Elevated TAT in COVID-19 Patients with Normal D-Dimer as a Predictor of Severe Respiratory Failure: A Retrospective Analysis of 797 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
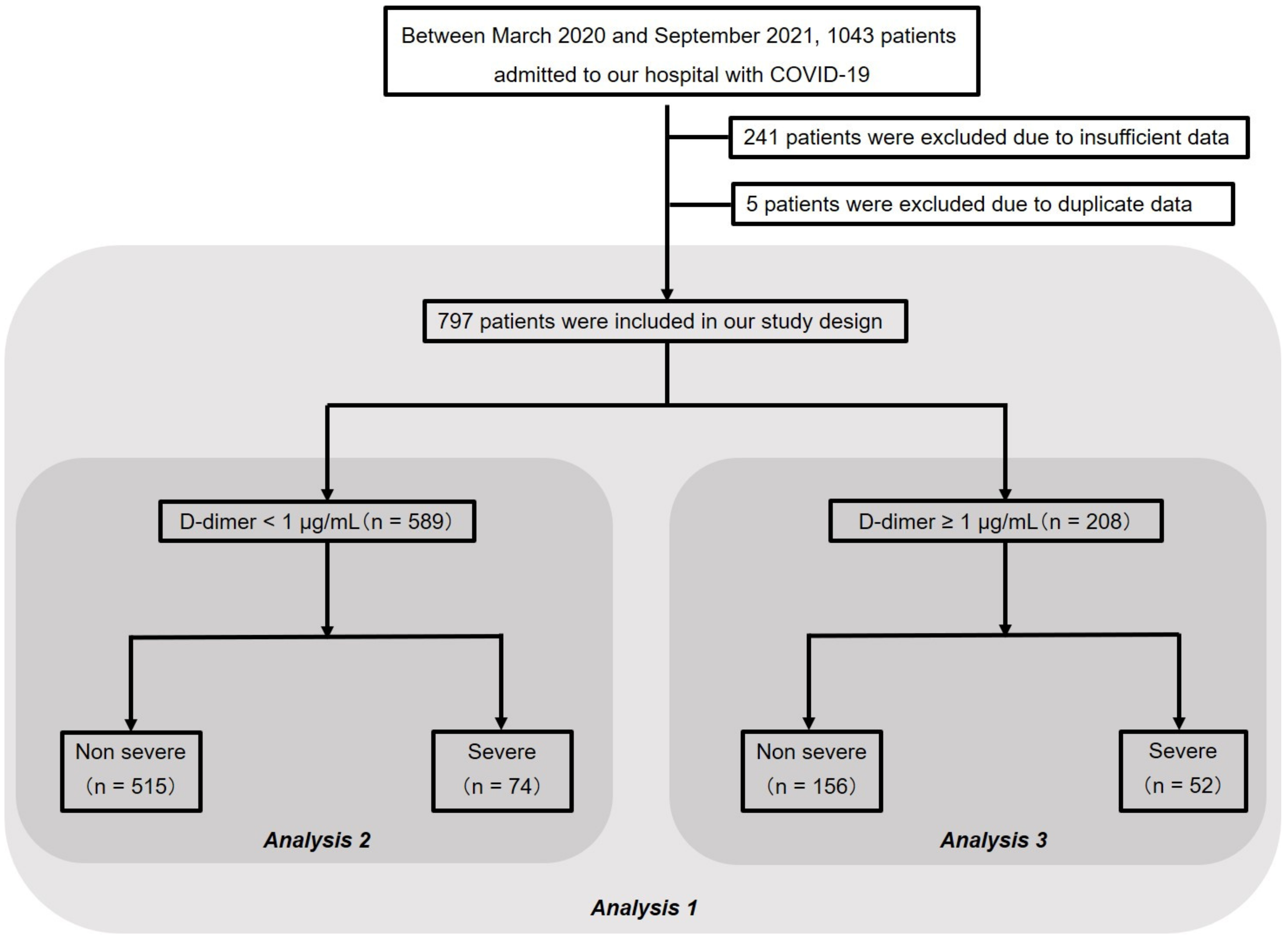
2.2. Study Design and Subjects
2.3. Clinical Assessment
2.4. Definition of Severe Respiratory Failure, Severe Group, and Non-Severe Group
2.5. The Setting of the Cutoff Value of Coagulation Factors
2.6. Statistical Analysis
2.7. Patient and Public Involvement
3. Results
3.1. Patient Characteristics (Analysis 1)
3.2. Percentage of Patients in the Severe Group with Abnormal Values of Coagulation/Fibrinolytic Markers
3.3. Respiratory Status on Admission When D-Dimer Is Divided into Two Groups
3.4. Analysis of Patients with Normal D-Dimer (Analysis 2)
3.5. Analysis of Patients with Elevated D-Dimer (Analysis 3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus Disease 2019 (COVID-19). Available online: https://covid19.who.int (accessed on 10 March 2020).
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Levi, M.; Iba, T. COVID-19 coagulopathy: Is it disseminated intravascular coagulation? Intern. Emerg. Med. 2021, 16, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Asakura, H. Classifying types of disseminated intravascular coagulation: Clinical and animal models. J. Intensive Care 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Micco, P.; Russo, V.; Carannante, N.; Imparato, M.; Cardillo, G.; Lodigiani, C. prognostic value of fibrinogen among COVID-19 patients admitted to an emergency department: An italian cohort study. J. Clin. Med. 2020, 9, 4134. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Warkentin, T.E.; Thachil, J.; Levi, M.; Levy, J.H. Proposal of the definition for COVID-19-Associated coagulopathy. J. Clin. Med. 2021, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Ballotta, A.; Di Dedda, U.; Baryshnikova, E.; Dei Poli, M.; Resta, M.; Falco, M.; Albano, G.; Menicanti, L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020, 18, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Zhou, F.; Luo, L.; Xu, M.; Wang, H.; Xia, J.; Gao, Y.; Cai, L.; Wang, Z.; Yin, P.; et al. Haematological characteristics classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020, 7, e671–e678. [Google Scholar] [CrossRef]
- He, X.; Yao, F.; Chen, J.; Wang, Y.; Fang, X.; Lin, X.; Long, H.; Wang, Q.; Wu, Q. The poor prognosis and influencing factors of high D-dimer levels for COVID-19 patients. Sci. Rep. 2021, 11, 1830. [Google Scholar] [CrossRef] [PubMed]
- Antunez Muiños, P.J.; López Otero, D.; Amat-Santos, I.J.; López País, J.; Aparisi, A.; Cacho Antonio, C.E.; Catalá, P.; González Ferrero, T.; Cabezón, G.; Otero García, O.; et al. The COVID-19 lab score: An accurate dynamic tool to predict in-hospital outcomes in COVID-19 patients. Sci. Rep. 2021, 11, 9361. [Google Scholar] [CrossRef] [PubMed]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A.; Rauch, A.; Staessens, S.; Moussa, M.; Rosa, M.; Corseaux, D.; Jeanpierre, E.; Goutay, J.; Caplan, M.; Varlet, P.; et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arter. Thromb. Vasc. Biol. 2021, 41, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H. Diversity of disseminated intravascular coagulation and selection of appropriate treatments. Int. J. Hematol. 2021, 113, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Takano, K.; Kayashima, M.; Kasahara, K.; Fukushima, H.; Matsumoto, M. Management of a COVID-19 patient during ecmo: Paying attention to acquired von willebrand syndrome. J. Atheroscler. Thromb. 2021, 28, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ogawa, H.; Asakura, H. Etiology and management of bleeding during ECMO in a COVID-19 patient. J. Atheroscler. Thromb. 2021, 28, 402–403. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Non-Severe (n = 671) | Severe (n = 126) | p Value |
|---|---|---|---|
| Age (year) | 54.8 (16.1) | 55.3 (13.6) | 0.7080 |
| Male | 436 (65.0%) | 106 (84.1%) | <0.0001 |
| BMI (kg/m2) | 25.40 (4.40), NA = 26 | 27.35 (5.42), NA = 2 | 0.0002 |
| PT (sec) | 8.7 (0.9), NA = 3 | 9.3 (4.4), NA = 1 | 0.1780 |
| APTT (sec) | 30.1 (4.3), NA = 6 | 32.7 (6.5), NA = 1 | <0.0001 |
| Fibrinogen (mg/dL) | 480.7 (137.7), NA = 163 | 582.2 (134.0), NA = 28 | <0.0001 |
| D-dimer (μg/mL) | 1.11 (2.56) | 1.39 (2.07) | 0.1770 |
| TAT (ng/mL) | 6.68 (5.36) | 9.88 (14.60) | 0.0235 |
| PIC (μg/mL) | 0.56 (0.84) | 0.70 (0.89) | 0.0935 |
| Variables | All Patients (n = 797) | Normal D-Dimer (n = 589) | Elevated D-Dimer (n = 208) | p Value |
|---|---|---|---|---|
| Pneumonia | 703 (89.1%, NA = 8) | 509 (87.3 %, NA = 6) | 194 (94.2 %, NA = 2) | 0.0060 |
| SpO2 ≤ 93% on room air at sea level | 21 (2.6 %) | 11 (1.9 %) | 10 (4.8 %) | 0.0397 |
| Conventional oxygen therapy | 253 (31.7 %) | 157/589 (26.7 %) | 96 (46.2 %) | <0.0001 |
| High-flow nasal cannula | 41(5.1 %) | 22/589 (3.7 %) | 19 (9.1 %) | 0.0053 |
| Mechanical ventilation | 3 (0.3 %) | 0 (0 %) | 3 (1.4 %) | 0.0176 |
| Characteristics | Non-Severe (n = 515) | Severe (n = 74) | p Value |
|---|---|---|---|
| Age (year) | 52.7 (15.4) | 52.6 (12.0) | 0.9570 |
| Male | 336 (65.2%) | 65 (87.8%) | <0.0001 |
| BMI (kg/m2) | 25.55 (4.43), NA = 14 | 27.66 (5.04) | 0.0002 |
| BMI ≥ 30 (kg/m2, %) | 77 (15.4%), NA = 14 | 15 (20.3%) | 0.3080 |
| Fibrinogen (mg/dL) | 465.8 (132.7), NA = 130 | 567.7 (114.4), NA = 16 | <0.0001 |
| D-dimer (μg/mL) | 0.61 (0.19) | 0.73 (0.16) | <0.0001 |
| TAT (ng/mL) | 5.74 (3.70) | 6.84 (3.40) | 0.0165 |
| PIC (μg/mL) | 0.47 (0.46) | 0.58 (0.53) | 0.0565 |
| Variables | Univariate Analysis OR (95% CI) | p Value | Multivariate Analysis OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (year) | - | - | 1.010 (0.984–1.030) | 0.6220 |
| Male | 3.841 (1.847–8.982) | <0.0001 | 2.440 (1.140–5.230) | 0.0219 |
| BMI ≥ 30 kg/m2 | 1.399 (0.700–2.656) | 0.3080 | 1.330 (0.630–2.810) | 0.4550 |
| Higher fibrinogen | 2.537 (1.311–4.806) | 0.0038 | 1.860 (0.989–3.520) | 0.0542 |
| Elevated TAT | 2.950 (1.554–6.011) | 0.0003 | 2.730 (1.360–5.500) | 0.0049 |
| Elevated PIC | 1.164 (0.124–5.391) | 0.6920 | 1.670 (0.309–9.050) | 0.5500 |
| Characteristics | Non-Severe (n = 156) | Severe (n = 52) | p Value |
|---|---|---|---|
| Age (year) | 61.7 (16.6) | 59.2 (14.9) | 0.3190 |
| Male | 100 (64.1%) | 41 (78.9%) | 0.0594 |
| BMI (kg/m2) | 24.87 (4.28), NA = 12 | 26.9 (5.95), NA = 2 | 0.0296 |
| Fibrinogen (mg/dL) | 527.4 (142.9), NA = 33 | 603.3 (157.2), NA = 12 | 0.0050 |
| D-dimer (μg/mL) | 2.76 (4.97) | 2.33 (3.00) | 0.4560 |
| TAT (ng/mL) | 9.76 (8.15) | 13.83 (21.25) | 0.1630 |
| PIC (μg/mL) | 0.86 (1.50) | 0.86 (1.22) | 0.9730 |
| Variables | Univariate Analysis OR (95% CI) | p Value | Multivariate Analysis OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (year) | 0.997 (0.971–1.020) | 0.8090 | ||
| Male | 2.080 (0.955–4.856) | 0.0594 | 1.670 (0.665–4.180) | 0.2750 |
| BMI ≥ 30 kg/m2 | 2.699 (1.056–6.796) | 0.0303 | 3.100 (1.180–8.150) | 0.0217 |
| Higher fibrinogen | 2.946 (1.317–6.639) | 0.0055 | 2.550 (1.150–5.650) | 0.0210 |
| Elevated TAT | 1.302 (0.438–4.713) | 0.8030 | 1.600 (0.423–6.080) | 0.4880 |
| Elevated PIC | 0.616 (0.173–1.788) | 0.4820 | 0.957 (0.277–3.310) | 0.9450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshita, Y.; Terada, J.; Hirasawa, Y.; Kinoshita, T.; Tajima, H.; Koshikawa, K.; Kinouchi, T.; Isaka, Y.; Shionoya, Y.; Fujikawa, A.; et al. Elevated TAT in COVID-19 Patients with Normal D-Dimer as a Predictor of Severe Respiratory Failure: A Retrospective Analysis of 797 Patients. J. Clin. Med. 2022, 11, 134. https://doi.org/10.3390/jcm11010134
Takeshita Y, Terada J, Hirasawa Y, Kinoshita T, Tajima H, Koshikawa K, Kinouchi T, Isaka Y, Shionoya Y, Fujikawa A, et al. Elevated TAT in COVID-19 Patients with Normal D-Dimer as a Predictor of Severe Respiratory Failure: A Retrospective Analysis of 797 Patients. Journal of Clinical Medicine. 2022; 11(1):134. https://doi.org/10.3390/jcm11010134
Chicago/Turabian StyleTakeshita, Yuichiro, Jiro Terada, Yasutaka Hirasawa, Taku Kinoshita, Hiroshi Tajima, Ken Koshikawa, Toru Kinouchi, Yuri Isaka, Yu Shionoya, Atsushi Fujikawa, and et al. 2022. "Elevated TAT in COVID-19 Patients with Normal D-Dimer as a Predictor of Severe Respiratory Failure: A Retrospective Analysis of 797 Patients" Journal of Clinical Medicine 11, no. 1: 134. https://doi.org/10.3390/jcm11010134
APA StyleTakeshita, Y., Terada, J., Hirasawa, Y., Kinoshita, T., Tajima, H., Koshikawa, K., Kinouchi, T., Isaka, Y., Shionoya, Y., Fujikawa, A., Tada, Y., Nakaseko, C., & Tsushima, K. (2022). Elevated TAT in COVID-19 Patients with Normal D-Dimer as a Predictor of Severe Respiratory Failure: A Retrospective Analysis of 797 Patients. Journal of Clinical Medicine, 11(1), 134. https://doi.org/10.3390/jcm11010134






