The Role of the Stromal Extracellular Matrix in the Development of Pterygium Pathology: An Update
Abstract
1. Introduction
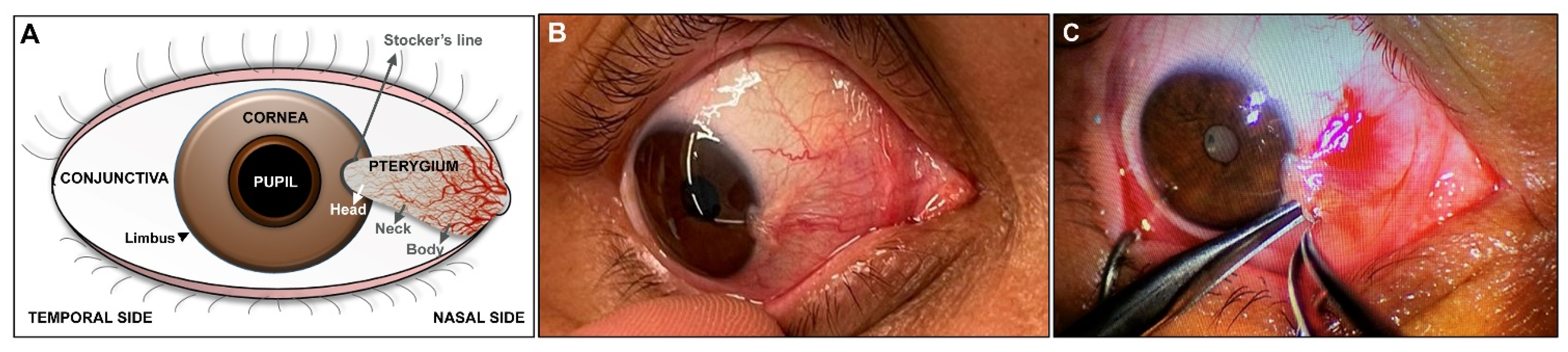
2. Clinical Diagnosis and Histopathological Characterization of Pterygium
3. Epidemiology and Pathogenesis of Pterygium
3.1. Oxidative Stress
3.2. Dysregulation of Cell Cycle Checkpoints
3.3. Induction of Inflammatory Mediators and Growth Factors
3.4. Angiogenic Stimulation
3.5. Viruses and Hereditary Changes
3.6. Extracellular Matrix Disorders
4. Role of ECM in Tissue Repair and Pathological Processes
5. ECM and Its Pathogenic Mechanisms in the Development of Pterygium
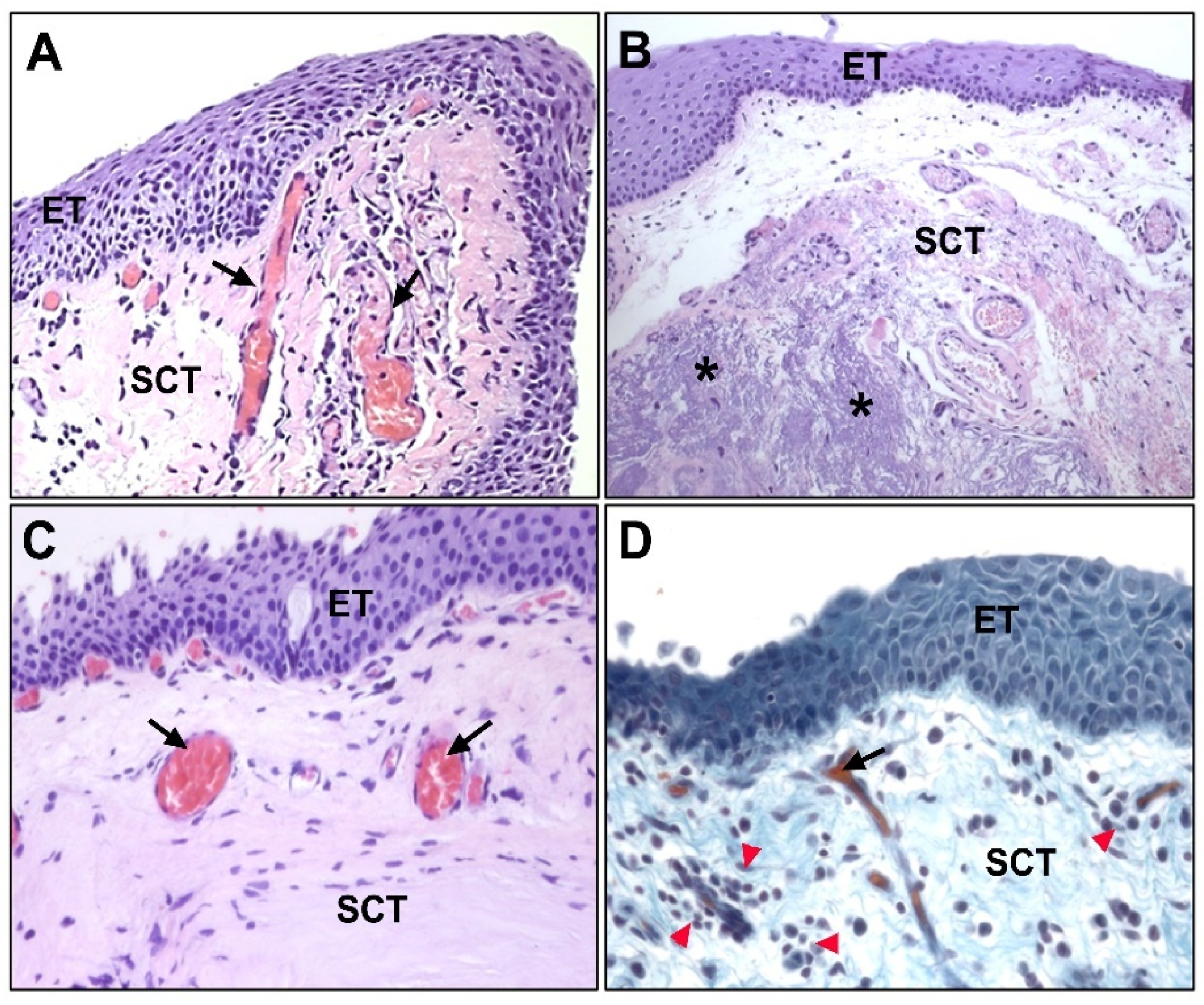
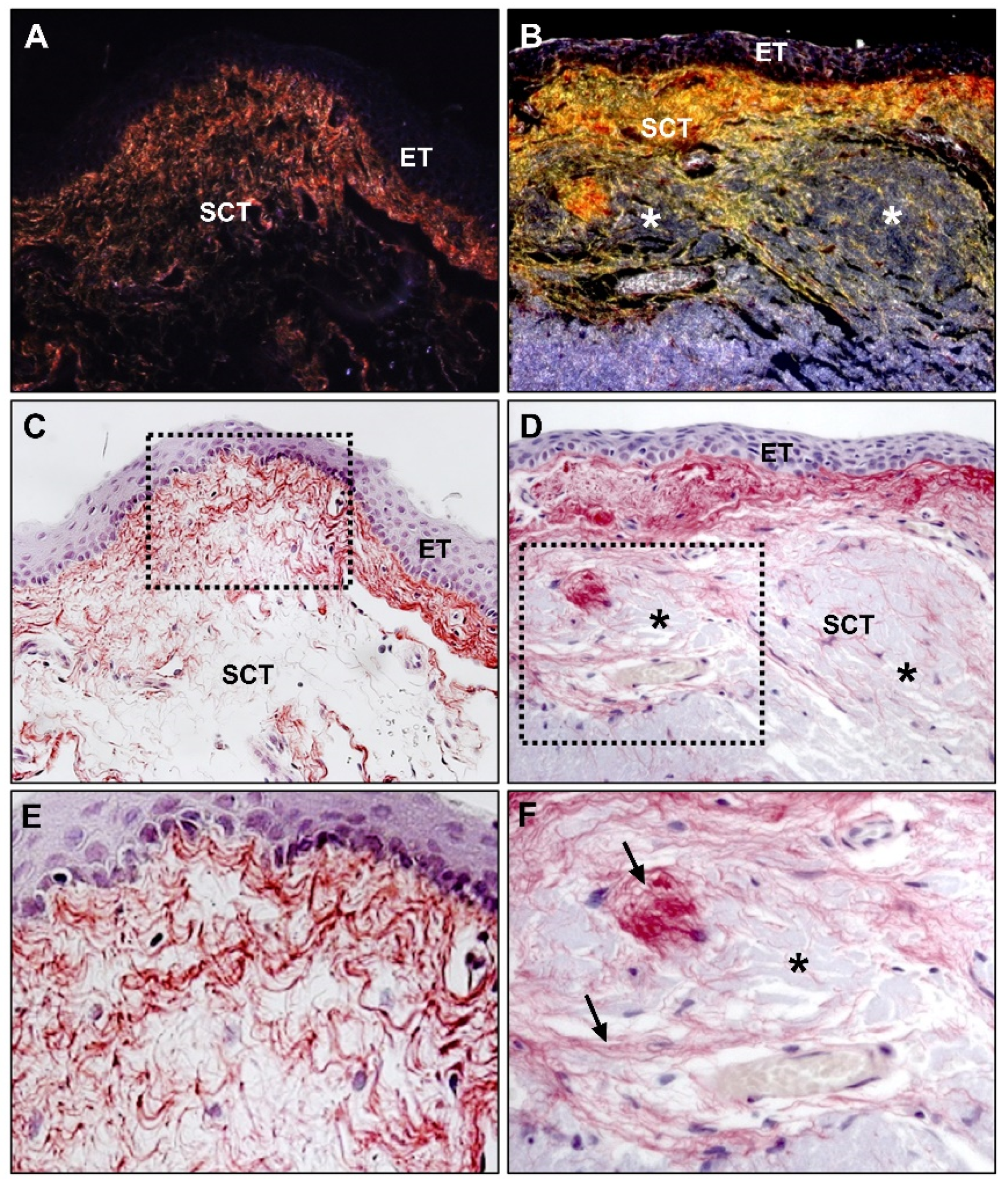
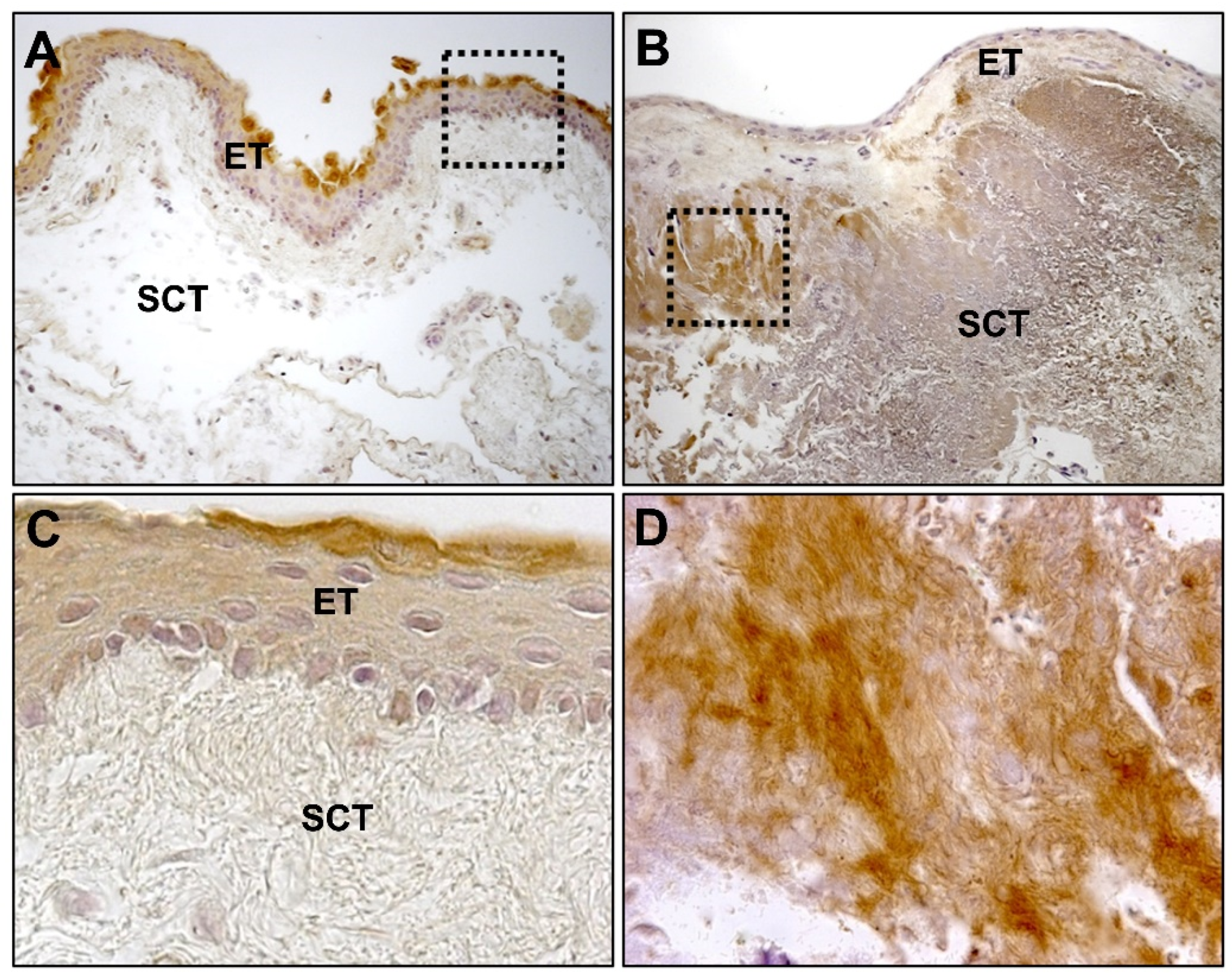
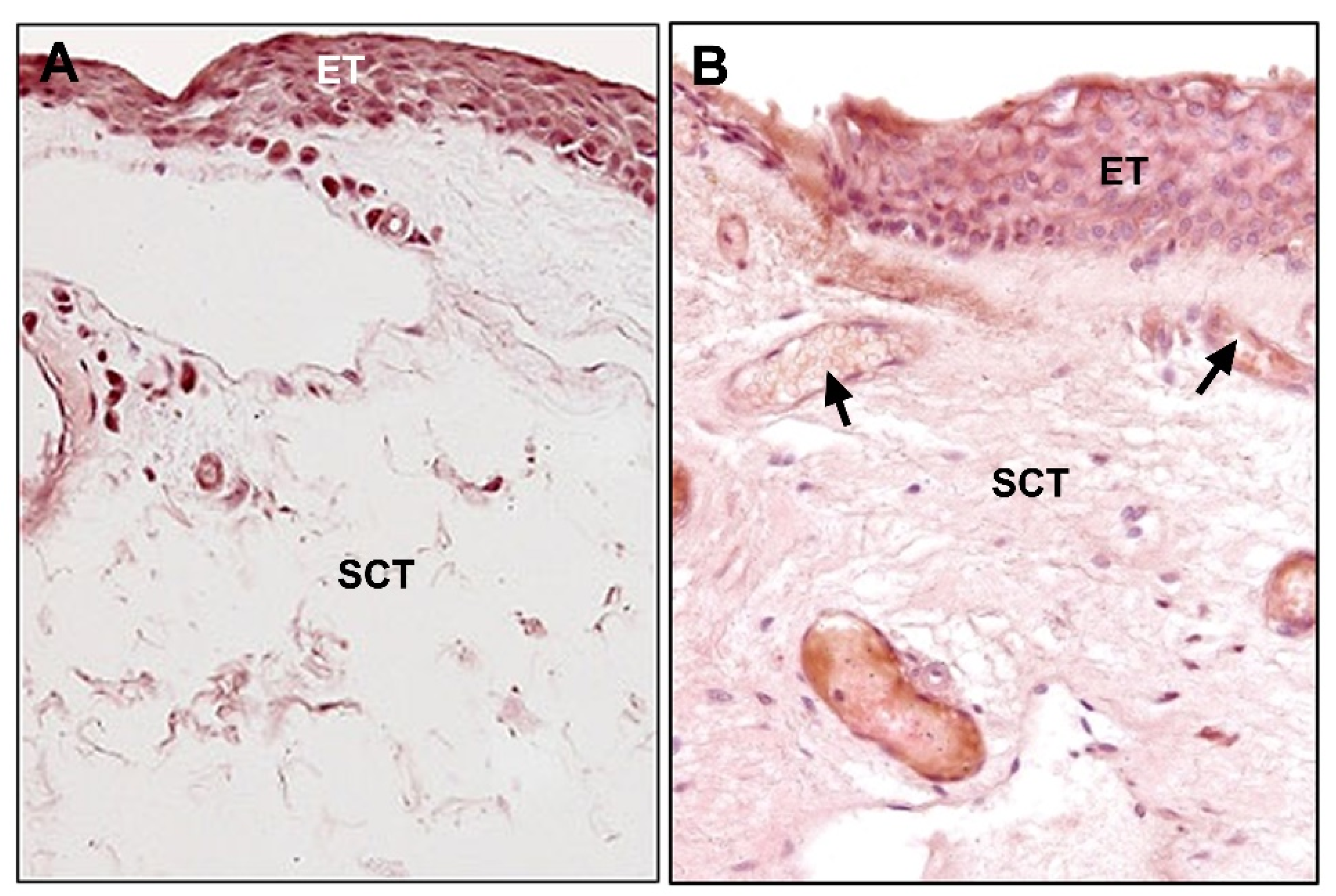
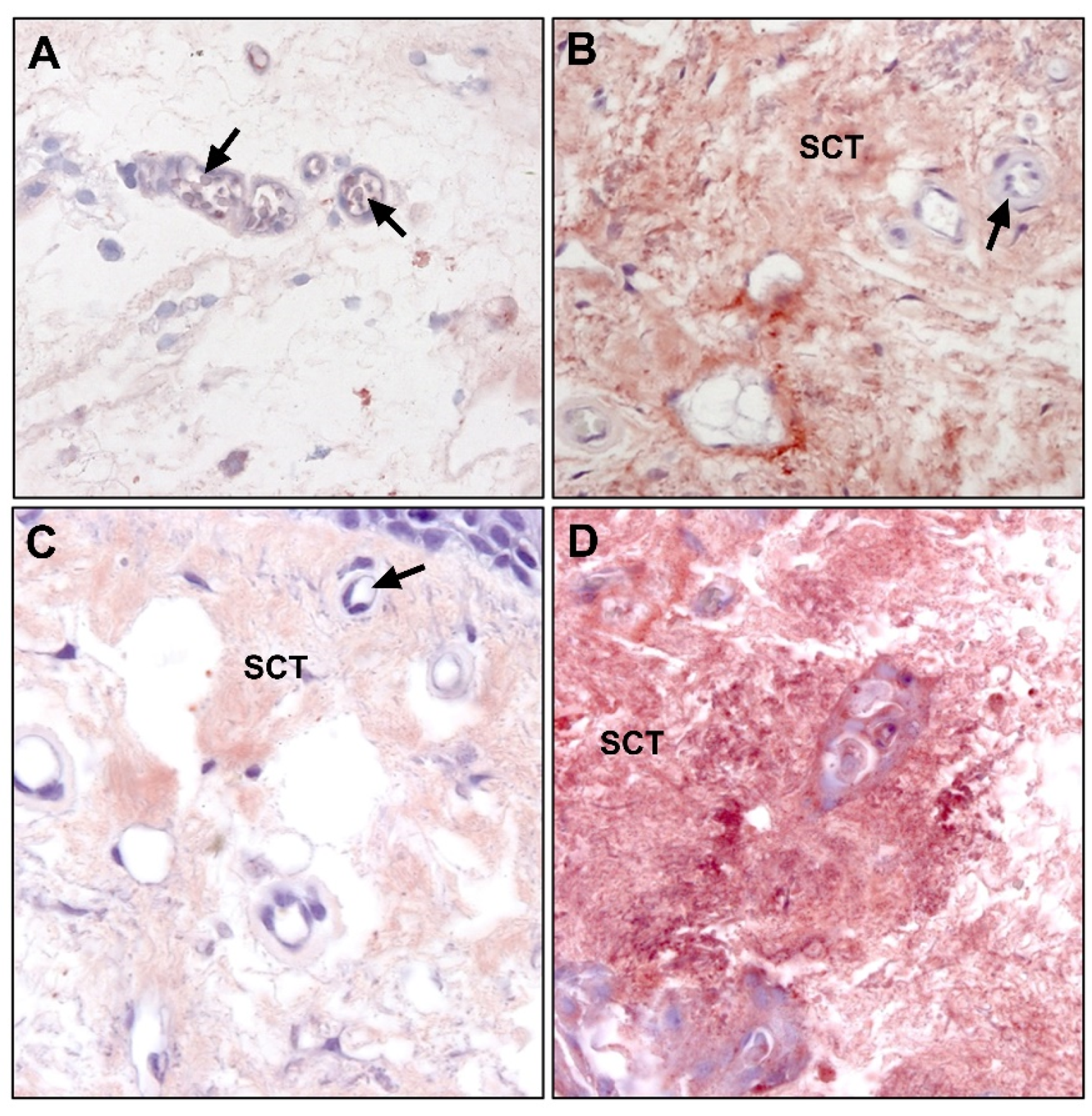
6. Fibroelastic Alterations in Pterygium ECM
6.1. Collagen
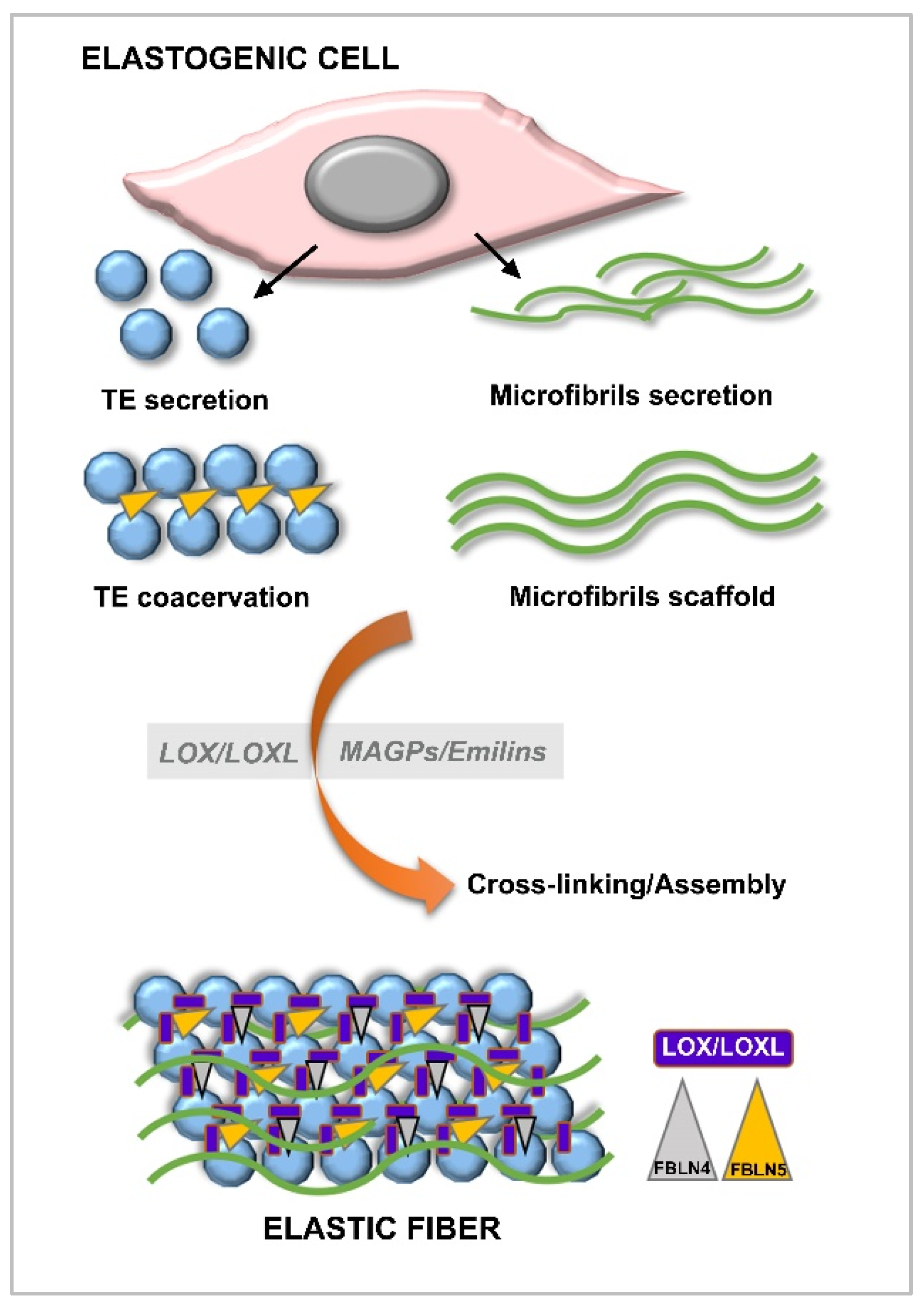
6.2. Elastin and Elastogenesis
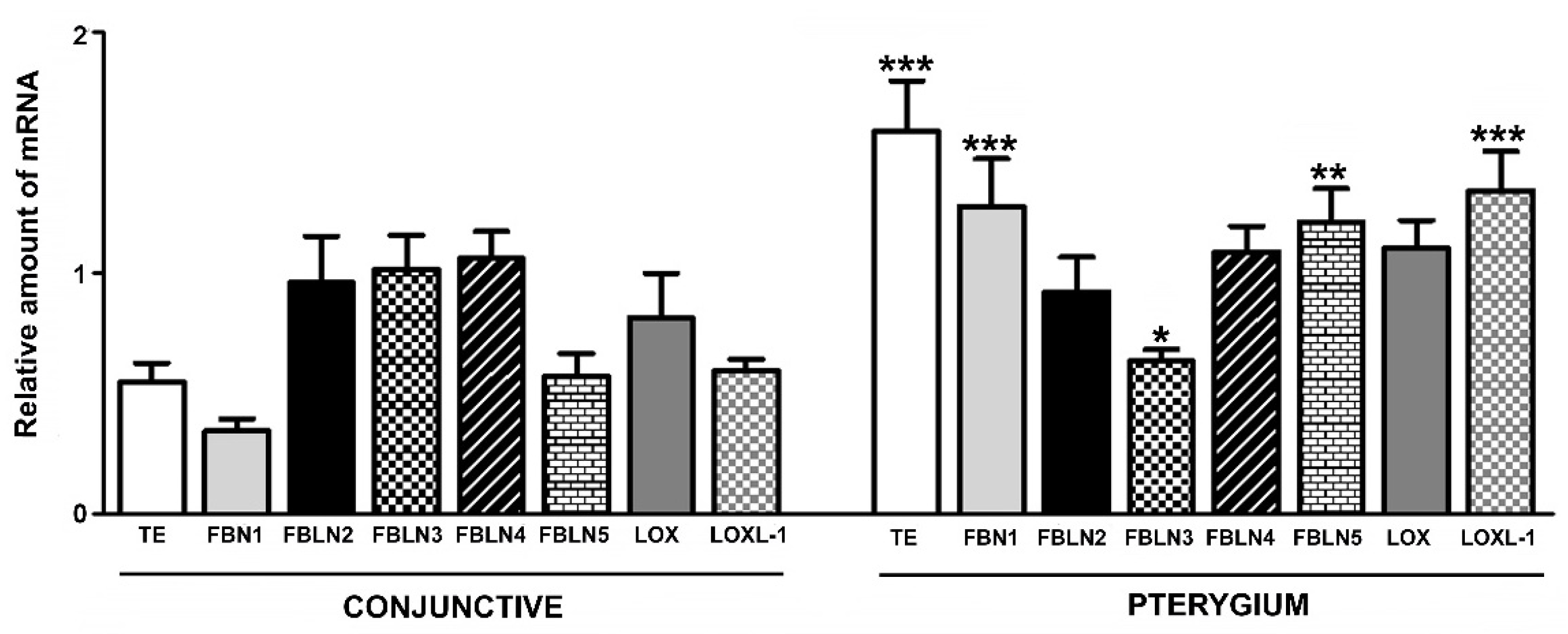
6.2.1. Tropoelastin
6.2.2. Fibrillins
6.2.3. Fibulins
6.2.4. Lysyl Oxydases
7. Discussion
- Increased synthesis and deposition of collagen fibers favor the immature form of collagen type III, and thus show a process of tissue remodeling;
- Increased protein levels in most of the constituents necessary for the development of elastic fibers, except FBLN4, whose biological roles are critical in the binding of the enzyme LOX and FBN1 for the development of stable elastin;
- Gene overexpression of TE, FBN1, FBLN5, and LOXL1, while the expression levels of LOX, as well as FBLN2 and -4, are comparable to those of controls.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, D.T.; Chee, S.P.; Dear, K.B.; Lim, A.S. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch. Ophthalmol. 1997, 115, 1235–1240, Erratum in Arch. Ophthalmol. 1998, 116, 552. [Google Scholar] [CrossRef]
- Saw, S.M.; Tan, D. Pterygium: Prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999, 6, 219–228. [Google Scholar] [CrossRef]
- Rezvan, F.; Khabazkhoob, M.; Hooshmand, E.; Yekta, A.; Saatchi, M.; Hashemi, H. Prevalence and risk factors of pterygium: A systematic review and meta-analysis. Surv. Ophthalmol. 2018, 63, 719–735. [Google Scholar] [CrossRef]
- Luthra, R.; Nemesure, B.B.; Wu, S.Y.; Xie, S.H.; Leske, M.C.; Barbados Eye Studies Group. Frequency and risk factors for pterygium in the Barbados Eye Study. Arch. Ophthalmol. 2001, 119, 1827–1832. [Google Scholar] [CrossRef]
- Chui, J.; Di Girolamo, N.; Wakefield, D.; Coroneo, M.T. The pathogenesis of pterygium: Current concepts and their therapeutic implications. Ocul. Surf. 2008, 6, 24–43. [Google Scholar] [CrossRef]
- Kau, H.C.; Tsai, C.C.; Lee, C.F.; Kao, S.C.; Hsu, W.M.; Liu, J.H.; Wei, Y.H. Increased oxidative DNA damage, 8-hydroxydeoxy- guanosine, in human pterygium. Eye 2006, 20, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.T.; Maxia, C.; Corbu, A.; Minerba, L.; Demurtas, P.; Colombari, R.; Murtas, D.; Bravo, S.; Piras, F.; Sirigu, P. Oxidative stress in pterygium: Relationship between p53 and 8- hydroxydeoxyguanosine. Mol. Vis. 2006, 12, 1136–1142. [Google Scholar]
- Tan, D.T.; Tang, W.Y.; Liu, Y.P.; Goh, H.S.; Smith, D.R. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br. J. Ophthalmol. 2000, 84, 212–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maxia, C.; Perra, M.T.; Demurtas, P.; Minerba, L.; Murtas, D.; Piras, F.; Corbu, A.; Gotuzzo, D.C.; Cabrera, R.G.; Ribatti, D.; et al. Expression of survivin protein in pterygium and relationship with oxidative DNA damage. J. Cell Mol. Med. 2008, 12, 2372–2380, Erratum in J. Cell Mol. Med. 2009, 13, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Detorakis, E.T.; Sourvinos, G.; Tsamparlakis, J.; Spandidos, D.A. Evaluation of loss of heterozygosity and microsatellite instability in human pterygium: Clinical correlations. Br. J. Ophthalmol. 1998, 82, 1324–1328. [Google Scholar] [CrossRef]
- Tan, D.T.; Lim, A.S.; Goh, H.S.; Smith, D.R. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am. J. Ophthalmol. 1997, 123, 404–405. [Google Scholar] [CrossRef]
- Chen, P.L.; Cheng, Y.W.; Chiang, C.C.; Tseng, S.H.; Chau, P.S.; Tsai, Y.Y. Hypermethylation of the p16 gene promoter in pterygia and its association with the expression of DNA methyltransferase 3b. Mol. Vis. 2006, 12, 1411–1416. [Google Scholar]
- Tong, L. Expression of p27(KIP1) and cyclin D1, and cell proliferation in human pterygium. Br. J. Ophthalmol. 2008, 92, 157. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Cheng, Y.W.; Lee, H.; Tsai, F.J.; Tseng, S.H.; Chang, K.C. P53 gene mutation spectrum and the relationship between gene mutation and protein levels in pterygium. Mol. Vis. 2005, 11, 50–55. [Google Scholar] [PubMed]
- Schneider, B.G.; John-Aryankalayil, M.; Rowsey, J.J.; Dushku, N.; Reid, T.W. Accumulation of p53 protein in pterygia is not accompanied by TP53 gene mutation. Exp. Eye Res. 2006, 82, 91–98. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Kumar, R.K.; Coroneo, M.T.; Wakefield, D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3430–3437. [Google Scholar]
- Hong, S.; Choi, J.Y.; Lee, H.K.; Seong, G.J.; Seo, K.Y.; Kim, E.K.; Byeon, S.H. Expression of neurotrophic factors in human primary pterygeal tissue and selective TNF-alpha induced stimulation of ciliary neurotrophic factor in pterygeal fibroblasts. Exp. Toxicol. Pathol. 2008, 60, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Di Girolamo, N.; Coroneo, M.T.; Wakefield, D. Proliferative effects of heparin binding epidermal growth factor-like growth factor on pterygium epithelial cells and fibroblasts. Investig. Ophthalmol. Vis. Sci. 2004, 45, 110–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, J.; Guan, M.; Sima, J.; Gao, G.; Zhang, M.; Liu, Z.; Fant, J.; Ma, J.X. Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea 2003, 22, 473–477. [Google Scholar] [CrossRef]
- Kria, L.; Ohira, A.; Amemiya, T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996, 98, 195–201. [Google Scholar] [CrossRef]
- Aspiotis, M.; Tsanou, E.; Gorezis, S.; Ioachim, E.; Skyrlas, A.; Stefaniotou, M.; Malamou-Mitsi, V. Angiogenesis in pterygium: Study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye 2007, 21, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Cho, H.J.; Kim, J.T.; Choi, J.S.; Joo, C.K. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea 2001, 20, 738–742. [Google Scholar] [CrossRef]
- Martín-López, J.; Pérez-Rico, C.; García-Honduvilla, N.; Buján, J.; Pascual, G. Elevated blood/lymphatic vessel ratio in pterygium and its relationship with vascular endothelial growth factor (VEGF) distribution. Histol. Histopathol. 2019, 34, 917–929. [Google Scholar] [CrossRef]
- Reid, T.W.; Dushku, N. Does human papillomavirus cause pterygium? Br. J. Ophthalmol. 2003, 87, 806–808. [Google Scholar] [CrossRef][Green Version]
- Detorakis, E.T.; Sourvinos, G.; Spandidos, D.A. Detection of herpes simplex virus and human papilloma virus in ophthalmic pterygium. Cornea 2001, 20, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Dake, Y.; Mukae, R.; Soda, Y.; Kaneko, M.; Amemiya, T. Immunohistochemical localization of collagen types I, II, III, and IV in pterygium tissues. Acta Histochem. 1989, 87, 71–74. [Google Scholar] [CrossRef]
- Naib-Majani, W.; Eltohami, I.; Wernert, N.; Watts, W.; Tschesche, H.; Pleyer, U.; Breipohl, W. Distribution of extracellular matrix proteins in pterygia: An immunohistochemical study. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 332–338. [Google Scholar] [CrossRef]
- Wang, I.J.; Hu, F.R.; Chen, P.J.; Lin, C.T. Mechanism of abnormal elastin gene expression in the pinguecular part of pterygia. Am. J. Pathol. 2000, 157, 1269–1276. [Google Scholar] [CrossRef]
- Di Girolamo, N.; McCluskey, P.; Lloyd, A.; Coroneo, M.T.; Wakefield, D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 671–679. [Google Scholar]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Kato, N.; Shimmura, S.; Kawakita, T.; Miyashita, H.; Ogawa, Y.; Yoshida, S.; Higa, K.; Okano, H.; Tsubota, K. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Parvani, J.G.; Schiemann, W.P. The pathophysiology of epithelialmesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia 2010, 15, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 429–1437. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 2016, 142, 110–118. [Google Scholar] [CrossRef]
- Chui, J.; Coroneo, M.T.; Tat, L.T.; Crouch, R.; Wakefield, D.; Di Girolamo, N. Ophthalmic pterygium: A stem cell disorder with premalignant features. Am. J. Pathol. 2011, 178, 817–827. [Google Scholar] [CrossRef]
- Chen, S.Y.; Mahabole, M.; Horesh, E.; Wester, S.; Goldberg, J.L.; Tseng, S.C. Isolation and characterization of mesenchymal progenitor cells from human orbital adipose tissue. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4842–4852. [Google Scholar] [CrossRef]
- Galligan, C.L.; Fish, E.N. The role of circulating fibrocytes in inflammation and autoimmunity. J. Leukoc. Biol. 2013, 93, 45–50. [Google Scholar] [CrossRef]
- Kria, L.; Ohira, A.; Amemiya, T. Growth factors in cultured pterygium fibroblasts: Immunohistochemical and ELISA analysis. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 702–708. [Google Scholar] [CrossRef]
- Lee, K.; Young Lee, S.; Park, S.Y.; Yang, H. Antifibrotic effect of pirfenidone on human pterygium fibroblasts. Curr. Eye Res. 2014, 39, 680–685. [Google Scholar] [CrossRef]
- Lee, S.B.; Li, D.Q.; Tan, D.T.; Meller, D.C.; Tseng, S.C. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr. Eye Res. 2000, 20, 325–334. [Google Scholar] [CrossRef]
- Sha, X.; Wen, Y.; Liu, Z.; Song, L.; Peng, J.; Xie, L. Inhibition of α-smooth muscle actin expression and migration of pterygium fibroblasts by coculture with amniotic mesenchymal stem cells. Curr. Eye Res. 2014, 39, 1081–1089. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Chui, J.; Coroneo, M.T.; Wakefield, D. Pathogenesis of pterygia: Role of cytokines, growth factors, and matrix metalloproteinases. Prog. Retin Eye Res. 2004, 23, 195–228. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.; Gotlinger, K.H.; Dunn, M.W.; Lee, O.L.; Milman, T.; Zaidman, G.; Schwartzman, M.L.; Bellner, L. Dysregulated heme oxygenase-ferritin system in pterygium pathogenesis. Cornea 2013, 32, 1276–1282. [Google Scholar] [CrossRef]
- Stone, E.M.; Braun, T.A.; Russell, S.R.; Kuehn, M.H.; Lotery, A.J.; Moore, P.A.; Eastman, C.G.; Casavant, T.L.; Sheffield, V.C. Missense variations in the fibulin 5 gene and age-related macular degeneration. N. Engl. J. Med. 2004, 35, 346–353. [Google Scholar] [CrossRef]
- Li, F.; Xu, H.; Zeng, Y.; Yin, Z.Q. Overexpression of fibulin-5 in retinal pigment epithelial cells inhibits cell proliferation and migration and downregulates VEGF, CXCR4, and TGFB1 expression in cocultured choroidal endothelial cells. Curr. Eye Res. 2012, 37, 540–548. [Google Scholar] [CrossRef]
- Ritch, R. Exfoliation syndrome-the most common identifiable cause of open-angle glaucoma. J. Glaucoma 1994, 3, 176–177. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Magnusson, K.P.; Sulem, P.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Jonasdottir, A.; Stefansdottir, G.; et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Dudakova, L.; Liskova, P.; Trojek, T.; Palos, M.; Kalasova, S.; Jirsova, K. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp. Eye Res. 2012, 104, 74–81. [Google Scholar] [CrossRef]
- Austin, P.; Jakobiec, F.A.; Iwamoto, T. Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pinguecula. Ophthalmology 1983, 90, 96–109. [Google Scholar] [CrossRef]
- Ihanamäki, T.; Pelliniemi, L.J.; Vuorio, E. Collagens and collagen-related matrix components in the human and mouse eye. Prog. Retin. Eye Res. 2004, 23, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Tuori, A.; Uusitalo, H.; Burgeson, R.E.; Terttunen, J.; Virtanen, I. The immunohistochemical composition of the human corneal basement membrane. Cornea 1996, 15, 286–294. [Google Scholar] [CrossRef]
- Fukuda, K.; Chikama, T.; Nakamura, M.; Nishida, T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea 1999, 18, 73–79. [Google Scholar] [CrossRef]
- Pérez-Rico, C.; Pascual, G.; Sotomayor, S.; Montes-Mollón, M.Á.; Trejo, C.; Sasaki, T.; Mecham, R.; Bellón, J.M.; Buján, J. Tropoelastin and fibulin overexpression in the subepithelial connective tissue of human pterygium. Am. J. Ophthalmol. 2011, 151, 44–52. [Google Scholar] [CrossRef]
- Mecham, R.P. Elastin synthesis and fiber assembly. Ann. N. Y. Acad. Sci. 1991, 624, 137–146. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. New insights into elastic fiber assembly. Birth Defects Res. C Embryo Today 2007, 81, 229–240. [Google Scholar] [CrossRef]
- Watanabe, M.; Sawai, T. Alteration of cross-linking amino acids of elastin in human aorta in association with dissecting aneurysm: Analysis using high performance liquid chromatography. Tohoku J. Exp. Med. 1999, 187, 291–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trask, T.M.; Trask, B.C.; Ritty, T.M.; Abrams, W.R.; Rosenbloom, J.; Mecham, R.P. Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J. Biol. Chem. 2000, 275, 24400–24406. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, K.; Bätge, B.; Müller, P.K.; Reinhardt, D.P. Interactions of fibrillin-1 with heparin/heparan sulfate, implications for microfibrillar assembly. J. Biol. Chem. 2001, 276, 36035–36042. [Google Scholar] [CrossRef] [PubMed]
- Argraves, W.S.; Dickerson, K.; Burgess, W.H.; Ruoslahti, E. Fibulin, a novel protein that interacts with the fibronectin receptor beta subunit cytoplasmic domain. Cell 1989, 58, 623–629. [Google Scholar] [CrossRef]
- Argraves, W.S.; Greene, L.M.; Cooley, M.A.; Gallagher, W.M. Fibulins: Physiological and disease perspectives. EMBO Rep. 2003, 4, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Davis, E.C. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int. J. Biochem. Cell Biol. 2010, 42, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Visconti, R.P.; Barth, J.L.; Keeley, F.W.; Little, C.D. Codistribution analysis of elastin and related fibrillar proteins in early vertebrate development. Matrix Biol. 2003, 22, 109–121. [Google Scholar] [CrossRef]
- McLaughlin, P.J.; Chen, Q.; Horiguchi, M.; Starcher, B.C.; Stanton, J.B.; Broekelmann, T.J.; Marmorstein, A.D.; McKay, B.; Mecham, R.; Nakamura, T.; et al. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol. Cell Biol. 2006, 26, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Inoue, T.; Ohbayashi, T.; Hirai, M.; Noda, K.; Marmorstein, L.Y.; Yabe, D.; Takagi, K.; Akama, T.O.; Kita, T.; et al. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc. Natl. Acad. Sci. USA 2009, 106, 19029–19034. [Google Scholar] [CrossRef]
- Huang, J.; Davis, E.C.; Chapman, S.L.; Budatha, M.; Marmorstein, L.Y.; Word, R.A.; Yanagisawa, H. Fibulin-4 deficiency results in ascending aortic aneurysms: A potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ. Res. 2010, 106, 583–592. [Google Scholar] [CrossRef]
- Nakamura, T.; Lozano, P.R.; Ikeda, Y.; Iwanaga, Y.; Hinek, A.; Minamisawa, S.; Cheng, C.F.; Kobuke, K.; Dalton, N.; Takada, Y.; et al. Fibulin5/DANCE is essential for elastogenesis in vivo. Nature 2002, 415, 171–175. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Davis, E.C.; Starcher, B.C.; Ouchi, T.; Yanagisawa, M.; Richardson, J.A.; Olson, E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 2002, 415, 168–171. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Timpl, R.; Sasaki, T.; Chu, M.L.; Ekblom, P. Fibulin-1 and fibulin-2 expression during organogenesis in the developing mouse embryo. Dev. Dyn. 1996, 205, 348–364. [Google Scholar] [CrossRef]
- Csiszar, K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar] [CrossRef]
- Thomassin, L.; Werneck, C.C.; Broekelmann, T.J.; Gleyzal, C.; Hornstra, I.K.; Mecham, R.P.; Sommer, P. The Pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers. J. Biol. Chem. 2005, 280, 42848–42855. [Google Scholar] [CrossRef]
- Hirai, M.; Horiguchi, M.; Ohbayashi, T.; Kita, T.; Chien, K.R.; Nakamura, T. Latent TGF-betabinding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. EMBO J. 2007, 26, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Gao, J.; Pawlyk, B.; Starcher, B.; Spencer, J.A.; Yanagisawa, H.; Zuo, J.; Li, T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004, 36, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Papke, C.L.; Yanagisawa, H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: Insights from mouse and human studies. Matrix Biol. 2014, 37, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Hornstra, I.K.; Birge, S.; Starcher, B.; Bailey, A.J.; Mecham, R.P.; Shapiro, S.D. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J. Biol. Chem. 2003, 278, 14387–14393. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; Kim, K.H.; Harten, B.; Brown, J.; Planck, S.; Meshul, C.; Edelhauser, H.; Rosenbaum, J.T.; Armstrong, C.A.; Ansel, J.C. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2483–2491. [Google Scholar]
- Di Girolamo, N.; Coroneo, M.T.; Wakefield, D. UVB-elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPKdependent pathway. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4705–4714. [Google Scholar] [CrossRef]
- Nolan, T.M.; DiGirolamo, N.; Sachdev, N.H.; Hampartzoumian, T.; Coroneo, M.T.; Wakefield, D. The role of ultraviolet irradiation and heparin-binding epidermal growth factor-like growth factor in the pathogenesis of pterygium. Am. J. Pathol. 2003, 162, 567–574. [Google Scholar] [CrossRef]
- Chen, J.K.; Tsai, R.J.; Lin, S.S. Fibroblasts isolated from human pterygia exhibit transformed cell characteristics. In Vitro Cell Dev. Biol. Anim. 1994, 30, 243–248. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, H.I.; Thapa, B.; Nuwormegbe, S.; Lee, K. Critical Role of mTORC2-Akt signaling in TGF-β1-induced myofibroblast differentiation of human pterygium fibroblasts. Investig. Ophthalmol. Vis. Sci. 2019, 60, 82–92. [Google Scholar] [CrossRef]
- Zhong, L.; Li, M. Transforming growth factor-beta1 induced cultured human trabecular cells to produce elastin. Zhonghua Yan Ke Za Zhi 1999, 35, 383–385. [Google Scholar] [PubMed]
- Dushku, N.; John, M.K.; Schultz, G.S.; Reid, T.W. Pterygia pathogenesis: Corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch. Ophthalmol. 2001, 119, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Chen, S.; Tong, L. MicroRNA-215 Regulates Fibroblast Function: Insights from a Human Fibrotic Disease. Cell Cycle 2015, 14, 1973–1984. [Google Scholar] [CrossRef]
- Han, S.; Chen, Y.; Gao, Y.; Sun, B.; Kong, Y. MicroRNA-218-5p inhibit the migration and proliferation of pterygium epithelial cells by targeting EGFR via PI3K/Akt/mTOR signaling pathway. Exp. Eye Res. 2019, 178, 37–45. [Google Scholar] [CrossRef]
- Li, X.; Dai, Y.; Xu, J. MiR-21 promotes pterygium cell proliferation through the PTEN/AKT pathway. Mol. Vis. 2018, 24, 485–494. [Google Scholar]
- Lee, J.H.; Jung, S.A.; Kwon, Y.A.; Chung, J.L.; Kim, U.S. Expression of microRNAs in fibroblast of pterygium. Int. J. Ophthalmol. 2016, 9, 967–972. [Google Scholar] [CrossRef]
- Riau, A.K.; Wong, T.T.; Lan, W.; Finger, S.N.; Chaurasia, S.S.; Hou, A.H.; Chen, S.; Yu, S.J.; Tong, L. Aberrant DNA methylation of matrix remodeling and cell adhesion related genes in pterygium. PLoS ONE 2011, 6, e14687, Erratum in PLoS ONE 2011, 6, doi:10.1371/annotation/814c14d4-eee6-44e2-bea8-cac11a0bae8f. [Google Scholar] [CrossRef][Green Version]
- Bernstein, E.F.; Chen, Y.Q.; Tamai, K.; Shepley, K.J.; Resnik, K.S.; Zhang, H.; Tuan, R.; Mauviel, A.; Uitto, J. Enhanced elastin and fibrillin gene expression in chronically photodamaged skin. J. Investig. Dermatol. 1994, 103, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Feinberg, E.; Lebwohl, M.; Mariani, T.J.; Boyd, C.D. Ultraviolet radiation increases tropoelastin accumulation by a post-transcriptional mechanism in dermal fibroblasts. J. Investig. Dermatol. 1995, 105, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.A.; Garner, A. Elastic and precursor fibres in the normal human eye. Exp. Eye Res. 1983, 36, 305–315. [Google Scholar] [CrossRef]
- Umihira, J.; Nagata, S.; Nohara, M.; Hanai, T.; Usuda, N.; Segawa, K. Localization of elastin in the normal and glaucomatous human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 1994, 35, 486–494. [Google Scholar]
- Wei, X.; Cai, S.P.; Zhang, X.; Li, X.; Chen, X.; Liu, X. Is low dose of estrogen beneficial for prevention of glaucoma? Med. Hypotheses 2012, 79, 377–380. [Google Scholar] [CrossRef]
- Damasceno, R.W.; Heindl, L.M.; Hofmann-Rummelt, C.; Belfort, R.; Schlötzer-Schrehardt, U.; Kruse, F.E.; Holbach, L.M. Pathogenesis of involutional ectropion and entropion: The involvement of matrix metalloproteinases in elastic fiber degradation. Orbit 2011, 30, 132–139. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Bellingham, C.M.; Davis, E.C.; Hubmacher, D.; Reinhardt, D.P.; Mecham, R.P.; Keeley, F.W. Fibrillins, fibulins, and matrix-associated glycoprotein modulate the kinetics and morphology of in vitro self-assembly of a recombinant elastin-like polypeptide. Biochemistry 2008, 47, 12601–12613. [Google Scholar] [CrossRef]
- Mohammad-Salih, P.A.; Sharif, A.F. Analysis of pterygium size and induced corneal astigmatism. Cornea 2008, 27, 434–438. [Google Scholar] [CrossRef]
- Kuchtey, J.; Chang, T.C.; Panagis, L.; Kuchtey, R.W. Marfan syndrome caused by a novel FBN1 mutation with associated pigmentary glaucoma. Am. J. Med. Genet. A 2013, 161, 880–883. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kostka, G.; Garbe, J.H.; Keene, D.R.; Bächinger, H.P.; Hanisch, F.G.; Markova, D.; Tsuda, T.; Timpl, R.; Chu, M.L.; et al. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J. Biol. Chem. 2007, 282, 11805–11816. [Google Scholar] [CrossRef]
- Hu, Q.; Loeys, B.L.; Coucke, P.J.; De Paepe, A.; Mecham, R.P.; Choi, J.; Davis, E.C.; Urban, Z. Fibulin-5 mutations: Mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum. Mol. Genet. 2006, 15, 3379–3386. [Google Scholar] [CrossRef] [PubMed]
- White, T.L.; Lewis, P.N.; Young, R.D.; Kitazawa, K.; Inatomi, T.; Kinoshita, S.; Meek, K.M. Elastic microfibril distribution in the cornea: Differences between normal and keratoconic stroma. Exp. Eye Res. 2017, 159, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.M.; Lotery, A.J.; Munier, F.L.; Héon, E.; Piguet, B.; Guymer, R.H.; Vandenburgh, K.; Cousin, P.; Nishimura, D.; Swiderski, R.E.; et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat. Genet. 1999, 22, 199–202. [Google Scholar] [CrossRef]
- Ducros, E.; Berthaut, A.; Mirshahi, P.; Lemarchand, S.; Soria, J.; Legeais, J.M.; Mirshahi, M. Expression of extracellular matrix proteins fibulin-1 and fibulin-2 by human corneal fibroblasts. Curr. Eye Res. 2007, 32, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Pasutto, F.; Sommer, P.; Hornstra, I.; Kruse, F.E.; Naumann, G.O.; Reis, A.; Zenkel, M. Genotype-correlated expression of lysyl oxidase-like 1 in ocular tissues of patients with pseudoexfoliation syndrome/glaucoma and normal patients. Am. J. Pathol. 2008, 173, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jia, L.; Wang, N.; Tang, G.; Zhang, C.; Fan, S.; Liu, W.; Meng, H.; Zeng, W.; Liu, N.; et al. Evaluation of LOXL1 polymorphisms in exfoliation syndrome in a Chinese population. Mol. Vis. 2009, 15, 2349–2357. [Google Scholar]
- Wiggs, J.L.; Pasquale, L.R. Expression and regulation of LOXL1 and elastin-related genes in eyes with exfoliation syndrome. J. Glaucoma 2014, 23, S62–S63. [Google Scholar] [CrossRef][Green Version]
- Zenkel, M.; Krysta, A.; Pasutto, F.; Juenemann, A.; Kruse, F.E.; Schlötzer-Schrehardt, U. Regulation of lysyl oxidase-like 1 (LOXL1) and elastin-related genes by pathogenic factors associated with pseudoexfoliation syndrome. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8488–8495. [Google Scholar] [CrossRef]
- Zenkel, M.; Schlötzer-Schrehardt, U. Expression and regulation of LOXL1 and elastinrelated genes in eyes with exfoliation syndrome. J. Glaucoma 2014, 23, S48–S50. [Google Scholar] [CrossRef]
- Gayathri, R.; Coral, K.; Sharmila, F.; Sripriya, S.; Sripriya, K.; Manish, P.; Shantha, B.; Ronnie, G.; Vijaya, L.; Narayanasamy, A. Correlation of Aqueous Humor Lysyl Oxidase Activity with TGF-ß Levels and LOXL1 Genotype in Pseudoexfoliation. Curr. Eye Res. 2016, 41, 1331–1338. [Google Scholar] [CrossRef]
- Padhy, B.; Kapuganti, R.S.; Hayat, B.; Mohanty, P.P.; Alone, D.P. De novo variants in an extracellular matrix protein coding gene, fibulin-5 (FBLN5) are associated with pseudoexfoliation. Eur. J. Hum. Genet. 2019, 27, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chew, J.; Yang, H.; Ang, L.P.; Tan, D.T.; Beuerman, R.W. Distinct gene subsets in pterygia formation and recurrence: Dissecting complex biological phenomenon using genome wide expression data. BMC Med. Genom. 2009, 2, 14. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Nie, D.; Zeng, K.; Hu, H.; Tie, J.; Sun, L.; Peng, L.; Liu, X.; Wang, J. Comparative Transcriptomic Analysis to Identify the Important Coding and Non-coding RNAs Involved in the Pathogenesis of Pterygium. Front. Genet. 2021, 15, 646550. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Jiang, Y.; Zhang, X.; Wang, Q. Transcriptional profiling to identify the key genes and pathways of pterygium. PeerJ 2020, 4, 8. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-López, J.; Pérez-Rico, C.; Benito-Martínez, S.; Pérez-Köhler, B.; Buján, J.; Pascual, G. The Role of the Stromal Extracellular Matrix in the Development of Pterygium Pathology: An Update. J. Clin. Med. 2021, 10, 5930. https://doi.org/10.3390/jcm10245930
Martín-López J, Pérez-Rico C, Benito-Martínez S, Pérez-Köhler B, Buján J, Pascual G. The Role of the Stromal Extracellular Matrix in the Development of Pterygium Pathology: An Update. Journal of Clinical Medicine. 2021; 10(24):5930. https://doi.org/10.3390/jcm10245930
Chicago/Turabian StyleMartín-López, Javier, Consuelo Pérez-Rico, Selma Benito-Martínez, Bárbara Pérez-Köhler, Julia Buján, and Gemma Pascual. 2021. "The Role of the Stromal Extracellular Matrix in the Development of Pterygium Pathology: An Update" Journal of Clinical Medicine 10, no. 24: 5930. https://doi.org/10.3390/jcm10245930
APA StyleMartín-López, J., Pérez-Rico, C., Benito-Martínez, S., Pérez-Köhler, B., Buján, J., & Pascual, G. (2021). The Role of the Stromal Extracellular Matrix in the Development of Pterygium Pathology: An Update. Journal of Clinical Medicine, 10(24), 5930. https://doi.org/10.3390/jcm10245930






