Association of Circulating Osteoprotegerin Level with Blood Pressure Variability in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection from Participants
2.3. Measurement of Serum OPG Concentration
2.4. Determination of Long-Term Visit-to-Visit BPV
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
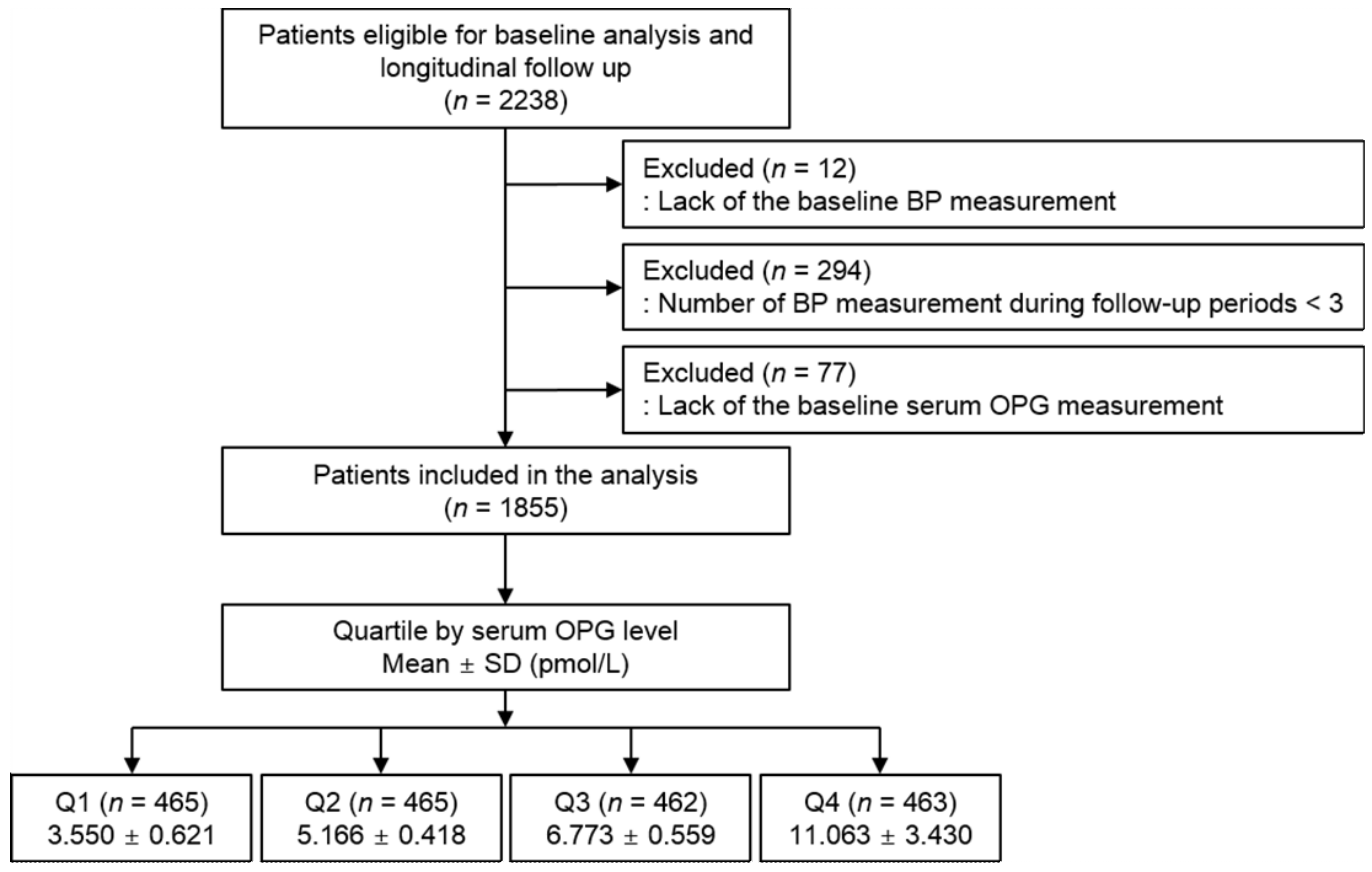
3.1. Baseline Characteristics
3.2. Association of Serum OPG Level with BPV in Patients with CKD
3.3. Sensitivity Analysis
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eguchi, K.; Hoshide, S.; Schwartz, J.E.; Shimada, K.; Kario, K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am. J. Hypertens. 2012, 25, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Shimbo, D.; Tonelli, M.; Reynolds, K.; Arnett, D.K.; Oparil, S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: Findings from NHANES III, 1988 to 1994. Hypertension 2011, 57, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Suchy-Dicey, A.M.; Wallace, E.R.; Mitchell, S.V.; Aguilar, M.; Gottesman, R.F.; Rice, K.; Kronmal, R.; Psaty, B.M.; Longstreth, W.T., Jr. Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am. J. Hypertens. 2013, 26, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Minutolo, R.; Leonardis, D.; D’Arrigo, G.; Tripepi, G.; Rapisarda, F.; Cicchetti, T.; Maimone, I.; Enia, G.; Postorino, M.; et al. Long-term visit-to-visit office blood pressure variability increases the risk of adverse cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2013, 84, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Song, Y.; Gao, L.; Fan, F.; Wang, B.; Liang, M.; Wang, G.; Li, J.; Zhang, Y.; et al. Visit-to-visit variability in blood pressure and the development of chronic kidney disease in treated general hypertensive patients. Nephrol. Dial. Transplant. 2020, 35, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Whittle, J.; Lynch, A.I.; Tanner, R.M.; Simpson, L.M.; Davis, B.R.; Rahman, M.; Whelton, P.K.; Oparil, S.; Muntner, P. Visit-to-Visit Variability of BP and CKD Outcomes: Results from the ALLHAT. Clin. J. Am. Soc. Nephrol. 2016, 11, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Fukuda, M.; Matsui, Y.; Hoshide, S.; Shimada, K.; Kario, K. Impact of visit-to-visit variability of blood pressure on deterioration of renal function in patients with non-diabetic chronic kidney disease. Hypertens. Res. 2013, 36, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Fukuda, M.; Matsui, Y.; Kario, K.; Kimura, K. Visit-to-visit variability of blood pressure and renal function decline in patients with diabetic chronic kidney disease. J. Clin. Hypertens. 2014, 16, 362–366. [Google Scholar] [CrossRef]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M.; Alberta Kidney Disease, N. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef]
- Montañez-Barragán, A.; Gómez-Barrera, I.; Sanchez-Niño, M.D.; Ucero, A.C.; González-Espinoza, L.; Ortiz, A. Osteoprotegerin and kidney disease. J. Nephrol. 2014, 27, 607–617. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Morony, S.; Tintut, Y.; Zhang, Z.; Cattley, R.C.; Van, G.; Dwyer, D.; Stolina, M.; Kostenuik, P.J.; Demer, L.L. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(-/-) mice. Circulation 2008, 117, 411–420. [Google Scholar] [CrossRef]
- Bennett, B.J.; Scatena, M.; Kirk, E.A.; Rattazzi, M.; Varon, R.M.; Averill, M.; Schwartz, S.M.; Giachelli, C.M.; Rosenfeld, M.E. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2117–2124. [Google Scholar] [CrossRef]
- Weiss, R.M.; Lund, D.D.; Chu, Y.; Brooks, R.M.; Zimmerman, K.A.; El Accaoui, R.; Davis, M.K.; Hajj, G.P.; Zimmerman, M.B.; Heistad, D.D. Osteoprotegerin inhibits aortic valve calcification and preserves valve function in hypercholesterolemic mice. PLoS ONE 2013, 8, e65201. [Google Scholar] [CrossRef]
- Morena, M.; Dupuy, A.M.; Jaussent, I.; Vernhet, H.; Gahide, G.; Klouche, K.; Bargnoux, A.S.; Delcourt, C.; Canaud, B.; Cristol, J.P. A cut-off value of plasma osteoprotegerin level may predict the presence of coronary artery calcifications in chronic kidney disease patients. Nephrol. Dial. Transplant. 2009, 24, 3389–3397. [Google Scholar] [CrossRef][Green Version]
- Mikami, S.; Hamano, T.; Fujii, N.; Nagasawa, Y.; Isaka, Y.; Moriyama, T.; Matsuhisa, M.; Ito, T.; Imai, E.; Hori, M. Serum osteoprotegerin as a screening tool for coronary artery calcification score in diabetic pre-dialysis patients. Hypertens. Res. 2008, 31, 1163–1170. [Google Scholar] [CrossRef]
- Marques, G.L.; Hayashi, S.; Bjallmark, A.; Larsson, M.; Riella, M.; Olandoski, M.; Lindholm, B.; Nascimento, M.M. Osteoprotegerin is a marker of cardiovascular mortality in patients with chronic kidney disease stages 3-5. Sci. Rep. 2021, 11, 2473. [Google Scholar] [CrossRef]
- Huang, Q.X.; Li, J.B.; Huang, N.; Huang, X.W.; Li, Y.L.; Huang, F.X. Elevated Osteoprotegerin Concentration Predicts Increased Risk of Cardiovascular Mortality in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2020, 45, 565–575. [Google Scholar] [CrossRef]
- Kamińska, J.; Stopiński, M.; Mucha, K.; Pac, M.; Gołębiowski, M.; Niewczas, M.A.; Pączek, L.; Foroncewicz, B. Circulating Osteoprotegerin in Chronic Kidney Disease and All-Cause Mortality. Int. J. Gen. Med. 2021, 14, 2413–2420. [Google Scholar] [CrossRef]
- Huang, Q.X.; Li, J.B.; Huang, X.W.; Jiang, L.P.; Huang, L.; An, H.W.; Yang, W.Q.; Pang, J.; Li, Y.L.; Huang, F.X. Circulating Osteoprotegerin Levels Independently Predict All-cause Mortality in Patients with Chronic Kidney Disease: A Meta-analysis. Int. J. Med. Sci. 2019, 16, 1328–1337. [Google Scholar] [CrossRef]
- Lin, W.C.; Tsai, J.P.; Lai, Y.H.; Lin, Y.L.; Kuo, C.H.; Wang, C.H.; Hsu, B.G. Serum osteoprotegerin level is positively associated with peripheral artery disease in patients with peritoneal dialysis. Ren. Fail. 2020, 42, 131–136. [Google Scholar] [CrossRef]
- Hou, J.S.; Lin, Y.L.; Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Subeq, Y.M.; Hsu, B.G. Serum osteoprotegerin is an independent marker of central arterial stiffness as assessed using carotid-femoral pulse wave velocity in hemodialysis patients: A cross sectional study. BMC Nephrol. 2019, 20, 184. [Google Scholar] [CrossRef]
- Csiky, B.; Sági, B.; Peti, A.; Lakatos, O.; Prémusz, V.; Sulyok, E. The Impact of Osteocalcin, Osteoprotegerin and Osteopontin on Arterial Stiffness in Chronic Renal Failure Patients on Hemodialysis. Kidney Blood Press. Res. 2017, 42, 1312–1321. [Google Scholar] [CrossRef]
- Avila, M.; Mora, C.; Prado, M.D.C.; Zavala, M.; Paniagua, R. Osteoprotegerin Is the Strongest Predictor for Progression of Arterial Calcification in Peritoneal Dialysis Patients. Am. J. Nephrol. 2017, 46, 39–46. [Google Scholar] [CrossRef]
- Bargnoux, A.S.; Dupuy, A.M.; Garrigue, V.; Deleuze, S.; Cristol, J.P.; Mourad, G. Renal transplantation decreases osteoprotegerin levels. Transplant. Proc. 2006, 38, 2317–2318. [Google Scholar] [CrossRef]
- Hjelmesaeth, J.; Ueland, T.; Flyvbjerg, A.; Bollerslev, J.; Leivestad, T.; Jenssen, T.; Hansen, T.K.; Thiel, S.; Sagedal, S.; Røislien, J.; et al. Early posttransplant serum osteoprotegerin levels predict long-term (8-year) patient survival and cardiovascular death in renal transplant patients. J. Am. Soc. Nephrol. 2006, 17, 1746–1754. [Google Scholar] [CrossRef]
- Oh, K.H.; Park, S.K.; Park, H.C.; Chin, H.J.; Chae, D.W.; Choi, K.H.; Han, S.H.; Yoo, T.H.; Lee, K.; Kim, Y.S.; et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): Design and methods. BMC Nephrol. 2014, 15, 80. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Bae, E.H.; Ma, S.K.; Han, S.H.; Choi, K.H.; Lee, J.; Chae, D.W.; Oh, K.H.; Ahn, C.; Kim, S.W.; et al. Association of Serum Osteoprotegerin Levels with Bone Loss in Chronic Kidney Disease: Insights from the KNOW-CKD Study. PLoS ONE 2016, 11, e0166792. [Google Scholar] [CrossRef] [PubMed]
- Rymarz, A.; Romejko, K.; Matyjek, A.; Bartoszewicz, Z.; Niemczyk, S. Serum Osteoprotegerin Is an Independent Marker of Metabolic Complications in Non-DialysisDependent Chronic Kidney Disease Patients. Nutrients 2021, 13, 3609. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, R.; Cavallari, I.; Tramontana, F.; Park, K.; Strollo, R.; Valente, L.; De Pascalis, M.; Grigioni, F.; Pozzilli, P.; Buzzetti, R.; et al. Association of bone biomarkers with advanced atherosclerotic disease in people with overweight/obesity. Endocrine 2021, 73, 339–346. [Google Scholar] [CrossRef]
- Alves-Lopes, R.; Neves, K.B.; Strembitska, A.; Harvey, A.P.; Harvey, K.Y.; Yusuf, H.; Haniford, S.; Hepburn, R.T.; Dyet, J.; Beattie, W.; et al. Osteoprotegerin regulates vascular function through syndecan-1 and NADPH oxidase-derived reactive oxygen species. Clin. Sci. 2021, 135, 2429–2444. [Google Scholar] [CrossRef]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef]
- Chae, S.Y.; Chung, W.; Kim, Y.H.; Oh, Y.K.; Lee, J.; Choi, K.H.; Ahn, C.; Kim, Y.S. The Correlation of Serum Osteoprotegerin with Non-Traditional Cardiovascular Risk Factors and Arterial Stiffness in Patients with Pre-Dialysis Chronic Kidney Disease: Results from the KNOW-CKD Study. J. Korean. Med. Sci. 2018, 33, e322. [Google Scholar] [CrossRef]
- Sigrist, M.K.; Levin, A.; Er, L.; McIntyre, C.W. Elevated osteoprotegerin is associated with all-cause mortality in CKD stage 4 and 5 patients in addition to vascular calcification. Nephrol. Dial. Transplant. 2009, 24, 3157–3162. [Google Scholar] [CrossRef][Green Version]
- Boardman, H.; Lewandowski, A.J.; Lazdam, M.; Kenworthy, Y.; Whitworth, P.; Zwager, C.L.; Francis, J.M.; Aye, C.Y.; Williamson, W.; Neubauer, S.; et al. Aortic stiffness and blood pressure variability in young people: A multimodality investigation of central and peripheral vasculature. J. Hypertens. 2017, 35, 513–522. [Google Scholar] [CrossRef]
- Hoshide, S. Clinical implication of visit-to-visit blood pressure variability. Hypertens. Res. 2018, 41, 993–999. [Google Scholar] [CrossRef]
- Mena, L.; Pintos, S.; Queipo, N.V.; Aizpúrua, J.A.; Maestre, G.; Sulbarán, T. A reliable index for the prognostic significance of blood pressure variability. J. Hypertens. 2005, 23, 505–511. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Howard, S.C.; Dolan, E.; O’Brien, E.; Dobson, J.E.; Dahlöf, B.; Sever, P.S.; Poulter, N.R. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010, 375, 895–905. [Google Scholar] [CrossRef]


| Serum OPG Level | p Value | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Age (Year) | 43.796 ± 10.698 | 51.159 ± 10.435 | 56.517 ± 10.170 | 62.702 ± 7.903 | <0.001 |
| Male | 300 (64.5) | 365 (57.0) | 262 (56.7) | 284 (59.9) | 0.044 |
| Charlson comorbidity index | <0.001 | ||||
| 0–3 | 411 (94.8) | 391 (84.1) | 366 (72.7) | 195 (42.1) | |
| 4–5 | 24 (5.2) | 71 (15.3) | 118 (25.5) | 248 (53.6) | |
| 6–7 | 0 (0.0) | 3 (0.6) | 8 (1.7) | 19 (4.1) | |
| ≥8 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | |
| DM | 47 (10.1) | 108 (23.2) | 161 (34.8) | 273 (59.0) | <0.001 |
| CAD | 3 (0.6) | 20 (4.3) | 26 (5.6) | 57 (12.3) | <0.001 |
| Arrhythmia | 5 (1.1) | 14 (3.0) | 12 (2.6) | 14 (3.0) | 0.091 |
| Primary renal disease | <0.001 | ||||
| DM | 21 (4.5) | 65 (14.0) | 116 (25.1) | 227 (49.0) | |
| HTN | 75 (16.1) | 95 (20.4) | 110 (23.8) | 95 (20.5) | |
| GN | 225 (48.4) | 181 (38.9) | 140 (30.3) | 63 (13.6) | |
| TID | 4 (0.9) | 1 (0.2) | 4 (0.9) | 5 (1.1) | |
| PKD | 111 (23.9) | 93 (20.0) | 69 (14.9) | 36 (7.8) | |
| Others | 29 (6.2) | 30 (6.5) | 23 (5.0) | 37 (8.0) | |
| Smoking history | 249 (53.5) | 260 (55.9) | 256 (55.4) | 239 (51.6) | 0.44 |
| Medication | |||||
| ACEi/ARBs | 405 (87.1) | 405 (87.1) | 402 (87.0) | 383 (82.7) | 0.142 |
| Diuretics | 92 (19.8) | 120 (25.8) | 154 (33.3) | 201 (43.4) | <0.001 |
| Number of anti-HTN drugs ≥ 3 | 92 (19.8) | 115 (24.7) | 135 (29.2) | 169 (36.5) | <0.001 |
| Statins | 189 (40.6) | 233 (50.1) | 271 (58.7) | 266 (57.5) | <0.001 |
| BMI (kg/m2) | 24.486 ± 3.588 | 24.688 ± 3.332 | 24.626 ± 3.452 | 24.417 ± 3.092 | 0.595 |
| SBP (mmHg) | 124.232 ± 14.459 | 126.062 ± 14.2741 | 127.089 ± 15.269 | 129.827 ± 17.244 | <0.001 |
| DBP (mmHg) | 77.531 ± 10.794 | 78.043 ± 10.429 | 76.513 ± 10.555 | 74.732 ± 11.772 | <0.001 |
| Laboratory findings | |||||
| Hemoglobin (g/dL) | 13.800 ± 1.827 | 13.291 ± 1.896 | 12.870 ± 1.869 | 11.902 ± 1.803 | <0.001 |
| Albumin (g/dL) | 4.303 ± 0.340 | 4.227 ± 0.368 | 4.204 ± 0.389 | 4.079 ± 0.407 | <0.001 |
| TC (mg/dL) | 176.630 ± 32.653 | 176.561 ± 40.129 | 174.583 ± 41.353 | 168.070 ± 36.628 | 0.001 |
| LDL-C (mg/dL) | 99.008 ± 27.956 | 99.376 ± 33.178 | 95.311 ± 32.248 | 92.201 ± 28.663 | 0.001 |
| HDL-C (mg/dL) | 50.799 ± 15.382 | 51.306 ± 15.425 | 49.312 ± 15.425 | 47.376 ± 15.703 | 0.001 |
| TG (mg/dL) | 156.615 ± 98.887 | 145.480 ± 81.129 | 166.894 ± 113.306 | 156.892 ± 98.735 | 0.014 |
| Fasting glucose (mg/dL) | 101.280 ± 22.310 | 104.455 ± 28.829 | 112.650 ± 40.133 | 121.763 ± 53.667 | <0.001 |
| hsCRP (mg/dL) | 0.450 (0.200, 1.200) | 0.700 (0.300, 1.500) | 0.635 ( 0.300, 1.600) | 0.700 (0.200, 2.100) | 0.019 |
| 25(OH) vitamin D (ng/dL) | 18.570 ± 7.498 | 18.176 ± 6.907 | 17.971 ± 7.494 | 17.890 ± 9.306 | 0.559 |
| 24 h urine protein (mg/day) | 368.000 (120.000, 888.000) | 486.000 (138.000, 1330.000) | 493.500 (191.925, 1274.125) | 759.000 (280.000, 4205.000) | <0.001 |
| Urine ACR (mg/g Cr) | 196.928 (30.800, 565.480) | 306.642 (55.529, 780.979) | 314.765 (77.804, 863.397) | 511.134 (144.603, 1490.550) | <0.001 |
| eGFR (mL/min/1.73 m2) | 68.717 ± 32.278 | 57.547 ± 30.177 | 47.749 ± 24.132 | 34.769 ± 18.409 | <0.001 |
| CKD stages | <0.001 | ||||
| Stage 1 | 157 (33.8) | 94 (20.2) | 47 (10.2) | 14 (3.0) | |
| Stage 2 | 129 (27.7) | 121 (26.0) | 91 (19.7) | 29 (6.3) | |
| Stage 3a | 82 (17.6) | 81 (17.4) | 82 (19.9) | 70 (15.1) | |
| Stage 3b | 61 (13.1) | 95 (20.4) | 130 (28.1) | 130 (28.1) | |
| Stage 4 | 33 (7.1) | 63 (13.5) | 89 (19.3) | 183 (39.5) | |
| Stage 5 | 3 (0.6) | 11 (2.4) | 13 (2.8) | 37 (8.0) | |
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β Coefficient (95% CIs) | p Value | β Coefficient (95% CIs) | p Value | |
| ARV of SBP | 0.412 (0.320, 0.505) | <0.001 | 0.143 (0.021, 0.264) | 0.021 |
| SD of SBP | 0.231 (0.162, 0.300) | <0.001 | 0.074 (−0.018, 0.165) | 0.113 |
| CoV of SBP | 0.001 (0.001, 0.002) | <0.001 | 0.000 (0.000, 0.001) | 0.203 |
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β Coefficient (95% CIs) | p Value | β Coefficient (95% CIs) | p Value | |
| ARV of SBP | 0.373 (0.270, 0.476) | <0.001 | 0.143 (0.008, 0.277) | 0.038 |
| SD of SBP | 0.200 (0.124, 0.275) | <0.001 | 0.081 (−0.018, 0.181) | 0.107 |
| CoV of SBP | 0.001 (0.001, 0.002) | <0.001 | 0.001 (0.000, 0.001) | 0.179 |
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β Coefficient (95% CIs) | p for Interaction | β Coefficient (95% CIs) | p for Interaction | |
| Age < 60 years | 0.499 (0.339, 0.658) | 0.151 | 0.018 (−0.179, 0.215) | 0.131 |
| Age ≥ 60 years | 0.377 (0.227, 0.528) | 0.171 (−0.009, 0.352) | ||
| Male | 0.480 (0.354, 0.606) | 0.955 | 0.224 (0.050, 0.399) | 0.465 |
| Female | 0.321 (0.184, 0.457) | 0.046 (−0.127, 0.220) | ||
| Charlson comorbidity index ≤ 3 | 0.361 (0.239, 0.484) | 0.03 | 0.143 (−0.008, 0.294) | 0.165 |
| Charlson comorbidity index ≥ 4 | 0.162 (−0.025, 0.348) | 0.174 (−0.060, 0.407) | ||
| History of DM (−) | 0.403 (0.265, 0.541) | 0.055 | 0.153 (−0.027, 0.333) | 0.228 |
| History of DM (+) | 0.218 (0.066, 0.370) | 0.185 (−0.003, 0.373) | ||
| BMI < 23 kg/m2 | 0.447 (0.279, 0.615) | 0.739 | 0.100 (−0.146, 0.347) | 0.801 |
| BMI ≥ 23 kg/m2 | 0.396 (0.284, 0.507) | 0.177 (0.037, 0.318) | ||
| Number of anti-HTN drugs ≤ 2 | 0.399 (0.291, 0.507) | 0.777 | 0.112 (−0.029, 0.253) | 0.871 |
| Number of anti-HTN drugs ≥ 3 | 0.377 (0.192, 0.562) | 0.194 (−0.050, 0.438) | ||
| eGFR ≥ 45 mL/min/1.73 m2 | 0.418 (0.255, 0.581) | 0.986 | 0.172 (−0.062, 0.347) | 0.281 |
| eGFR < 45 mL/min/1.73 m2 | 0.339 (0.201, 0.477) | 0.106 (−0.073, 0.284) | ||
| 24 h urine protein < 200 mg | 0.530 (0.340, 0.720) | 0.108 | 0.366 (0.124, 0.607) | 0.149 |
| 24 h urine protein ≥ 200 mg | 0.363 (0.253, 0.472) | 0.103 (−0.040, 0.246) | ||
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β Coefficient (95% CIs) | p for Interaction | β Coefficient (95% CIs) | p for Interaction | |
| Age < 60 years | 0.272 (0.151, 0.393) | 0.040 | −0.001 (−0.153, 0.150) | 0.388 |
| Age ≥ 60 years | 0.167 (0.059, 0.275) | 0.095 (−0.036, 0.225) | ||
| Male | 0.258 (0.168, 0.348) | 0.800 | 0.142 (0.016, 0.268) | 0.630 |
| Female | 0.195 (0.089, 0.302) | −0.007 (−0.144, 0.130) | ||
| Charlson comorbidity index ≤ 3 | 0.261 (0.167, 0.355) | <0.001 | 0.127 (0.009, 0.245) | 0.005 |
| Charlson comorbidity index ≥ 4 | 0.003 (−0.127, 0.133) | 0.062 (−0.100, 0.225) | ||
| History of DM (−) | 0.263 (0.158, 0.369) | 0.007 | 0.145 (0.006, 0.284) | 0.043 |
| History of DM (+) | 0.067 (−0.041, 0.175) | 0.051 (−0.084, 0.186) | ||
| BMI < 23 kg/m2 | 0.237 (0.112, 0.362) | 0.998 | −0.007 (−0.193, 0.178) | 0.868 |
| BMI ≥ 23 kg/m2 | 0.228 (0.146, 0.311) | 0.118 (0.013, 0.224) | ||
| Number of anti-HTN drugs ≤ 2 | 0.229 (0.149, 0.310) | 0.411 | 0.057 (−0.049, 0.163) | 0.973 |
| Number of anti-HTN drugs ≥ 3 | 0.204 (0.067, 0.341) | 0.105 (−0.074, 0.285) | ||
| eGFR ≥ 45 mL/min/1.73 m2 | 0.355 (0.228, 0.483) | 0.033 | 0.145 (−0.016, 0.305) | 0.421 |
| eGFR < 45 mL/min/1.73 m2 | 0.171 (0.073, 0.270) | 0.074 (−0.055, 0.203) | ||
| 24 h urine protein < 200 mg | 0.354 (0.213, 0.496) | 0.055 | 0.194 (0.011, 0.377) | 0.103 |
| 24 h urine protein ≥ 200 mg | 0.186 (0.105, 0.267) | 0.060 (−0.048, 0.167) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Oh, K.-H.; Lee, J.; Oh, Y.K.; Jung, J.Y.; Choi, K.H.; Ma, S.K.; et al. Association of Circulating Osteoprotegerin Level with Blood Pressure Variability in Patients with Chronic Kidney Disease. J. Clin. Med. 2022, 11, 178. https://doi.org/10.3390/jcm11010178
Suh SH, Oh TR, Choi HS, Kim CS, Oh K-H, Lee J, Oh YK, Jung JY, Choi KH, Ma SK, et al. Association of Circulating Osteoprotegerin Level with Blood Pressure Variability in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2022; 11(1):178. https://doi.org/10.3390/jcm11010178
Chicago/Turabian StyleSuh, Sang Heon, Tae Ryom Oh, Hong Sang Choi, Chang Seong Kim, Kook-Hwan Oh, Joongyub Lee, Yun Kyu Oh, Ji Yong Jung, Kyu Hun Choi, Seong Kwon Ma, and et al. 2022. "Association of Circulating Osteoprotegerin Level with Blood Pressure Variability in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 11, no. 1: 178. https://doi.org/10.3390/jcm11010178
APA StyleSuh, S. H., Oh, T. R., Choi, H. S., Kim, C. S., Oh, K.-H., Lee, J., Oh, Y. K., Jung, J. Y., Choi, K. H., Ma, S. K., Bae, E. H., Kim, S. W., & on behalf of the Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD) Investigators. (2022). Association of Circulating Osteoprotegerin Level with Blood Pressure Variability in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 11(1), 178. https://doi.org/10.3390/jcm11010178






