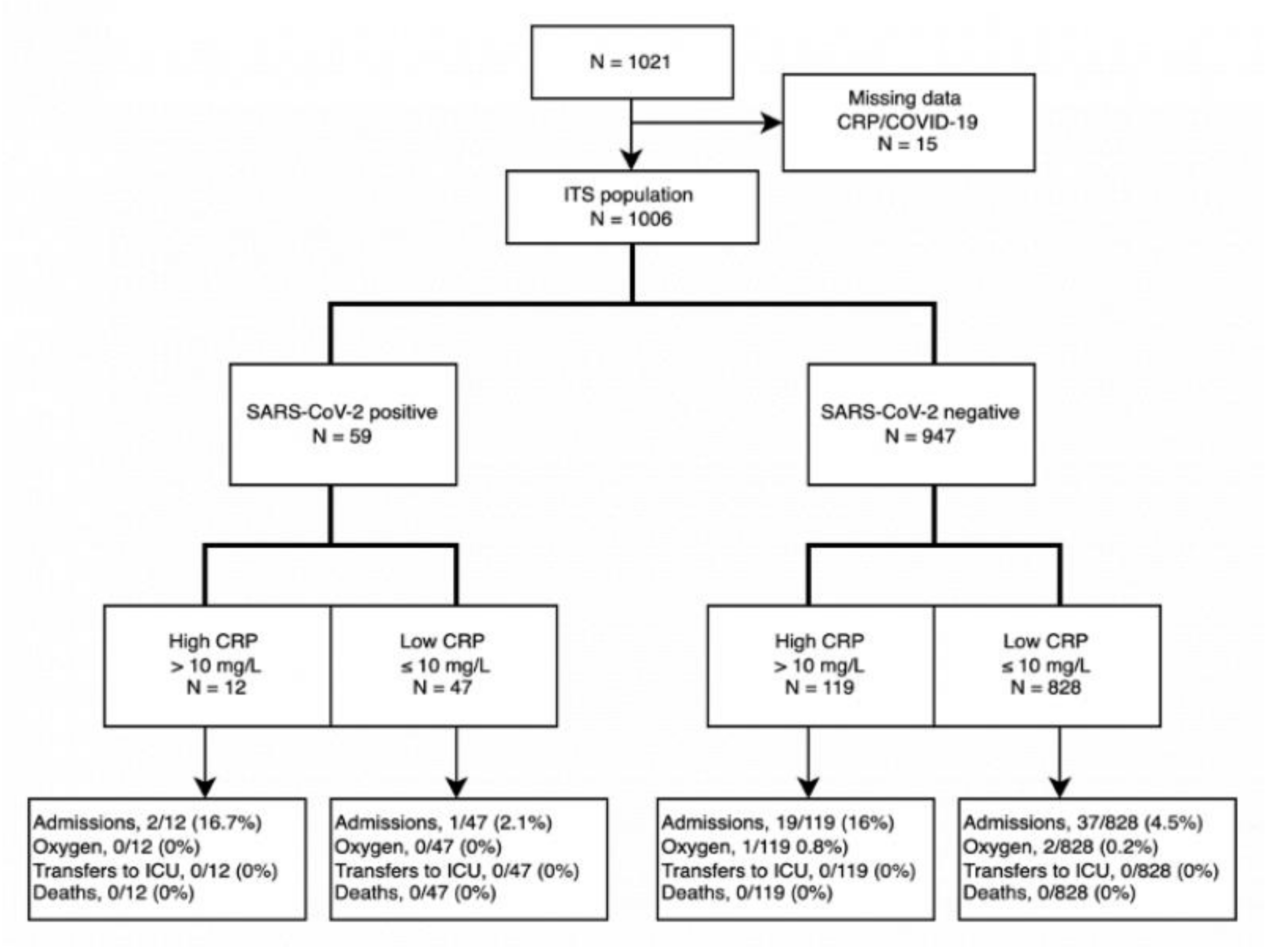
3. Results
3.1. Clinical Characteristics of Patients
3.2. Baseline CRP Levels and Clinical Characteristics
3.3. Outcomes in Individuals with Suspected COVID-19
3.4. Hospitalizations and Risk Factors
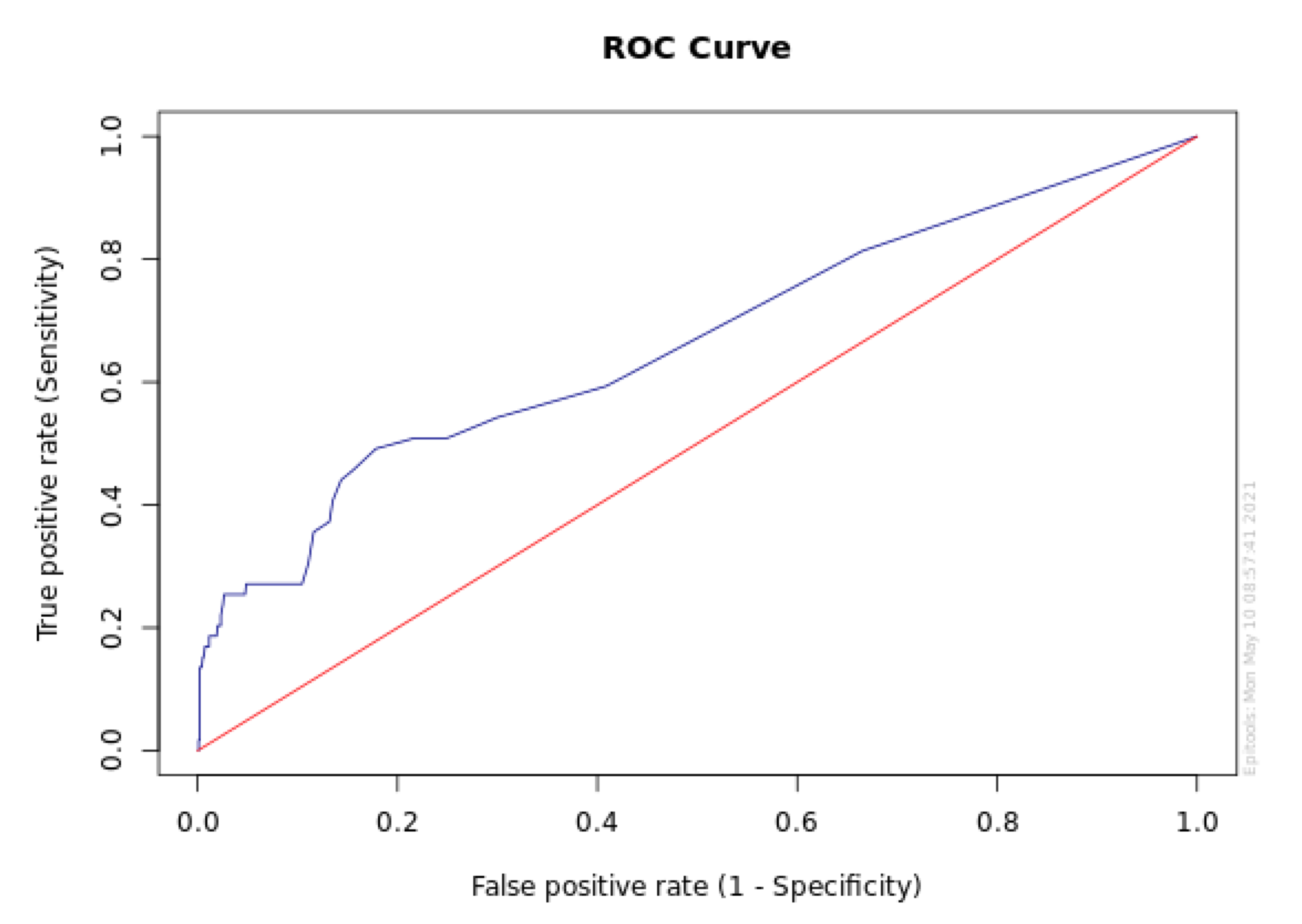
3.5. Receiver Operating Characteristic Curve
4. Discussion
4.1. Handling of SARS-CoV-2
4.2. CRP in the Different Stages of COVID-19 Infections
4.3. Reflections on Investigation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pulia, M.S.; O’Brien, T.P.; Hou, P.C.; Schuman, A.; Sambursky, R. Multi-tiered screening and diagnosis strategy for COVID-19: A model for sustainable testing capacity in response to pandemic. Ann. Med. 2020, 52, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chilimuri, S.; Sun, H.; Alemam, A.; Manthri, N.; Shehi, E.; Tejada, J.; Yugay, A.; Nayudu, S.K. Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study from New York City. West. J. Emerg. Med. 2020, 21, 779–784. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 November 2021).
- sst.dk. tal-og-overvaagning @ www.sst.dk 2021. Available online: https://www.sst.dk/da/corona/status-for-epidemien/tal-og-overvaagning (accessed on 1 November 2021).
- Zhang, X.; Li, S.; Niu, S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020, 96, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 2021, 74, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534.e1. [Google Scholar] [CrossRef] [PubMed]
- Slaats, J.; ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog. 2016, 12, e1005973. [Google Scholar] [CrossRef]
- Volanakis, E.J. Human C-reactive protein: Expression, structure, and function. Mol. Immunol. 2001, 38, 189–197. [Google Scholar] [CrossRef]
- Escadafal, C.; Incardona, S.; Fernandez-Carballo, B.L.; Dittrich, S. The good and the bad: Using C reactive protein to distinguish bacterial from non-bacterial infection among febrile patients in low-resource settings. BMJ Glob. Health 2020, 5, e002396. [Google Scholar] [CrossRef]
- Arcari, L.; Luciani, M.; Cacciotti, L.; Musumeci, M.B.; Spuntarelli, V.; Pistella, E.; Martolini, D.; Manzo, D.; Pucci, M.; Marone, C.; et al. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern. Emerg. Med. 2020, 15, 1467–1476. [Google Scholar] [CrossRef]
- Keddie, S.; Ziff, O.; Chou, M.; Taylor, R.; Heslegrave, A.; Garr, E.; Lakdawala, N.; Church, A.; Ludwig, D.; Manson, J.; et al. Laboratory biomarkers associated with COVID-19 severity and management. Clin. Immunol. 2020, 221, 108614. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, W.; Yan, X.; Guo, T.; Wang, B.; Xia, H.; Ye, L.; Xiong, J.; Jiang, Z.; Liu, Y.; et al. Prognostic Value of C-Reactive Protein in Patients with Coronavirus 2019. Clin. Infect. Dis. 2020, 71, 2174–2179. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Li, G.-M.; He, J.; Liu, Y.; Li, M.; Zhang, R.; Li, Y.-L.; Wu, Y.-Z.; Diao, B. Combined use of the neutrophil-to-lymphocyte ratio and CRP to predict 7-day disease severity in 84 hospitalized patients with COVID-19 pneumonia: A retrospective cohort study. Ann. Transl. Med. 2020, 8, 635. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Corman, V.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.; Bleicker, T.; Brünink, S.; Schneider, J.; Luisa Schmidt, M.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Von Eckardstein, A.; Roth, H.J.; Jones, G.; Preston, S.; Szekeres, T.; Imdahl, R.; Conti, M.; Blanckaert, N.; Jose, D.; Thiery, J.; et al. Cobas 8000 Modular Analyzer Series Evaluated under Routine-like Conditions at 14 Sites in Australia, Europe, and the United States. J. Lab. Autom. 2013, 18, 306–327. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Horton, N.; Carpenter, J.; Pocock, S.J. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011, 342, d40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.; Walter, S.; Shannon, H.; Cook, D.; Jaeschke, R.; Heddle, N. Basic statistics for clinicians: 4. Correlation and regression. Can. Med. Assoc. J. 1995, 152, 497–504. [Google Scholar]
- Jeon, J.-S.; Rheem, I.; Kim, J.K. C-Reactive Protein and Respiratory Viral Infection. Korean J. Clin. Lab. Sci. 2017, 49, 15–21. [Google Scholar] [CrossRef]
- Mahat, R.K.; Panda, S.; Rathore, V.; Swain, S.; Yadav, L.; Sah, S.P. The dynamics of inflammatory markers in coronavirus disease-2019 (COVID-19) patients: A systematic review and meta-analysis. Clin. Epidemiol. Glob. Health 2021, 11, 100727. [Google Scholar] [CrossRef]
- Wang, L. C-reactive protein levels in the early stage of COVID-19. Med. Mal. Infect. 2020, 50, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B. C-reactive protein predicts outcome in COVID-19: Is it also a therapeutic target? Eur Heart J. 2021, 42, 2280–2283. [Google Scholar] [CrossRef] [PubMed]
- Ali, N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J. Med. Virol. 2020, 92, 2409–2411. [Google Scholar] [CrossRef] [PubMed]
| SARS-CoV-2 Result | BFH | RH | Total | Overall Total | |||
|---|---|---|---|---|---|---|---|
| Positive n = 37 | Negative n = 586 | Positive n = 22 | Negative n = 361 | Positive n = 59 | Negative n = 947 | All Participant n = 1006 | |
| Males, no. (%) | 10 (27) | 220 (37.5) | 7 (31.2) | 92 (25.5) | 17 (28.8) | 312 (32.9) | 329 (32.7) |
| Age, years (mean) | 39.7 | 46.5 | 40.3 | 43.3 | 39.9 | 45.3 | 45.0 |
| CRP level, mg/L (mean) | 11.6 | 9.9 | 5.0 | 5.1 | 9.1 | 8.1 | 8.1 |
| CRP < 1 mg/L, no. (%) | 2 (5.4) | 134 (22.9) | 6 (27.3) | 186 (51.2) | 8 (13.6) | 320 (33.8) | 328 (32.6) |
| CRP between 1 mg/L and 10 mg/L, no. (%) | 26 (70.2) | 367 (62.6) | 13 (59.0) | 141 (39.1) | 39 (66.1) | 508 (53.6) | 547 (54.4) |
| High CRP > 10 mg/L, no. (%) | 9 (24.3) | 85 (14.5) | 3 (13.6) | 34 (9.4) | 12 (20.3) | 119 (12.6) | 131 (13.0) |
| SARS-CoV-2 positive, no. (%) | 37 (100) | 0 (0) | 22 (100) | 0 (0) | 59 (100) | 0 (0) | 59 (5.9) |
| High CRP + SARS-CoV-2 positive, no. (%) | 9 (24.3) | 0 (0) | 9 (15.3) | 9 (0.9) | |||
| Outcomes 1 | Odds Ratio (95%CI; p Value) | ||
|---|---|---|---|
| Simple Logistic Regression | Logistic Regression (With 2 Independent Variables) | Multiple Regression Analysis (Adjusted for the Other Variables) | |
| Y: Hospitalization | |||
| High CRP (>10 mg/L) | 4.21 (2.38–7.43; p < 0.0001) | 4.26 (2.41–7.53; p < 0.0001) | 3.48 (1.91–6.33; p < 0.0001) |
| SARS-CoV-2 positive | 0.85 (0.26–2.81; p = 0.79) | 0.72 (0.22–2.43; p = 0.60) | 0.81 (0.23–2.92; p = 0.75) |
| X1: Male sex | 2.09 (1.23–3.54; p = 0.006) | 1.59 (0.91–2.76; p = 0.10) | |
| X2: Age in years | 1.04 (1.03–1.06; p < 0.0001) | 1.04 (1.02–1.05; p < 0.0001) | |
| X3: Hospital 2 | 0.31 (0.16–0.63; p = 0.001) | 0.42 (0.20–0.85; p = 0.02) | |
| Y: Oxygen treatment | |||
| High CRP (>10 mg/L) | 3.36 (0.30–37.29; p = 0.32) | 3.50 (0.32–38.90; p = 0.31) | 2.85 (0.25–33.01; p = 0.40) |
| SARS-CoV-2 positive | n.a. (<0.98) | n.a. (p = 0.98) | n.a. (<0.98) |
| X1: Males | 4.14 (0.37–45.76; p = 0.25) | 3.65 (0.31–42.33; p = 0.30) | |
| X2: Age in years | 1.02 (0.96–1.10; p = 0.49) | 1.02 (0.95–1.09; p = 0.58) | |
| X3: Hospital2 | 0.81 (0.07–8.99; p = 0.87) | 1.18 (0.10–14.10; p = 0.89) | |
| Outcome Stratified by SARS-CoV-2 Test | Odds Ratio (95%CI; p Value) | |
|---|---|---|
| Simple Regression Analysis | Multiple Regression Analysis | |
| Hospitalization among the SARS-CoV-2 positive individuals (N = 59) | ||
| High CRP (>10 mg/L) | 9.20 (0.76–111.63; p = 0.08) | 1.00 (0.01–70.50; p = 0.99) |
| X1: Males | 5.47 (0.46–64.77; p = 0.18) | 3.91 (0.20–74.77; p = 0.37) |
| X2: Age in years | 1.11 (1.02–1.21; p = 0.02) | 1.09 (0.97–1.23; p = 0.16) |
| X3: Hospital 1 | n.a. (0.96) | n.a. (0.96) |
| Hospitalization among the SARS-CoV-2 negative individuals (N = 947) | ||
| High CRP (>10 mg/L) | 4.06 (2.25–7.34; p < 0.0001) | 3.47 (1.88–6.41; p < 0.0001) |
| X1: Males | 1.98 (1.15–3.41; p = 0.01) | 1.53 (0.87–2.69; p = 0.14) |
| X2: Age in years | 1.04 (1.02–1.06; p < 0.0001) | 1.03 (1.02–1.05; p = 0.0001) |
| X3: Hospital 1 | 0.34 (0.17–0.67; p = 0.002) | 0.44 (0.21–0.90; p = 0.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyesen, E.O.; Balsby, I.M.; Henriksen, M.; Christensen, R.; Rasmussen, J.H.; Nielsen, F.E.; Nygaard, H.; Friis-Hansen, L.J.; Nielsen, S.D.; Thudium, R.F.; et al. Triage Strategies Based on C-Reactive Protein Levels and SARS-CoV-2 Tests among Individuals Referred with Suspected COVID-19: A Prospective Cohort Study. J. Clin. Med. 2022, 11, 201. https://doi.org/10.3390/jcm11010201
Boyesen EO, Balsby IM, Henriksen M, Christensen R, Rasmussen JH, Nielsen FE, Nygaard H, Friis-Hansen LJ, Nielsen SD, Thudium RF, et al. Triage Strategies Based on C-Reactive Protein Levels and SARS-CoV-2 Tests among Individuals Referred with Suspected COVID-19: A Prospective Cohort Study. Journal of Clinical Medicine. 2022; 11(1):201. https://doi.org/10.3390/jcm11010201
Chicago/Turabian StyleBoyesen, Erika Olivia, Ida Maria Balsby, Marius Henriksen, Robin Christensen, Jens Henning Rasmussen, Finn Erland Nielsen, Hanne Nygaard, Lennart Jan Friis-Hansen, Susanne Dam Nielsen, Rebekka Faber Thudium, and et al. 2022. "Triage Strategies Based on C-Reactive Protein Levels and SARS-CoV-2 Tests among Individuals Referred with Suspected COVID-19: A Prospective Cohort Study" Journal of Clinical Medicine 11, no. 1: 201. https://doi.org/10.3390/jcm11010201
APA StyleBoyesen, E. O., Balsby, I. M., Henriksen, M., Christensen, R., Rasmussen, J. H., Nielsen, F. E., Nygaard, H., Friis-Hansen, L. J., Nielsen, S. D., Thudium, R. F., Porsberg, C., Kristensen, L. E., & Bliddal, H. (2022). Triage Strategies Based on C-Reactive Protein Levels and SARS-CoV-2 Tests among Individuals Referred with Suspected COVID-19: A Prospective Cohort Study. Journal of Clinical Medicine, 11(1), 201. https://doi.org/10.3390/jcm11010201






