Heart Team for Left Atrial Appendage Occlusion: A Patient-Tailored Approach
Abstract
1. Introduction
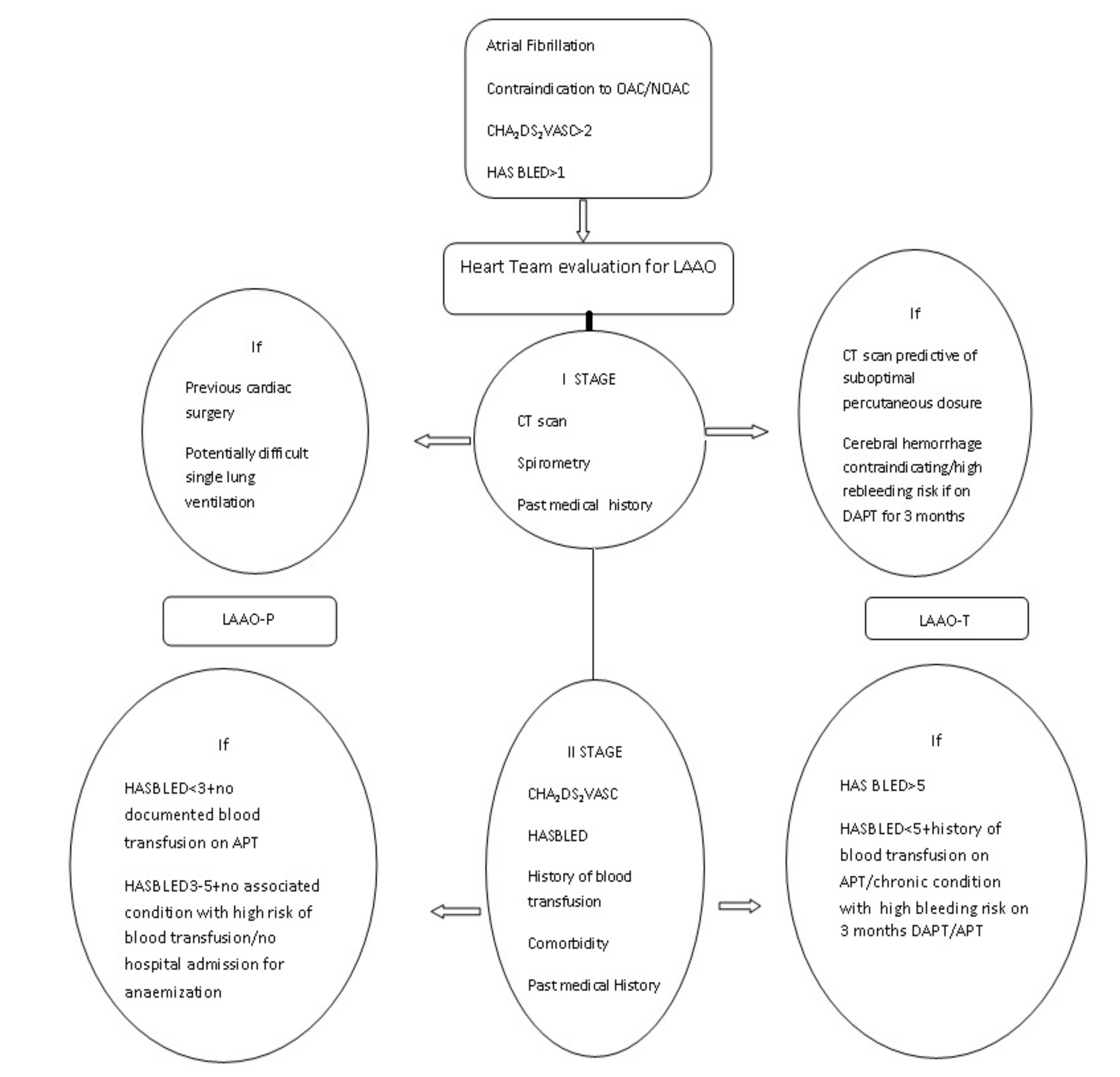
2. Materials and Methods
2.1. Statistics
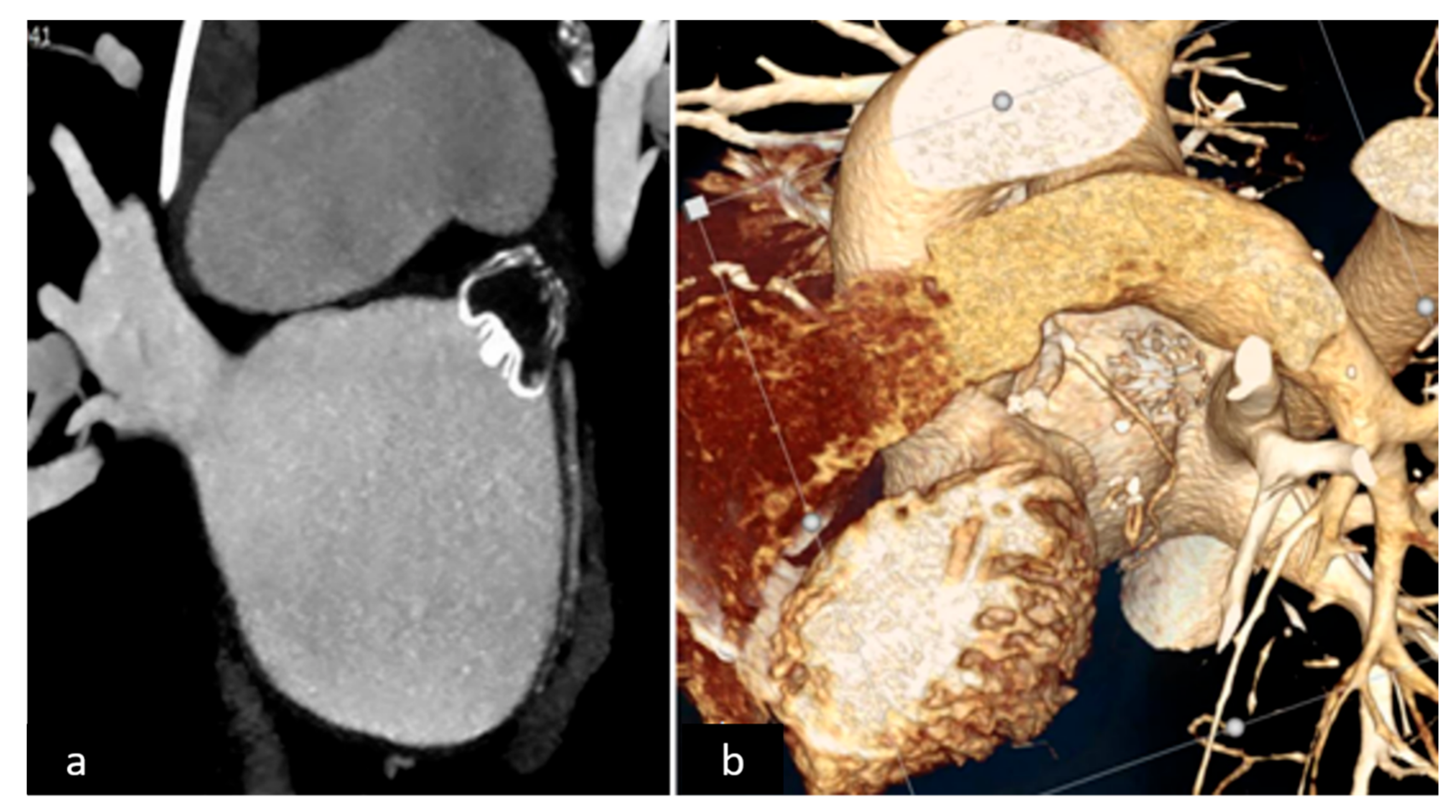
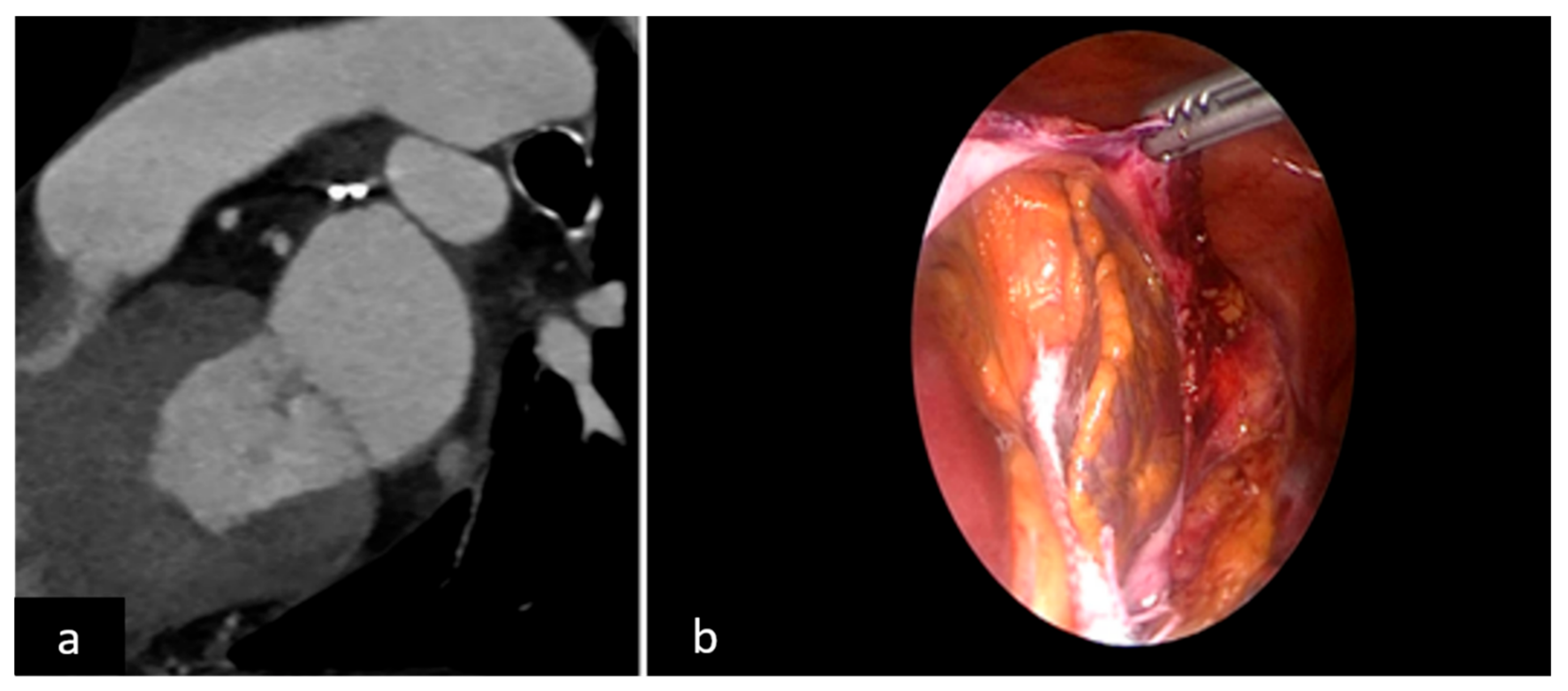
2.2. LAAO-T Procedure
2.3. LAAO-P Procedure
2.4. LAAO-T and LAAO-P Procedures: Role of Echo Imaging
2.5. Post Procedural Anticoagulation Regimen
2.6. Follow Up
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackshear, J.; Odell, J. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L.; Ad, N.; Palazzo, T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J. Thor. Cardiovasc. Surg. 1999, 118, 833–840. [Google Scholar] [CrossRef]
- Tsai, Y.; Phan, K.; Larsen-Munkholm, S.; Tian, D.H.; La Meir, M.; Yan, T.D. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: A meta-analysis. Eur. J. Card. Thorac. Surg. 2014, 47, 847–857. [Google Scholar] [CrossRef]
- Kanderian, A.; Gillinov, A.M.; Pettersson, G.B.; Blackstone, E.; Klein, A.L. Success of left atrial appendage closure assessment by transesophageal echocardiography. J. Am. Coll. Cardiol. 2008, 59, 924–929. [Google Scholar] [CrossRef]
- Lee, R.; Vassallo, P.; Kruse, J.; Malaisrie, S.C.; Rigolin, V.; Andrei, A.C.; McCarthy, P. A randomized prospective pilot comparison of 3 atrial appendage elimination techniques: Internal ligation, stapled excision, and surgical excision. J. Thorac. Cardiovasc. Surg. 2016, 152, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Ninomiya, M.; Nonaka, T.; Hisagi, M.; Ota, T.; Mizutani, T. Thoracoscopic standalone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J. Am. Coll. Cardiol. 2013, 62, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, C.; Verberkmoes, N.; van Es, H.W.; Lewalter, T.; Dunnington, G.; Stark, S.; Longoria, J.; Hofman, F.H.; Pierce, C.M.; Kotecha, D.; et al. Thoracoscopic left atrial appendage clipping: A multicenter Cohort Analysis. JACC Clin. Electrophysiol. 2018, 4, 893–901. [Google Scholar] [CrossRef]
- Branzoli, S.; Marini, M.; Guarracini, F.; Pederzolli, C.; Pomarolli, C.; D’Onghia, G.; Centonze, M.; Fantinel, M.; Corsini, F.; Bonmassari, R.; et al. Epicadial standalone left atrial appendage clipping for prevention of ischemic stroke in patients with atrial fibrillation contraindicated for oral anticoagulation. J. Cardiovasc. Electrophysiol. 2020, 31, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, N.; Beigel, R.; Swaans, M.J.; Ho, S.Y.; Siegel, R.J. Percutaneous interventions for left atrial appendage exclusion: Options assessment, and imaging using 2D and 3D Echocardiography. JACC 2015, 8, 472–488. [Google Scholar]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5 Years outcomes after left atrial appendage closure: From the PREVAIL and PROTECT AF Trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef]
- Boersma, C.; Schmidt, B.; Betts, T.R.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; Stein, K.M.; et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri procedural outcomes form the EWOLUTION registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.V.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; Betts, T.; et al. Evaluating Real-World Clinical Outcomes in atrail fibrillation patients receiving the WATCHMAN left atrial appendage closure technology. Circ. Arrhythm. Electrophysiol. 2019, 12, e006841. [Google Scholar] [CrossRef]
- Saw, J.; Lempereur, M. Percutaneous left atrial appendage closure: Procedural techniques and outcomes. JACC Cardiovasc. Interv. 2014, 7, 1205–1220. [Google Scholar] [CrossRef]
- Murthy, S.; Gupta, A.; Merkler, A.E.; Navi, B.B.; Mandava, P.; Iadecola, C.; Sheth, K.N.; Hanley, D.F.; Ziai, W.C.; Kamel, H. Restarting anticoagulant therapy after intracranial hemorrhage a systematic review and meta analysis. Stroke 2017, 48, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, S.; Grunenfelder, J.; Emmert, M. Left atrial appendage closure to prevent stroke in patients with atrial fibrillation: A call for the heart team approach. Europace 2015, 12, 1880–1881. [Google Scholar] [CrossRef][Green Version]
- Kany, S.; Metzner, A.; Lubos, E.; Kirchhof, P. The Atrial Fibrillation Heart Team-guiding therapy in left atrial appendage occlusion with increasingly complex patients and little evidence. Eur. Heart J. 2021, ehab744. [Google Scholar] [CrossRef]
- Lip, G.Y. Implication of the CHA2DS2-VASc and HAS BLED scores for thromboprophylaxis in atrial fibrillation. Am. J. Med. 2011, 2, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Santangeli, P.; Anselmino, M.; Mohanty, P.; Salbetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; et al. Does the Left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from multicenter study. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar] [CrossRef]
- Branzoli, S.; Marini, M.; Guarracini, F.; Pederzolli, C.; D’Onghia, G.; Centonze, M.; Pomarolli, C.; Graffigna, A.; La Meir, M. Standalone totally thoracoscopic left appendage clipping: Safe, Simple, Standardized. Ann. Thor. Surg. 2021, 111, 61–63. [Google Scholar] [CrossRef]
- Holmes, D.; Reddy, D.; Turi, Z.; Doshi, S.; Sievert, H.; Buchbinder, M.; Mullin, C.; Sick, P. PROTECT AF Investigators Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomized non inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Vainrib, A.; Harb, S.; Jaber, W.; Benenstein, R.J.; Aizer, A.; Chinitz, L.A.; Saric, M. Left atrial appendage occlusion/exclusion: Procedural image guidance with transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 454–464. [Google Scholar] [CrossRef]
- Patel, A.; Venkataraman, R.; Schurmann, P.; Dave, A.; Valderrabano, M. Left atrial appendage occlusion using intracardiac echocardiography. Heart Rhythm 2021, 18, 313–317. [Google Scholar] [CrossRef]
- Jones, W.; Williams, L.; Meschia, J.F. Validating the Questionnaire for Verifying stroke free status QSVS by Neurological history and examination. Stroke 2001, 32, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Emmert, M.; Puippe, G.; Baumuller, S.; Alkadhi, H.; Landmesser, U.; Plass, A.; Bettex, D.; Scherman, J.; Grunenfelder, J.; Genoni, M.; et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: First long term results from a prospective device trial. Eur. J. Cardiothorac. Surg. 2014, 45, 126–131. [Google Scholar] [CrossRef]
- Jackson, L.; Kim, S.; Blanco, R.; Thomas, L.; Ansell, J.; Fonarow, G.C.; Gersh, B.J.; Go, A.; Kowey, P.; Mahaffey, K.; et al. Discontinuation rates of warfarin versus direct acting oral anticoagulants in US clinical practice: Results from Outcomes Registry for Better Informed treatment of Atrial Fibrillation II (ORBIT-AFII). Am. Heart J. 2020, 226, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.; Giugliano, R.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, J.M.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A metanalysis of randomized trial. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Connolly, S.; Ezekowitz, M.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in patients with atrial fibrillation. N. Eng. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.A.; Gao, H.; Shrader, P.; Pieper, K.; Thomas, L.; Camm, A.J.; Ezekowitz, M.D.; Fanarow, G.; Gersh, B.; Goldhaber, S.; et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: Results from the GAREFIELD-AF, ORBIT-AF I, anf ORBIT AF II registries. Am. Heart J. 2017, 194, 132–140. [Google Scholar] [CrossRef]
- Huisman, M.V.; Rothman, K.J.; Paquette, M.; Teutsch, T.; Diener, H.C.; Dubner, S.; Halperin, J.; Ma, C.S.; Zint, K.; Elsaesser, A.; et al. The changing landscape for stroke prevention in AF: Finding from the GLORIA–AF phase 2. J. Am. Coll. Cardiol. 2017, 69, 777–785. [Google Scholar] [CrossRef]
- Sarajlic, P.; Simonsson, M.; Jernberg, T.; Back, M.; Hofmann, R. Incidence, associated outcomes, and predictors of upper gastrointestinal bleeding following acute myocardial infarction: A SWEDEHEART-based nationwide cohort study. Eur. Heart J. Cardiovasc. Pharm. 2021, pvab059. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Doshi, S.; Sievert, H.; Buchbinder, M.; Neuzil, P.; Huber, K.; Halperin, J.; Holmes, D. PROTECT AF Investigators Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3 years follow up of the PROTECT AF. Circulation 2013, 127, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczec, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrail Appendage Closure Versus Direct Oral Anticoagulants in High-Risk patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Mc Neil, J.J.; Woods, R.L.; Nelson, M.; Reid, C.; Kirpach, B.; Wolfe, R.; Storey, E.; Saha, R.; Lockery, J.; Tonkin, A.; et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- The ACTIVE Investigators. Effect of clopidrogrel added to aspirin in patient with atrial fibrillation. N. Engl. J. Med. 2009, 360, 2066–2067. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Nonaka, T.; Hisagi, M.; Ninomya, M.; Masukawa, A.; Ota, T. Thoracoscopic stapler-and-loop technique for left atrial appendage closure in nonvalvular atrial fibrillation: Mid-term outcomes in 201 patients. Heart Rhythm 2018, 15, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
| Clinical Variable | Watchman | Atriclip ProII | p Value |
|---|---|---|---|
| Age mean (±SD); median (Q1–Q3) | 72.3 (±7.5); 75.5 (65.5–79) | 74.9 ± (8); 75 (69.5–79.5) | 0.828 |
| Gender (M) (%) | 13 (65) | 13 (65) | 1.000 |
| CHA2DS2VASc mean(±SD); median (Q1–Q3) | 4.2 (±1.5); 4 (3–5) | 6 (±1.5); 6.5 (4.5–7) | 0.002 |
| HASBLEDmean ± SD; median (Q1–Q3) | 3.5 (±1.1); 3 (3–4) | 5.4 (±1.4); 5(4–7) | <0.0001 |
| Previous ischemic stroke (%) | 3 (15%) | 3 (15%) | 1.000 |
| Diabetes mellitus (%) | 3 (15) | 4 (20) | 1.000 |
| Hypertension (%) | 14 (70) | 15 (75) | 0.723 |
| Previous cerebral hemorrhage (%) | 3 (15) | 9 (45) | 0.038 |
| GI bleeding | 6 (30) | 6 (30) | 1.000 |
| non cerebral/GI | 9 (45) | 5 (25) | 0.185 |
| Renal disease (CrCl < 30mL/min) (%) | 4 (20) | 5 (25) | 1.000 |
| Dialysis (%) | 1 (0.5) | 1 (0.5) | 1.000 |
| Previous cardiac surgery (%) | 4 (20) | 0 (0) | 0.106 |
| COPD (%) | 1 (0.5) | 6 (30) | 0.092 |
| Carotid stenosis 100% (%) | 1 (0.5) | 1 (0.5) | 1.000 |
| Unilateral carotid stenosis >50% (%) | 2 (10) | 6 (30) | 0.235 |
| Bilateral Carotid stenosis <50% (%) | 17 (85) | 13 (65) | 0.144 |
| Disease with known bleeding risk on aspirin (%) | 0 (0) | 11 (55) | <0.0001 |
| Type of AF permanent (%) | 13 (65) | 12 (60) | 0.744 |
| Paroxysmal | 3 (15) | 1 (0.5) | 0.605 |
| LS Persistent | 4 (20) | 7 (35) | 0.288 |
| Previous AF ablation | 0 | 0 | |
| EjectionFraction (EF)mean (±SD); median (Q1–Q3) | 57 ± 8.7; 58.8 (54.5–60) | 53 ± 6.3; 55 (50–56) | 0.067 |
| Total | 20 | 20 |
| Type of Appendage | Watchman | Atriclip ProII |
|---|---|---|
| Windsock | 6 | 3 |
| Cauliflower | 5 | 6 |
| Cactus | 4 | 5 |
| Chicken Wing | 5 | 6 |
| Total | 20 | 20 |
| Watchman | AtriclipProII | p-Value | |
|---|---|---|---|
| Operative time mean ± SD; med(IQR) | 54.4 ± 12.7; 54 (46.5–57.5) | 52.2 ± 14.3; 47.5 (45–55) | 0.232 |
| Ventilation time mean ± SD; med(IQR) | 11.2 ± 6.4; 11.5 (9.5–13.5) | 15.8 ± 16.4; 11 (9.5–13) | 0.643 |
| Hospital stay mean ± SD;med(IQR) | 3.4 ± 0.7; 3 (3–4) | 3.8 ± 0.9; 3.5 (3–4) | 0.289 |
| Complication | 1groin hematoma | 1 pericarditis | |
| Post-procedure therapy | DAPT for 3 months then SAPT | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branzoli, S.; Guarracini, F.; Marini, M.; D’Onghia, G.; Penzo, D.; Piffer, S.; Peterlana, D.; Graffigna, A.; Gulizia, M.M.; Gelsomino, S.; et al. Heart Team for Left Atrial Appendage Occlusion: A Patient-Tailored Approach. J. Clin. Med. 2022, 11, 176. https://doi.org/10.3390/jcm11010176
Branzoli S, Guarracini F, Marini M, D’Onghia G, Penzo D, Piffer S, Peterlana D, Graffigna A, Gulizia MM, Gelsomino S, et al. Heart Team for Left Atrial Appendage Occlusion: A Patient-Tailored Approach. Journal of Clinical Medicine. 2022; 11(1):176. https://doi.org/10.3390/jcm11010176
Chicago/Turabian StyleBranzoli, Stefano, Fabrizio Guarracini, Massimiliano Marini, Giovanni D’Onghia, Daniele Penzo, Silvio Piffer, Dimitri Peterlana, Angelo Graffigna, Michele Massimo Gulizia, Sandro Gelsomino, and et al. 2022. "Heart Team for Left Atrial Appendage Occlusion: A Patient-Tailored Approach" Journal of Clinical Medicine 11, no. 1: 176. https://doi.org/10.3390/jcm11010176
APA StyleBranzoli, S., Guarracini, F., Marini, M., D’Onghia, G., Penzo, D., Piffer, S., Peterlana, D., Graffigna, A., Gulizia, M. M., Gelsomino, S., & La Meir, M. (2022). Heart Team for Left Atrial Appendage Occlusion: A Patient-Tailored Approach. Journal of Clinical Medicine, 11(1), 176. https://doi.org/10.3390/jcm11010176






