New Perspectives in the Pathophysiology and Treatment of Pain in Patients with Dry Eye Disease
Abstract
1. Introduction
2. Ocular Discomfort and Eye Pain
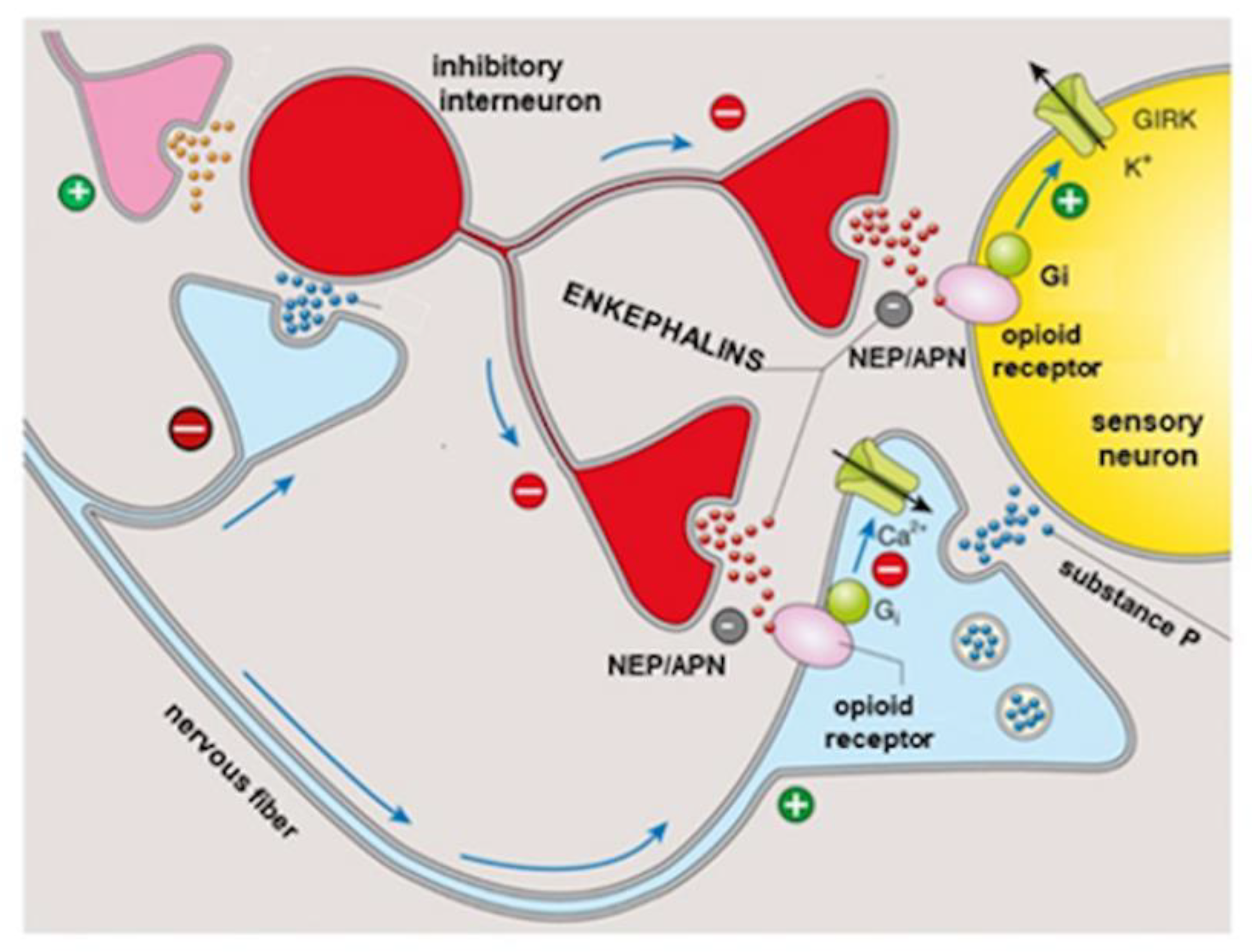
3. Opiorphin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Giannaccare, G.; Vukatana, G.; Mulè, R.; Malavolta, N.; Campos, E.C. Predictive role of tear protein expression in the early diagnosis of Sjögren’s syndrome. Ann. Clin. Biochem. Int. J. Lab. Med. 2018, 55, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; Li, W. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int. J. Mol. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef] [PubMed]
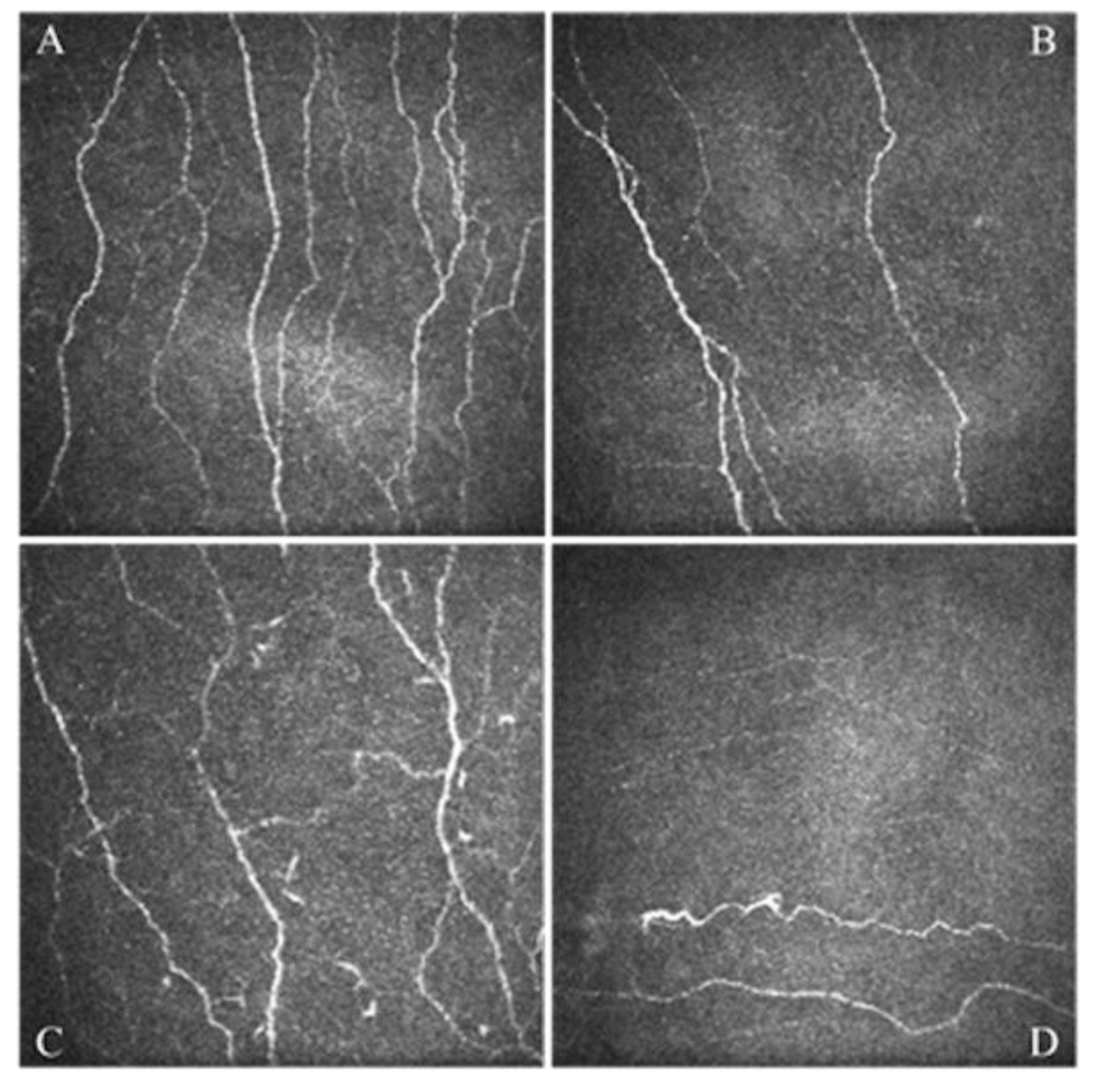
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Moscardelli, F.; Versura, P.; Campos, E.C. In vivo confocal microscopy morphometric analysis of corneal subbasal nerve plexus in dry eye disease using newly developed fully automated system. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 583–589. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Moscardelli, F.; Buzzi, M.; Versura, P.; Campos, E.C. In Vivo Confocal Microscopy Automated Morphometric Analysis of Corneal Subbasal Nerve Plexus in Patients with Dry Eye Treated With Different Sources of Homologous Serum Eye Drops. Cornea 2019, 38, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, P.; Steeds, C.S.; Stern, L.S.; Wiederkehr, D.P.; Doyle, J.J.; Katz, L.M.; Figueiredo, F.C. Utility assessment to measure the impact of dry eye disease. Ocul. Surf. 2006, 4, 155–161. [Google Scholar] [CrossRef]
- Morthen, M.K.; Magno, M.S.; Utheim, T.P.; Snieder, H.; Hammond, C.J.; Vehof, J. The physical and mental burden of dry eye dis-ease: A large population-based study investigating the relationship with health-related quality of life and its determinants. Ocul. Surf. 2021, 21, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Dufour, E.; Villard-Saussine, S.; Mellon, V.; Leandri, R.; Jouannet, P.; Ungeheuer, M.N.; Rougeot, C. Opiorphin secretion pattern in healthy volunteers: Gender difference and organ specificity. Biochem. Anal. Biochem. 2013, 2, 2–11. [Google Scholar]
- Puri, S.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Coulson-Thomas, V.J. Distribution and function of glycosaminoglycans and proteoglycans in the development, homeostasis and pathology of the ocular surface. Front. Cell Dev. Biol. 2020, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Spierer, O.; Felix, E.; McClellan, A.L.; Parel, J.M.; Gonzalez, A.; Feuer, W.J.; Sarantopoulos, C.D.; Levitt, R.C.; Ehrmann, K.; Galor, A. Corneal Mechanical Thresholds Negatively Associate with Dry Eye and Ocular Pain Symptoms. Investig. Opthalmol. Vis. Sci. 2016, 57, 617–625. [Google Scholar] [CrossRef]
- Levitt, A.E.; Galor, A.; Weiss, J.S.; Felix, E.R.; Martin, E.R.; Patin, D.J.; Sarantopoulos, K.D.; Levitt, R.C. Chronic Dry Eye Symptoms after LASIK: Parallels and Lessons to be Learned from other Persistent Post-Operative Pain Disorders. Mol. Pain 2015, 11, 21. [Google Scholar] [CrossRef]
- Rosenthal, P.; Baran, I.; Jacobs, D.S. Corneal pain without stain: Is it real? Ocul. Surf. 2009, 7, 28–40. [Google Scholar] [CrossRef]
- Mc Monnies, C.W. The potential role of neuropathic mechanisms in dry eye syndromes. J. Optom. 2017, 10, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Moreno, A.; Baudouin, C.; Parsadaniantz, S.M.; Goazigo, A.R.-L. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Front. Cell. Neurosci. 2020, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Pflugfelder, S.C.; Liu, Z.; Baudouin, C.; Kim, H.M.; Messmer, E.M.; Kruse, F.; Liang, L.; Carreno-Galeano, J.T.; Rolando, M.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271. [Google Scholar] [CrossRef]
- Dieckmann, G.; Borsook, D.; Moulton, E. Neuropathic corneal pain and dry eye: A continuum of nociception. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Moshirfar, M.; Benstead, E.E.; Sorrentino, P.M.; Tripathy, K. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review. Br. J. Ophthalmol. 2020, 105, 446–453. [Google Scholar] [CrossRef]
- Giannaccare, G.; Versura, P.; Buzzi, M.; Primavera, L.; Pellegrini, M.; Campos, E.C. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus. Apher. Sci. 2017, 56, 595–604. [Google Scholar] [CrossRef]
- Bernabei, F.; Roda, M.; Buzzi, M.; Pellegrini, M.; Giannaccare, G.; Versura, P. Blood-Based Treatments for Severe Dry Eye Disease: The Need of a Consensus. J. Clin. Med. 2019, 8, 1478. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, J.P.; Hoffmann, F.; Dumitrescu, L. Side-effects of local anesthetics on the corneal epithelium of the rabbit eye. Graefe's Arch. Clin. Exp. Ophthalmol. 1975, 197, 71–81. [Google Scholar] [CrossRef]
- Joubert, F.; Guerrero-Moreno, A.; Fakih, D.; Reboussin, E.; Gaveriaux-Ruff, C.; Acosta, M.C.; Gallar, J.; Sahel, J.A.; Bodineau, L.; Baudouin, C.; et al. Topical treatment with a mu opioid receptor agonist alleviates corneal allodynia and corneal nerve sensitization in mice. Biomed. Pharmacother. 2020, 132, 110794. [Google Scholar] [CrossRef]
- Reaux-Le Goazigo, A.; Poras, H.; Ben-Dhaou, C.; Ouimet, T.; Baudouin, C.; Wurm, M.; Melik Parsadaniantz, S. Dual enkephalinase inhibitor PL265: A novel topical treatment to alleviate corneal pain and inflammation. Pain 2019, 160, 307–321. [Google Scholar] [CrossRef]
- Yang, D.J.; Lee, K.S.; Ko, C.M.; Moh, S.H.; Song, J.; Hur, L.C.; Cheon, Y.W.; Yang, S.H.; Choi, Y.H.; Kim, K.W. Leucine-enkephalin pro-motes wound repair through the regulation of hemidesmosome dynamics and matrix metalloprotease. Peptides 2016, 76, 57–64. [Google Scholar] [CrossRef]
- Hossain, A.; Heron, D.; Davenport, I.; Huckaba, T.; Graves, R.; Mandal, T.; Muniruzzaman, S.; Wang, S.; Bhattacharjee, P.S. Protective effects of bestatin in the retina of streptozotocin-induced diabetic mice. Exp. Eye Res. 2016, 149, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Campos, E.C. Neurotrophic keratitis: Current challenges and future prospects. Eye Brain 2018, 10, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Acosta, M.C.; Borderie, V.; Borras, F.; Gallar, J.; Bury, T.; Laroche, L.; Belmonte, C. Decreased corneal sensitivity in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2341–2345. [Google Scholar] [CrossRef]
- Peyman, G.A.; Rahimy, M.H.; Fernandes, M.L. Effects of morphine on corneal sensitivity and epithelial wound healing: Impli-cations for topical ophthalmic analgesia. Br. J. Ophthalmol. 1994, 78, 138–141. [Google Scholar] [CrossRef]
- Chen, H.-T.; Chen, K.-H.; Hsu, W.-M. Toxic keratopathy associated with abuse of low-dose anesthetic: A case report. Cornea 2004, 23, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Wisner, A.; Dufour, E.; Messaoudi, M.; Nejdi, A.; Marcel, A.; Ungeheuer, M.N.; Rougeot, C. Human Opiorphin, a natural antino-ciceptive modulator of opioid-dependent pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 17979–17984. [Google Scholar] [CrossRef] [PubMed]
- Rougeot, C.; Messaoudi, M. Identification of human opiorphin, a natural antinociceptive modulator of opioid dependent pathways. Med. Sci. 2007, 23, 33–35. [Google Scholar]
- Power, I. An update on analgesics. Br. J. Anaesth. 2011, 107, 19–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salarić, I.; Sabalic, M.; Alajbeg, I. Opiorphin in burning mouth syndrome patients: A case-control study. Clin. Oral Investig. 2016, 21, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Ozdogan, M.S.; Gungormus, M.; Yusufoglu, S.I.; Ertem, S.Y.; Sonmez, C.; Orhan, M. Salivary opiorphin in dental pain: A potential biomarker for dental disease. Arch. Oral Biol. 2018, 99, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ozdogan, S.; Sonmez, C.; Yolcu, D.; Gungormus, M. Tear Opiorphin Levels in Ocular Pain Caused by Corneal Foreign Body. Cornea 2020, 39, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Mennini, N.; Mura, P.; Nativi, C.; Richichi, B.; Mannelli, L.D.C.; Ghelardini, C. Injectable liposomal formulations of opiorphin as a new therapeutic strategy in pain management. Futur. Sci. OA 2015, 1, FSO2. [Google Scholar] [CrossRef]
- Popik, P.; Kamysz, E.; Kreczko, J.; Wrobel, M. Human opiorphin: The lack of physiological dependence, tolerance to antino-ciceptive effects and abuse liability in laboratory mice. Behav. Brain Res. 2010, 213, 88–93. [Google Scholar] [CrossRef]
- Rougeot, C.; Robert, F.; Menz, L.; Bisson, J.-F.; Messaoudi, M. Systemically active human opiorphin is a potent yet non-addictive analgesic without drug tolerance effects. J. Physiol. Pharmacol. 2020, 61, 483–490. [Google Scholar]
- Ghelardini, C.; Giotti, A.; Gualtieri, F.; Matucci, R.; Romanelli, M.; Scapecchi, S.; Teodori, E.; Bartolini, A. Presynaptic auto- and hetero-receptors in the cholinergic regulation of pain. In Trends in Receptor Research; Angeli, P., Gulini, U., Quaglia, W., Eds.; Edizioni Elsevier: Amsterdam, The Netherlands, 1992; pp. 95–114. [Google Scholar]
- Tian, X.-Z.; Chen, J.; Xiong, W.; He, T.; Chen, Q. Effects and underlying mechanisms of human opiorphin on colonic motility and nociception in mice. Peptides 2009, 30, 1348–1354. [Google Scholar] [CrossRef]
- Mencucci, R.; Strazzabosco, G.; Cristofori, V.; Alogna, A.; Bortolotti, D.; Gafà, R.; Cennamo, M.; Favuzza, E.; Trapella, C.; Gentili, V.; et al. GlicoPro, Novel Standardized and Sterile Snail Mucus Extract for Multi-Modulative Ocular Formulations: New Perspective in Dry Eye Disease Management. Pharmaceutics 2021, 13, 2139. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannaccare, G.; Ghelardini, C.; Mancini, A.; Scorcia, V.; Di Cesare Mannelli, L. New Perspectives in the Pathophysiology and Treatment of Pain in Patients with Dry Eye Disease. J. Clin. Med. 2022, 11, 108. https://doi.org/10.3390/jcm11010108
Giannaccare G, Ghelardini C, Mancini A, Scorcia V, Di Cesare Mannelli L. New Perspectives in the Pathophysiology and Treatment of Pain in Patients with Dry Eye Disease. Journal of Clinical Medicine. 2022; 11(1):108. https://doi.org/10.3390/jcm11010108
Chicago/Turabian StyleGiannaccare, Giuseppe, Carla Ghelardini, Alessandra Mancini, Vincenzo Scorcia, and Lorenzo Di Cesare Mannelli. 2022. "New Perspectives in the Pathophysiology and Treatment of Pain in Patients with Dry Eye Disease" Journal of Clinical Medicine 11, no. 1: 108. https://doi.org/10.3390/jcm11010108
APA StyleGiannaccare, G., Ghelardini, C., Mancini, A., Scorcia, V., & Di Cesare Mannelli, L. (2022). New Perspectives in the Pathophysiology and Treatment of Pain in Patients with Dry Eye Disease. Journal of Clinical Medicine, 11(1), 108. https://doi.org/10.3390/jcm11010108








